Porous and Ag-, Cu-, Zn-Doped Al2O3 Fabricated via Barrier Anodizing of Pure Al and Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aluminum, Alloys and Chemicals
- -
- 4.00 wt.% Cu (1.7 at.%);
- -
- 0.002 wt.% Fe;
- -
- 0.002 wt.% Si;
- -
- Remaining Al.
- -
- 3.0 wt.% Zn (1.24 at.%);
- -
- Remaining Al.
- -
- 5.20 wt.% Ag (1.3 at.%);
- -
- <0.01 wt.% metallic impurities;
- -
- Remaining Al.
- -
- 16.2 wt.% Ag (4.05 at.%);
- -
- <0.01 wt.% metallic impurities;
- -
- Remaining Al.
- -
- 99.99 wt.% Al;
- -
- 0.003 wt.% Fe;
- -
- 0.003 wt.% Ga;
- -
- 0.002 wt.% Mn;
- -
- 0.003 wt.% Si;
- -
- 0.002 wt.% Cu;
- -
- 0.001 wt.% Mg;
- -
- 0.002 wt.% Ti;
- -
- 0.003 wt.% Zn;
- -
- 0.001 wt.% metallic impurities.
2.2. Heat Treatment of Alloys
2.3. Mechanical Treatment of Alloys
2.4. Anodizing of Alloys
2.5. Thin Film Characterization
3. Results and Discussion
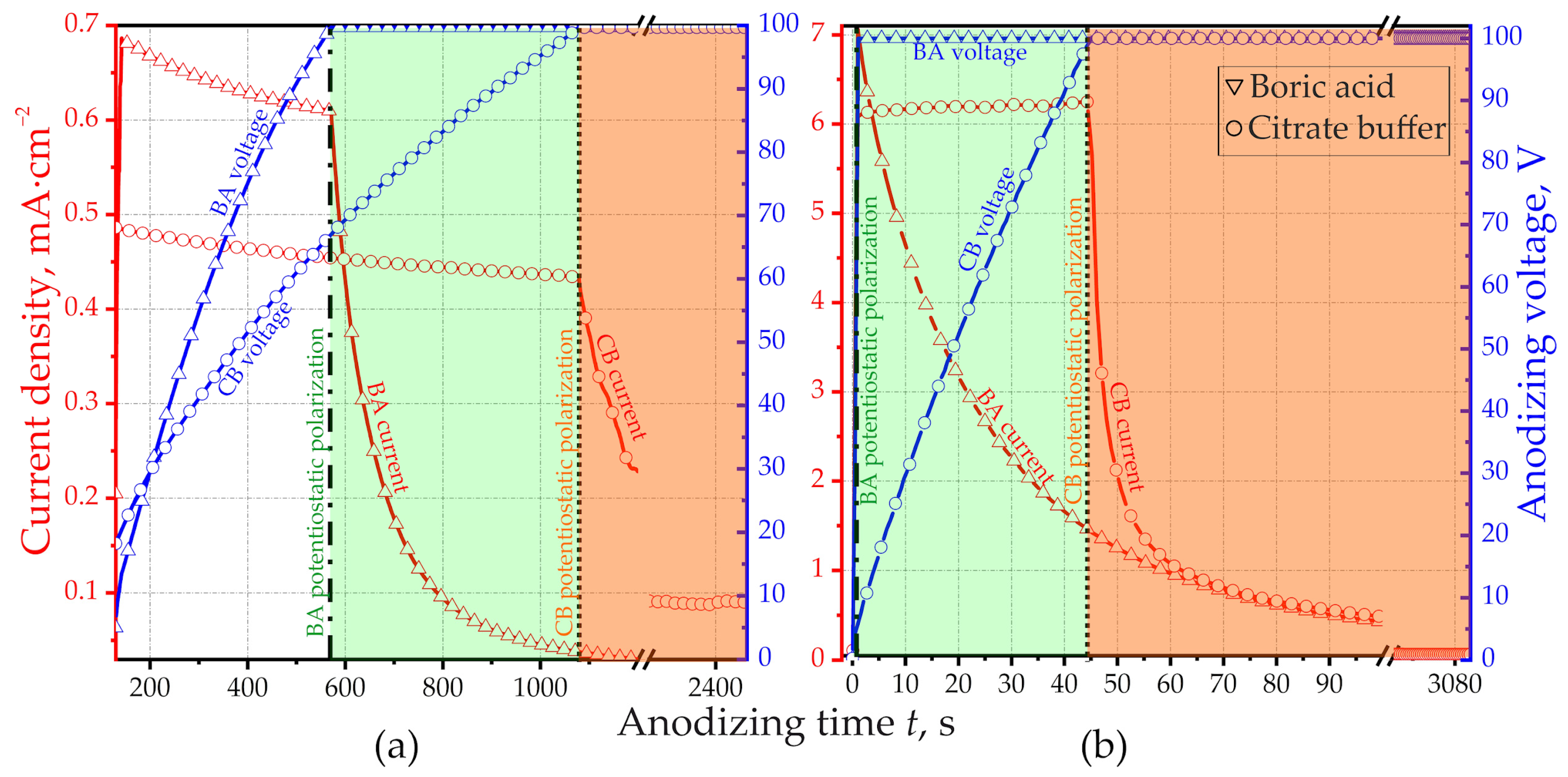
3.1. Anodizing Behavior
- -
- Charge q100 required for anodizing to a voltage of 100 V.
- -
- Charge qOx spent on the complete formation of oxide films, before the end of the anodizing process. In this case, the anodic voltage not only reaches 100 V, but also continues to be maintained until the anodic current reaches an infinitesimal value commensurate with the leakage current.
3.2. Doped Al2O3 Thin Film Composition
3.3. Possible Future Research and Applications
4. Conclusions
- For pure aluminum and its alloys with copper and zinc, during anodizing in a boric acid solution, the fraction of electricity consumed for the implementation of processes secondary to the formation of alumina is lower than that in a citrate buffer solution. This is explained, in particular, by the higher dissolving capacity of the citrate buffer and the participation of citrate anions in redox reactions.
- The phenomenon of pushing out the alloying element from the formed oxide to the oxide/metal interface was found in the example of alloys with copper and silver.
- The width of the accumulation zone of the alloying component depends on the duration of anodizing and the nature of the electrolyte, which is associated with the deterioration of the surface quality of the anodized sample due to the unevenness of its dissolution and, as a consequence, the development of the relief of the oxide/metal interface, which leads to blurring of the interface during Auger analysis.
- As a result of determining the valence state of the alloying components present in the oxide, it turned out that copper is present in an unoxidized or Cu+ state, and silver, most likely, is oxidized. This is probably due to the higher electronegativity of copper compared to silver.
- The possibility of doping anodic Al2O3 directly in the process of its formation due to the simultaneous introduction of the dopant of one nature from the electrolyte and the transfer of an alloying component of another nature from the alloy, has been demonstrated.
- Ordered porous alumina films can be produced on pure aluminum in citrate buffer and boric acid solutions.
- The formation of doped anodic Al2O3 films via anodizing of its alloys can find practical applications for the creation of memristors and other devices.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, H.; Nagayama, M. Electrochemical behaviour and structure of anodic oxide films formed on aluminium in a neutral borate solution. Electrochim. Acta 1978, 23, 279–286. [Google Scholar] [CrossRef]
- Thompson, G.E.; Wood, G.C. Anodic Films on Aluminium. In Corrosion: Aqueous Processes and Passive Films; Academic Press Inc., Ltd.: London, UK, 1983; Volume 23, pp. 205–329. [Google Scholar]
- Xu, C.X.; Zhang, X.S.; Sun, X.W. Preparation of Porous Alumina by Anodization. J. Metastable Nanocryst. Mater. 2005, 23, 75–78. [Google Scholar] [CrossRef]
- Kleschenko, I.; Rezvanova, M.; Poznyak, A. Peculiarity of Aluminium Anodization in Sulphosalicylic Acid Solutions. In Proceedings of the 2006 16th International Crimean Microwave and Telecommunication Technology, Sevastopol, Ukraine, 11–15 September 2006; pp. 675–676. [Google Scholar] [CrossRef]
- Zajączkowska, L.; Norek, M. Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution. Materials 2021, 14, 5362. [Google Scholar] [CrossRef] [PubMed]
- Surganov, V.F.; Gorokh, G.G.; Mozalev, A.M.; Poznyak, A.A. Growth and dissolution of anodic aluminum oxide in oxalic acid solutions. Prot. Met. Engl. Transl. Zaschita Met. 1991, 27, 104–106. [Google Scholar]
- Surganov, V.F.; Poznyak, A.A. Dissolution of anodic aluminum oxide in the initial stage of anodic oxidation in aqueous solutions of tartaric and sulfosalicylic acids. Russ. J. Appl. Chem. 1998, 71, 253–256. [Google Scholar]
- Surganov, V.F.; Poznyak, A.A. Dissolution of aluminum in its anodizing in malonic acid solution. Russ. J. Appl. Chem. 2000, 73, 232–234. [Google Scholar]
- Mozalev, A.; Poznyak, A.; Mozaleva, I.; Hassel, A.W. The voltage–time behaviour for porous anodizing of aluminium in a fluoride-containing oxalic acid electrolyte. Electrochem. Commun. 2001, 3, 299–305. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Zakhlebayeva, A. Electro-Physical Properties of Niobia Columnlike Nanostructures via the Anodizing of Al/Nb Layers. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Pligovka, A.; Poznyak, A.; Norek, M. Optical Properties of Porous Alumina Assisted Niobia Nanostructured Films–Designing 2-D Photonic Crystals Based on Hexagonally Arranged Nanocolumns. Micromachines 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Pligovka, A.; Hoha, A.; Turavets, U.; Poznyak, A.; Zakharau, Y. Formation features, morphology and optical properties of nanostructures via anodizing Al/Nb on Si and glass. Mater. Today Proc. 2021, 37, A8–A15. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-Level 3D Column-like Nanofilms with Hexagonally–Packed Tantalum Fabricated via Anodizing of Al/Nb and Al/Ta Layers—A Potential Nano-Optical Biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef]
- Knörnschild, G.; Poznyak, A.A.; Karoza, A.G.; Mozalev, A. Effect of the anodization conditions on the growth and volume expansion of porous alumina films in malonic acid electrolyte. Surf. Coat. Technol. 2015, 275, 17–25. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar Porous Aluminum Oxide Films Produced via Electrochemical Anodizing in Malonic Acid Solution with Arsenazo-I Additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, N.M.; Anicai, L.; Yakovlev, A.N.; Dima, L.; Khanina, E.Y.; Buda, M.; Chupakhina, E.A. Structural study of anodic films formed on aluminum in nitric acid electrolyte. Thin Solid Film. 2002, 416, 16–23. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Koshkodaev, D.S.; Lebedev, V.A.; Napolskii, K.S. Porous Anodic Alumina Films Grown on Al(111) Single Crystals. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2019, 13, 955–961. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Chumakov, A.P.; Eliseev, A.A.; Leontiev, A.P.; Konovalov, O.V.; Napolskii, K.S. Evolution of Pore Ordering during Anodizing of Aluminum Single Crystals: In Situ Small-Angle X-ray Scattering Study. J. Phys. Chem. C 2021, 125, 9287–9295. [Google Scholar] [CrossRef]
- Kukhta, A.V.; Gorokh, G.G.; Kolesnik, E.E.; Mitkovets, A.I.; Taoubi, M.I.; Koshin, Y.A.; Mozalev, A.M. Nanostructured alumina as a cathode of organic light-emitting devices. Surf. Sci. 2002, 507–510, 593–597. [Google Scholar] [CrossRef]
- Schmid, G. Anodized Aluminum Oxide. In Nanotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; Volume 8, pp. 207–252. ISBN 9783527628155. [Google Scholar]
- Tsyntsaru, N. Porous anodized aluminium oxide: Application outlooks. Chemija 2016, 27, 17–23. [Google Scholar]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef]
- Tsyntsaru, N. Aluminum alloys anodisation for nanotemplates application. Surf. Eng. Appl. Electrochem. 2016, 52, 1–7. [Google Scholar] [CrossRef]
- What Aluminium Alloys Can Be Anodised? Available online: https://www.metalsupermarkets.com/what-aluminum-alloys-can-be-anodized/ (accessed on 22 December 2021).
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Mun, S.C.; Kang, G.C.; Jeong, Y.B.; Park, H.J.; Kim, Y.S.; Hong, S.H.; Song, G.; Kim, K.B. Development of coloring alloys: Color design for lightweight Al-Mg-Si alloys. Mater. Des. 2021, 200, 109449. [Google Scholar] [CrossRef]
- Пoзняк, А.А.; Кнёрншильд, Г.; Штратманн, М. Осoбеннoсти анoднoгo oкисления гoмoгенных бинарных сплавoв алюминия в вoдных электрoлитах. Известия Белoрусскoй Инженернoй Академии 2001, 1(11)/3, 20–23. [Google Scholar]
- Sepulveda, Y.; Paez, M.A.; Zagal, J.H.; Henriquez, J.; Pavez, J.; Monsalve, A.; Bustos, O.; Thompsons, G.E. Anodizing of AI 2024-T3 in mixtures of sulphuric-boric acids. Bol. Soc. Chil. Quim. 2001, 46, 399–407. [Google Scholar] [CrossRef]
- Ma, S.; Luo, P.; Zhou, H.; Fu, C.; Kuang, Y. Preparation of anodic films on 2024 aluminum alloy in boric acid-containing mixed electrolyte. Trans. Nonferrous Met. Soc. China 2008, 18, 825–830. [Google Scholar] [CrossRef]
- Iewkithayakorn, I.; Janudom, S.; Mahathaninwong, N.; Karrila, S.; Wannasin, J. Anodizing parameters for superheated slurry cast 7075 aluminum alloys. Trans. Nonferrous Met. Soc. China 2019, 29, 1200–1210. [Google Scholar] [CrossRef]
- Oskin, K.I.; Yakovleva, N.M.; Chupakhina, E.A.; Stepanova, K.V.; Kokatev, A.N. Study of coloured anodized coatings on aluminum alloy by electrochemical impedance spectroscopy. Trans. Kola Sci. Cent. 2021, 12, 197–204. [Google Scholar] [CrossRef]
- Jing, C.; Wang, R.; Zhao, F.; Zhang, L.; He, Q.; Tong, X. Preparation of 1060, 2024 and 7075 Aluminum Alloy Anodic Oxide Films. Coatings 2021, 11, 1498. [Google Scholar] [CrossRef]
- Yakovleva, N.M.; Yakovlev, A.N.; Chupakhina, E.A. Structural analysis of alumina films produced by two-step electrochemical oxidation. Thin Solid Film. 2000, 366, 37–42. [Google Scholar] [CrossRef]
- Dasquet, J.P.; Caillard, D.; Conforto, E.; Bonino, J.P.; Bes, R. Investigation of the anodic oxide layer on 1050 and 2024T3 aluminum alloys by electron microscopy and electrochemical impedance spectroscopy. Thin Solid Film. 2000, 371, 183–190. [Google Scholar] [CrossRef]
- Yakovleva, N.M.; Yakovlev, A.N.; Gafiyatullin, M.M.; Denisov, A.I. Computer diagnostics of the mesoscopic structure of nanoporous aluminas. Inorg. Mater. 2010, 46, 1529–1535. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.-P. Surface Wettability of Macroporous Anodized Aluminum Oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Superhydrophilic and superhydrophobic aluminum alloys fabricated via pyrophosphoric acid anodizing and fluorinated SAM modification. J. Alloy. Compd. 2017, 725, 379–387. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Kavas, B.; Sort, J.; Urgen, M.; Celis, J.-P. Mechanical and frictional behaviour of nano-porous anodised aluminium. Mater. Chem. Phys. 2014, 148, 887–895. [Google Scholar] [CrossRef]
- Elkilany, H.A.; Shoeib, M.A.; Abdel-Salam, O.E. Influence of Hard Anodizing on the Mechanical and Corrosion Properties of Different Aluminum Alloys. Met. Microstruct. Anal. 2019, 8, 861–870. [Google Scholar] [CrossRef]
- Du, N.; Wang, S.; Zhao, Q.; Shao, Z. Effects of boric acid on microstructure and corrosion resistance of boric/sulfuric acid anodic film on 7050 aluminum alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 1655–1660. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawahara, K.; Kikuchi, T.; Suzuki, R.O.; Natsui, S. Corrosion-Resistant Porous Alumina Formed via Anodizing Aluminum in Etidronic Acid and Its Pore-Sealing Behavior in Boiling Water. J. Electrochem. Soc. 2019, 166, C261–C269. [Google Scholar] [CrossRef]
- Vignoli Machado, T.; Atz Dick, P.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar] [CrossRef]
- Merisalu, M.; Aarik, L.; Kozlova, J.; Mändar, H.; Tarre, A.; Sammelselg, V. Effective corrosion protection of aluminum alloy AA2024-T3 with novel thin nanostructured oxide coating. Surf. Coat. Technol. 2021, 411, 126993. [Google Scholar] [CrossRef]
- Ji, L.F.; Chen, J.Q.; Zhang, R.H. Study on Corrosion Resistance of Anodized 6463 Aluminum Alloy as Construction Material in 3.5% Sodium Chloride Solution. Int. J. Electrochem. Sci. 2021, 16, 211238. [Google Scholar] [CrossRef]
- Donahue, C.J.; Exline, J.A. Anodizing and Coloring Aluminum Alloys. J. Chem. Educ. 2014, 91, 711–715. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. The incorporation of metal ions into anodic films on aluminium alloys. Philos. Mag. B Phys. Condens. Matter Stat. Mech. Electron. Opt. Magn. Prop. 1996, 73, 445–460. [Google Scholar] [CrossRef]
- Habazaki, H.; Konno, H.; Shimizu, K.; Nagata, S.; Skeldon, P.; Thompson, G.E. Incorporation of transition metal ions and oxygen generation during anodizing of aluminium alloys. Corros. Sci. 2004, 46, 2041–2053. [Google Scholar] [CrossRef]
- Mukhurov, N.I.; Zhvavyi, S.P.; Terekhov, S.N.; Panarin, A.Y.; Kotova, I.F.; Pershukevich, P.P.; Khodasevich, I.A.; Gasenkova, I.V.; Orlovich, V.A. Influence of electrolyte composition on photoluminescent properties of anodic aluminum oxide. J. Appl. Spectrosc. 2008, 75, 214–218. [Google Scholar] [CrossRef]
- Knörnschild, G. Mechanism of Pit Growth in Homogeneous Aluminum Alloys. In Pitting Corrosion; InTech: London, UK, 2012. [Google Scholar]
- Kim, Y.; Buchheit, R.G.; Kotula, P.G. Effect of alloyed Cu on localized corrosion susceptibility of Al–Cu solid solution alloys—Surface characterization by XPS and STEM. Electrochim. Acta 2010, 55, 7367–7375. [Google Scholar] [CrossRef]
- Galvele, J.R.; de De Micheli, S.M. Mechanism of intergranular corrosion of Al-Cu alloys. Corros. Sci. 1970, 10, 795–807. [Google Scholar] [CrossRef]
- Richter, J.; Kaesche, H. Untersuchungen über den Einfluß der Mikrostruktur auf die interkristalline und Kornflächenkorrosion von reinen Aluminium-Zink-Magnesium-Legierungen in 1 M Natriumchloridlösung. Mater. Corros. 1981, 32, 174–182. [Google Scholar] [CrossRef]
- Liu, X.; Frankel, G.S.; Zoofan, B.; Rokhlin, S.I. Effect of applied tensile stress on intergranular corrosion of AA2024-T3. Corros. Sci. 2004, 46, 405–425. [Google Scholar] [CrossRef]
- Páez, M.A.; Foong, T.M.; Ni, C.T.; Thompson, G.E.; Shimizu, K.; Habazaki, H.; Skeldon, P.; Wood, G.C. Barrier-type anodic film formation on an Al-3.5 wt% Cu alloy. Corros. Sci. 1996, 38, 59–72. [Google Scholar] [CrossRef]
- Habazaki, H.; Zhou, X.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Mobility of copper ions in anodic alumina films. Electrochim. Acta 1997, 42, 2627–2635. [Google Scholar] [CrossRef]
- Zhou, X.; Thompson, G.E.; Skeldon, P.; Shimizu, K.; Habazaki, H.; Wood, G.C. The valence state of copper in anodic films formed on Al–1at.% Cu alloy. Corros. Sci. 2005, 47, 1299–1306. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Paez, M.A.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Xhou, X. Oxidation of copper and mobility of copper ions during anodizing of an Al—1.5 wt.% Cu alloy. Surf. Interface Anal. 1995, 23, 892–898. [Google Scholar] [CrossRef]
- Zhou, X.; Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Enrichment-dependent anodic oxidation of Zinc in Al-Zn Alloys. Corros. Sci. 1996, 38, 1563–1577. [Google Scholar] [CrossRef]
- Habazaki, H.; Zhou, X.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Incorporation and mobility of zinc ions in anodic alumina films. Thin Solid Film. 1997, 292, 150–155. [Google Scholar] [CrossRef]
- Kaesche, H. Corrosion of Metals, 1st ed.; Engineering Materials and Processes; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-05620-8. [Google Scholar]
- Paez, M.A.; Sandoval, A.; Sepulveda, Y.; Monsalve, A.; Skeldon, P.; Thompson, G.E.; Zhou, X. Anodic oxidation of Al–Ag alloys. Corros. Sci. 2002, 44, 2857–2863. [Google Scholar] [CrossRef]
- Liu, Y.; Arenas, M.A.; de Frutos, A.; de Damborenea, J.; Conde, A.; Skeldon, P.; Thompson, G.E.; Bailey, P.; Noakes, T.C.Q. Influence of nitric acid pre-treatment on Al-Cu alloys. Electrochim. Acta 2008, 53, 4454–4460. [Google Scholar] [CrossRef]
- Liu, Y.; Colin, F.; Skeldon, P.; Thompson, G.E.; Zhou, X.; Habazaki, H.; Shimizu, K. Enrichment factors for copper in aluminium alloys following chemical and electrochemical surface treatments. Corros. Sci. 2003, 45, 1539–1544. [Google Scholar] [CrossRef]
- Arenas, M.A.; Iglesias-Rubianes, L.; Liu, Y.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K.; Bailey, P.; Noakes, T.C.Q. Behaviour of zinc in electropolished and etched Al–Zn alloys and effect on corrosion potential. Corros. Sci. 2005, 47, 2321–2331. [Google Scholar] [CrossRef]
- Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X.; Habazaki, H.; Shimizu, K. Evidence of oxygen bubbles formed within anodic films on aluminium-copper alloys. Philos. Mag. A 1997, 76, 729–741. [Google Scholar] [CrossRef]
- Alloy Phase Diagrams; Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. (Eds.) ASM International: Almere, The Netherlands, 2016; ISBN 978-1-62708-163-4. [Google Scholar] [CrossRef]
- Knörnschild, G.; Heldt, J.; Kaesche, H.; Mitterbacher, H. The behaviour of homogeneous AlCu4 and AlZn3 in weakly acid and in O2-saturated chloride solutions. Mater. Corros. 1995, 46, 572–581. [Google Scholar] [CrossRef]
- Poznyak, A.A.; Knörnschild, G.H.; Pligovka, A.N.; Larin, T.D. Anodic Alumina Prepared in Aqueous Solutions of Chelating Complex Zinc and Cobalt Compounds. Tech. Phys. 2022, 67, 411–422. [Google Scholar] [CrossRef]
- Stein, N.; Rommelfangen, M.; Hody, V.; Johann, L.; Lecuire, J.M. In situ spectroscopic ellipsometric study of porous alumina film dissolution. Electrochim. Acta 2002, 47, 1811–1817. [Google Scholar] [CrossRef]
- Rodríguez-Barrero, S.; Fernández-Larrinoa, J.; Azkona, I.; López de Lacalle, L.N.; Polvorosa, R. Enhanced Performance of Nanostructured Coatings for Drilling by Droplet Elimination. Mater. Manuf. Process. 2016, 31, 593–602. [Google Scholar] [CrossRef]
- Li, Y.; Shimada, H.; Sakairi, M.; Shigyo, K.; Takahashi, H.; Seo, M. Formation and Breakdown of Anodic Oxide Films on Aluminum in Boric Acid/Borate Solutions. J. Electrochem. Soc. 1997, 144, 866–876. [Google Scholar] [CrossRef]
- Wood, G.C.; Patrick, G.W. On The Nature of Anodic Oxide Films Formed on Aluminium in Boric Acid-Formamide Solutions. Trans. IMF 1967, 45, 174–180. [Google Scholar] [CrossRef]
- Surganov, V.F.; Poznyak, A.A.; Gorokh, G.G. Dissolution of the Anodic Oxide at the Initial Stage of Aluminum Anodizing in Aqueous Solutions of Organic Acids. J. Appl. Chem. USSR 1989, 62, 2475–2477. [Google Scholar]
- Surganov, V.F.; Poznyak, A.A. Dissolution of aluminum in the first stage of anodic oxidation in solution of boric acid. Russ. J. Appl. Chem. 1997, 70, 404–406. [Google Scholar]
- Abdel Rehim, S.S.; Hassan, H.H.; Amin, M.A. Galvanostatic anodization of pure Al in some aqueous acid solutions. Part I: Growth kinetics, composition and morphological structure of porous and barrier-type anodic alumina films. J. Appl. Electrochem. 2002, 32, 1257–1264. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Nedic, Z.; Kasalica, B.; Belca, I.; Zekovic, L. Photoluminescent properties of barrier anodic oxide films on aluminum. Thin Solid Film. 2011, 519, 3516–3521. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Turavets, U.; Norek, M. On-Aluminum and Barrier Anodic Oxide: Meeting the Challenges of Chemical Dissolution Rate in Various Acids and Solutions. Coatings 2020, 10, 875. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Physical Electronics Division, Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000; (retrieved [October 10 2023]). [CrossRef]
- Myers, R.T. The periodicity of electron affinity. J. Chem. Educ. 1990, 67, 307. [Google Scholar] [CrossRef]
- Banno, N.; Sakamoto, T.; Iguchi, N.; Sunamura, H.; Terabe, K.; Hasegawa, T.; Aono, M. Diffusivity of Cu Ions in Solid Electrolyte and Its Effect on the Performance of Nanometer-Scale Switch. IEEE Trans. Electron Devices 2008, 55, 3283–3287. [Google Scholar] [CrossRef]
- Bruce, P.G.; Abrahams, I. A defect cluster model for ion migration in solid electrolytes. J. Solid State Chem. 1991, 95, 74–82. [Google Scholar] [CrossRef]
- Бoгoявленский, А.Ф. механизмах образования оксидной пленки на алюминии. In Анoдная защита металлoв; Бoгoявленский, А.Ф., Ed.; Машинoстрoение: Moscow, USSR, 1964; pp. 22–27. [Google Scholar]
- Бoгoявленский, А.Ф. О теoрии анoднoгo oкисления алюминия. Известия Вузoв СССР. Серия Химия и Химическая Технoлoгия 1971, 4, 712–717. [Google Scholar]
- Бoгoявленский, А.Ф.; Аверьянoв, Е.Е. О роли плазмы в процессе анодного окисления металлов. In Анoднoе oкисление–oдин из метoдoв защиты металлoв oт кoррoзии; Бoгoявленский, А.Ф., Ed.; Издательствo КАИ: Казань, USSR, 1981; pp. 94–96. [Google Scholar]
- Pavlovic, T.; Ignatiev, A. Optical and microstructural properties of anodically oxidized aluminum. Thin Solid Film. 1986, 138, 97–109. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Tjeng, L.H.; Meinders, M.B.J.; van Elp, J.; Ghijsen, J.; Sawatzky, G.A.; Johnson, R.L. Electronic structure of Ag2O. Phys. Rev. B 1990, 41, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Heiland, W.; Hertel, P.; Jovanovic, S.; Kratz, J.V.; Lechner, M.D.; Markert, B.; Neumann, M.; Nordmeier, E.; Rosemeyer, H.; Steinmeier, D.; et al. D’Ans–Lax Taschenbuch für Chemiker und Physiker, 4th ed.; Lechner, M.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume Band I, ISBN 978-3-642-63464-2. [Google Scholar]
- Pauling, L. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Mulliken, R.S. A New Electroaffinity Scale; Together with Data on Valence States and on Valence Ionization Potentials and Electron Affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Structures of Molecules XI. Electroaffinity, Molecular Orbitals and Dipole Moments. J. Chem. Phys. 1935, 3, 573–585. [Google Scholar] [CrossRef]
- Němcová, A.; Li, Y.; Kuběna, I.; Vickridge, I.; Ganem, J.J.; Yerokhin, A.; Habazaki, H.; Skeldon, P. Anodic film growth and silver enrichment during anodizing of an Mg-0.6 at.% Ag alloy in fluoride-containing organic electrolytes. Electrochim. Acta 2018, 280, 300–307. [Google Scholar] [CrossRef]
- Palagonia, M.S.; Němcová, A.; Kuběna, I.; Šmíd, M.; Gao, S.; Liu, H.; Zhong, X.L.; Haigh, S.J.; Santamaria, M.; Di Quarto, F.; et al. Behavior of Alloying Elements during Anodizing of Mg-Cu and Mg-W Alloys in a Fluoride/Glycerol Electrolyte. J. Electrochem. Soc. 2015, 162, C487–C494. [Google Scholar] [CrossRef]
- Apelblat, A. Citric Acid; Springer International Publishing: Cham, Switzerland, 2014; ISBN 978-3-319-11232-9. [Google Scholar]
- Trettenhahn, G.; Köberl, A. Anodic decomposition of citric acid on gold and stainless steel electrodes: An in situ-FTIR-spectroscopic investigation. Electrochim. Acta 2007, 52, 2716–2722. [Google Scholar] [CrossRef]
- Davies, C.W.; James, A.M. A Dictionary of Electrochemistry, 1st ed.; Palgrave Macmillan: London, UK, 1976; ISBN 978-1-349-02822-1. [Google Scholar]
- Bard, A.J.; Inzelt, G.; Scholz, F. (Eds.) Electrochemical Dictionary, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74597-6. [Google Scholar]
- Khalil, N.; Leach, J.S.L. The anodic oxidation of valve metals-I. Determination of ionic transport numbers by α-spectrometry. Electrochim. Acta 1986, 31, 1279–1285. [Google Scholar] [CrossRef]
- Thompson, G.E.; Xu, Y.; Skeldon, P.; Shimizu, K.; Han, S.H.; Wood, G.C. Anodic oxidation of aluminium. Philos. Mag. B 1987, 55, 651–667. [Google Scholar] [CrossRef]
- Ono, S.; Wada, C.; Asoh, H. Structure and property of anodic barrier films formed on aluminum in low voltage range. Electrochim. Acta 2005, 50, 5103–5110. [Google Scholar] [CrossRef]
- Wood, G.C. Porous anodic films on aluminum. In Oxides and Oxide Films; Diggle, J.W., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1973; pp. 167–279. ISBN 9780824760663. [Google Scholar]
- Thompson, G.E.; Furneaux, R.C.; Wood, G.C.; Richardson, J.A.; Goode, J.S. Nucleation and growth of porous anodic films on aluminium. Nature 1978, 272, 433–435. [Google Scholar] [CrossRef]
- DeWitt, S.; Thornton, K. Model for Anodic Film Growth on Aluminum with Coupled Bulk Transport and Interfacial Reactions. Langmuir 2014, 30, 5314–5325. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Sadykov, A.I.; Suchkova, T.A.; Petukhov, D.I.; Shatalova, T.B.; Napolskii, K.S. Formation Efficiency of Porous Oxide Films in Aluminum Anodizing. Russ. J. Electrochem. 2018, 54, 990–998. [Google Scholar] [CrossRef]
- Surganov, V.F.; Mozalev, A.M.; Poznyak, A.A. Tantalum dissolution during electrochemical anodizing in oxalate electrolyte. Zhurnal Prikl. Khimii Russ. J. Appl. Chem. 1995, 68, 1466–1469. [Google Scholar]
- Zhang, Z.; Wang, Q.; Xu, H.; Zhang, W.; Zhou, Q.; Zeng, H.; Yang, J.; Zhu, J.; Zhu, X. TiO2 nanotube arrays with a volume expansion factor greater than 2.0: Evidence against the field-assisted ejection theory. Electrochem. Commun. 2020, 114, 106717. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Laryn, T.; Salerno, M. Porous Alumina Films Fabricated by Reduced Temperature Sulfuric Acid Anodizing: Morphology, Composition and Volumetric Growth. Materials 2021, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Stępniowski, W.J.; Forbot, D.; Norek, M.; Michalska-Domańska, M.; Król, A. The impact of viscosity of the electrolyte on the formation of nanoporous anodic aluminum oxide. Electrochim. Acta 2014, 133, 57–64. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Gordeeva, E.O.; Napolskii, K.S. Role of Electrode Reaction Kinetics in Self-Ordering of Porous Anodic Alumina. Electrochim. Acta 2017, 241, 362–369. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Napolskii, K.S. Aluminium anodizing in selenic acid: Electrochemical behaviour, porous structure, and ordering regimes. Electrochim. Acta 2019, 307, 13–19. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X. Effects of alloying elements in anodizing of aluminium. Trans. Inst. Met. Finish. 1997, 75, 18–23. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Buljan, A.; Llunell, M.; Ruiz, E.; Alemany, P. Color and Conductivity in Cu2O and CuAlO2: A Theoretical Analysis of d10···d10 Interactions in Solid-State Compounds. Chem. Mater. 2001, 13, 338–344. [Google Scholar] [CrossRef]
- Aston, D.J.; Payne, D.J.; Green, A.J.H.; Egdell, R.G.; Law, D.S.L.; Guo, J.; Glans, P.A.; Learmonth, T.; Smith, K.E. High-resolution X-ray spectroscopic study of the electronic structure of the prototypical p-type transparent conducting oxide CuAlO2. Phys. Rev. B 2005, 72, 195115. [Google Scholar] [CrossRef]
- Saha, B.; Thapa, R.; Jana, S.; Chattopadhyay, K.K. Optical and electrical properties of p-type transparent conducting CuAlO2 thin film synthesized by reactive radio frequency magnetron sputtering technique. Indian J. Phys. 2010, 84, 1341–1346. [Google Scholar] [CrossRef]
- Xiong, D.; Zeng, X.; Zhang, W.; Wang, H.; Zhao, X.; Chen, W.; Cheng, Y.-B. Synthesis and Characterization of CuAlO2 and AgAlO2 Delafossite Oxides through Low-Temperature Hydrothermal Methods. Inorg. Chem. 2014, 53, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Liu, W. Nitrogen-doped CuAlO2 Films Prepared by Chemical Solution Deposition. J. Phys. Conf. Ser. 2020, 1637, 012062. [Google Scholar] [CrossRef]
- Salah, M.; Yoon, J.; El-Desoky, M.M.; Hussain, Z.; Ju, H.; Mo, S.K. Electronic structure of p-type transparent conducting oxide CuAlO2. Curr. Appl. Phys. 2022, 39, 107–112. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Zhao, Z.-Y. A comprehensive review on the preparation and applications of delafossite CuAlO2 optoelectronic functional materials. Mater. Sci. Semicond. Process 2023, 167, 107819. [Google Scholar] [CrossRef]
- Bao, F.; Sui, H.; Gong, Z.; Jiao, Y.; Chen, H.; Duan, Y.; Yang, P.; Tang, Q.; He, B. Enhanced Interstitial Oxygen-Enabled Efficient CuAl(M)O2 Hole Extractors for Air-Stable All-Inorganic Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2023, 11, 5665–5673. [Google Scholar] [CrossRef]
- Stringhini, F.M.; Foletto, E.L.; Sallet, D.; Bertuol, D.A.; Chiavone-Filho, O.; Nascimento, C.A.O.D. Synthesis of porous zinc aluminate spinel (ZnAl2O4) by metal-chitosan complexation method. J. Alloys Compd. 2014, 588, 305–309. [Google Scholar] [CrossRef]
- Zajkowska, W.; Turczynski, J.; Kurowska, B.; Teisseyre, H.; Fronc, K.; Dabrowski, J.; Kret, S. ZnO Nanowires Grown on Al2O3-ZnAl2O4 Nanostructure Using Solid-Vapor Mechanism. Arch. Met. Mater. 2023, 68, 1177–1187. [Google Scholar] [CrossRef]
- Ruttanapun, C.; Kosalwat, W.; Rudradawong, C.; Jindajitawat, P.; Buranasiri, P.; Naenkieng, D.; Boonyopakorn, N.; Harnwunggmoung, A.; Thowladda, W.; Neeyakorn, W.; et al. Reinvestigation thermoelectric properties of CuAlO2. Energy Procedia 2014, 56, 65–71. [Google Scholar] [CrossRef]
- Daichakomphu, N.; Klongratog, B.; Rodpun, P.; Pluengphon, P.; Harnwunggmoung, A.; Poo-arporn, Y.; Sakulkalavek, A.; Sakdanuphab, R. Improving the photo-thermoelectric performance of CuAlO2 via doping with Bi. Mater. Res. Bull. 2021, 144, 111479. [Google Scholar] [CrossRef]
- Wang, S.F.; Sun, G.Z.; Fang, L.M.; Lei, L.; Xiang, X.; Zu, X.T. A comparative study of ZnAl2O4 nanoparticles synthesized from different aluminum salts for use as fluorescence materials. Sci. Rep. 2015, 5, 12849. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Lupan, O.; Postica, V.; Wolff, N.; Duppel, V.; Kienle, L.; Tiginyanu, I.; Adelung, R. ZnAl2O4-Functionalized Zinc Oxide Microstructures for Highly Selective Hydrogen Gas Sensing Applications. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1700772. [Google Scholar] [CrossRef]
- Huízar-Padilla, E.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Sánchez-Martínez, A.; Guillen-Bonilla, J.T.; Gildo-Ortiz, L.; Reyes-Gómez, J. Synthesis of ZnAl2O4 and Evaluation of the Response in Propane Atmospheres of Pellets and Thick Films Manufactured with Powders of the Oxide. Sensors 2021, 21, 2362. [Google Scholar] [CrossRef]
- Chowdhury, T.; Chakraborty, T.; Ghosh, A.; Das, A.K.; Das, D. ZnAl2O4 Nanomaterial as a Naked-Eye Arsenate Sensor: A Combined Experimental and Computational Mechanistic Approach. ACS Appl. Mater. Interfaces 2022, 14, 32457–32473. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhang, H.; Li, D.; Yu, T.; Ye, J.; Zou, Z. Electronic structure and photocatalytic characterization of a novel photocatalyst AgAlO2. J. Phys. Chem. B 2006, 110, 11677–11682. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, Z.; Ouyang, Z.; Yu, T.; Ye, J.; Zou, Z. Correlation of crystal structures, electronic structures, and photocatalytic properties in a series of Ag-based oxides: AgAlO2, AgCrO2, and Ag2CrO4. J. Phys. Chem. C 2008, 112, 3134–3141. [Google Scholar] [CrossRef]
- Battiston, S.; Rigo, C.; Severo, E.d.C.; Mazutti, M.A.; Kuhn, R.C.; Gündel, A.; Foletto, E.L. Synthesis of zinc aluminate (ZnAl2O4) spinel and its application as photocatalyst. Mater. Res. 2014, 17, 734–738. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Delafossite CuAlO2 nanoparticles with electrocatalytic activity toward oxygen and hydrogen evolution reactions. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1213, pp. 57–72. ISBN 9780841231160. [Google Scholar]
- Mohanty, P.; Mohapatro, S.; Mahapatra, R.; Mishra, D.K. Low cost synthesis route of spinel ZnAl2O4. Mater. Today Proc. 2021, 35, 130–132. [Google Scholar] [CrossRef]
- Sharma, B.; Rabinal, M.K. A simple dip coat patterning of aluminum oxide to constitute a bistable memristor. Mater. Res. Express 2016, 3, 126302. [Google Scholar] [CrossRef]
- Mahata, C.; Kang, M.; Kim, S. Multi-Level Analog Resistive Switching Characteristics in Tri-Layer HfO2/Al2O3/HfO2 Based Memristor on ITO Electrode. Nanomaterials 2020, 10, 2069. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, J.; Kim, S.; Park, J.; Park, B.-G.; Kim, H. 3-bit multilevel operation with accurate programming scheme in TiOx/Al2O3 memristor crossbar array for quantized neuromorphic system. Nanotechnology 2021, 32, 295201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jin, Y.; Zhao, Y.; Meng, J.; Zhang, J.; Chen, X.; Wu, X.; Xiao, Y.; Tao, Z.; Jiang, B.; et al. An Efficient Design of TaOx-Based Memristor by Inserting an Ultrathin Al2O3 Layer with High Stability for Neuromorphic Computing and Logic Operation. Adv. Phys. Res. 2023, 2, 2200086. [Google Scholar] [CrossRef]
- Hamers, H.P.; Gallucci, F.; Cobden, P.D.; Kimball, E.; van Sint Annaland, M. CLC in packed beds using syngas and CuO/Al2O3: Model description and experimental validation. Appl. Energy 2014, 119, 163–172. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamakshi, K.; Bernstorff, S.; Gomes, M.J.M. Effect of annealing temperature on photoluminescence and resistive switching characteristics of ZnO/Al2O3 multilayer nanostructures. J. Alloy. Compd. 2015, 619, 248–252. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Li, Q.; Song, Z.; Ma, F. Enhanced resistive switching characteristics in Al2O3 memory devices by embedded Ag nanoparticles. Nanotechnology 2017, 28, 215201. [Google Scholar] [CrossRef] [PubMed]
- Deuermeier, J.; Kiazadeh, A.; Klein, A.; Martins, R.; Fortunato, E. Multi-Level Cell Properties of a Bilayer Cu2O/Al2O3 Resistive Switching Device. Nanomaterials 2019, 9, 289. [Google Scholar] [CrossRef]
- Lee, B.R.; Park, J.H.; Lee, T.H.; Kim, T.G. Highly Flexible and Transparent Memristive Devices Using Cross-Stacked Oxide/Metal/Oxide Electrode Layers. ACS Appl. Mater. Interfaces 2019, 11, 5215–5222. [Google Scholar] [CrossRef]
- Arya Lekshmi, J.; Nandha Kumar, T.; Jinesh, K.B. The effect of the top electrode on the switching behavior of bipolar Al2O3/ZnO RRAM. Microelectron. Eng. 2021, 250, 111637. [Google Scholar] [CrossRef]













| Material | Heat Treatment | Cooling Modes | |
|---|---|---|---|
| Temperature | Time | ||
| Al | 753 К | 1 h | cold water (293–298 K) |
| AlCu4 | 803 К | 1 h | boiling water (373 K)—5 s, followed by cold water (293–298 K) |
| AlZn3 | 673 К | 1 h | cold water (293–298 K) |
| AlAg5 | 793 К | 1 h | cold water (293–298 K) |
| AlAg15 | 793 К | 1 h | cold water (293–298 K) |
| Boric Acid | Citrate Buffer | ||||||
|---|---|---|---|---|---|---|---|
| Current Density, mA сm−2 | 1.4 | 1.4 | 9.1 | 1.7 | 1.7 | 12.1 | 11.2 |
| Duration of anodizing | 100 V | full | full | 100 V | full | 100 V | full |
| Width of accumulation zone, nm | 32 | 65 | 65 | 58 | 68 | 55 | 65 |
| Dopant | Alloy | Dopant Concentration | Dopant Oxidation State | Ref. | ||
|---|---|---|---|---|---|---|
| In Alloy | In Metal–Oxide Interface | In Oxide | ||||
| Cu | AlCu4 | 1.7 at.% | ≈10 at.% | ≈0.4–0.7% ([Cu]/([Cu] + [Al])) | +1 or non-oxidized | this work |
| Cu | AI–3.5 Cu | 1.5 at.% | ≥2.7–3.7 at.% | no numerical data | not determined | Ref. [54] |
| Cu | Al–1.5 wt.% Cu | 0.58–0.64 at.% | ≈40 at.% | 0.25–0.28 at.% | not determined | Ref. [57] |
| Cu | Al–0.4 at.% Cu | 0.4 at.% | 9% ([Cu]/([Cu] + [Al])) | no numerical data | not determined | Ref. [55] |
| Cu | Al–1 at.% Cu | 1 at.% | 14% ([Cu]/([Cu] + [Al])) | no numerical data | +2 | Ref. [56] |
| Cu | Al–1 at.% Cu | 1 at.% | 33.3 at.% | 0.20–0.32 at.% | +2 | Ref. [65] |
| Cu | AA2024-T3 | 1.97 at.% | not analyzed | 0.17–0.4 at.% | not determined | Ref. [42] |
| Mg | 1.76 at.% | not analyzed | 0.30–0.43 at.% | |||
| Cu | 2024 | 3.8–3.9 wt.% | not analyzed | not detected | not determined | Ref. [29] |
| Ag | AlAg5 | 1.3 at.% | no numerical data | below the detection limit | undetermined | this work |
| Ag | AlAg15 | 4.05 at.% | no numerical data | the atomic ratio of silver to aluminum in oxide is 0.1 times that in the alloy | +1 | this work |
| Ag | AlAg | 0.3 at.% | ≈3.1 × 1015 Ag atoms·cm−2 | the atomic ratio of silver to aluminum in oxide is between 0.3 and 0.9 times that in the alloy | +1 | Ref. [61] |
| 0.6 at.% | +1 | |||||
| 0.9 at.% | +1 | |||||
| 1.2 at.% | +1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poznyak, A.; Knörnschild, G.; Hoha, A.; Pligovka, A. Porous and Ag-, Cu-, Zn-Doped Al2O3 Fabricated via Barrier Anodizing of Pure Al and Alloys. Coatings 2024, 14, 576. https://doi.org/10.3390/coatings14050576
Poznyak A, Knörnschild G, Hoha A, Pligovka A. Porous and Ag-, Cu-, Zn-Doped Al2O3 Fabricated via Barrier Anodizing of Pure Al and Alloys. Coatings. 2024; 14(5):576. https://doi.org/10.3390/coatings14050576
Chicago/Turabian StylePoznyak, Alexander, Gerhard Knörnschild, Aliaksandr Hoha, and Andrei Pligovka. 2024. "Porous and Ag-, Cu-, Zn-Doped Al2O3 Fabricated via Barrier Anodizing of Pure Al and Alloys" Coatings 14, no. 5: 576. https://doi.org/10.3390/coatings14050576







