Preparation and Capacitive Properties of Ni-Doped Zinc Cobaltate/Carbon Fiber Composite Porous Mesh Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Preparation of ZnCo2O4
2.3. Preparation of Ni-ZnCo2O4/CF Materials
2.4. Nitrogen Adsorption and Desorption Test
2.5. Assembly of Asymmetric Supercapacitors
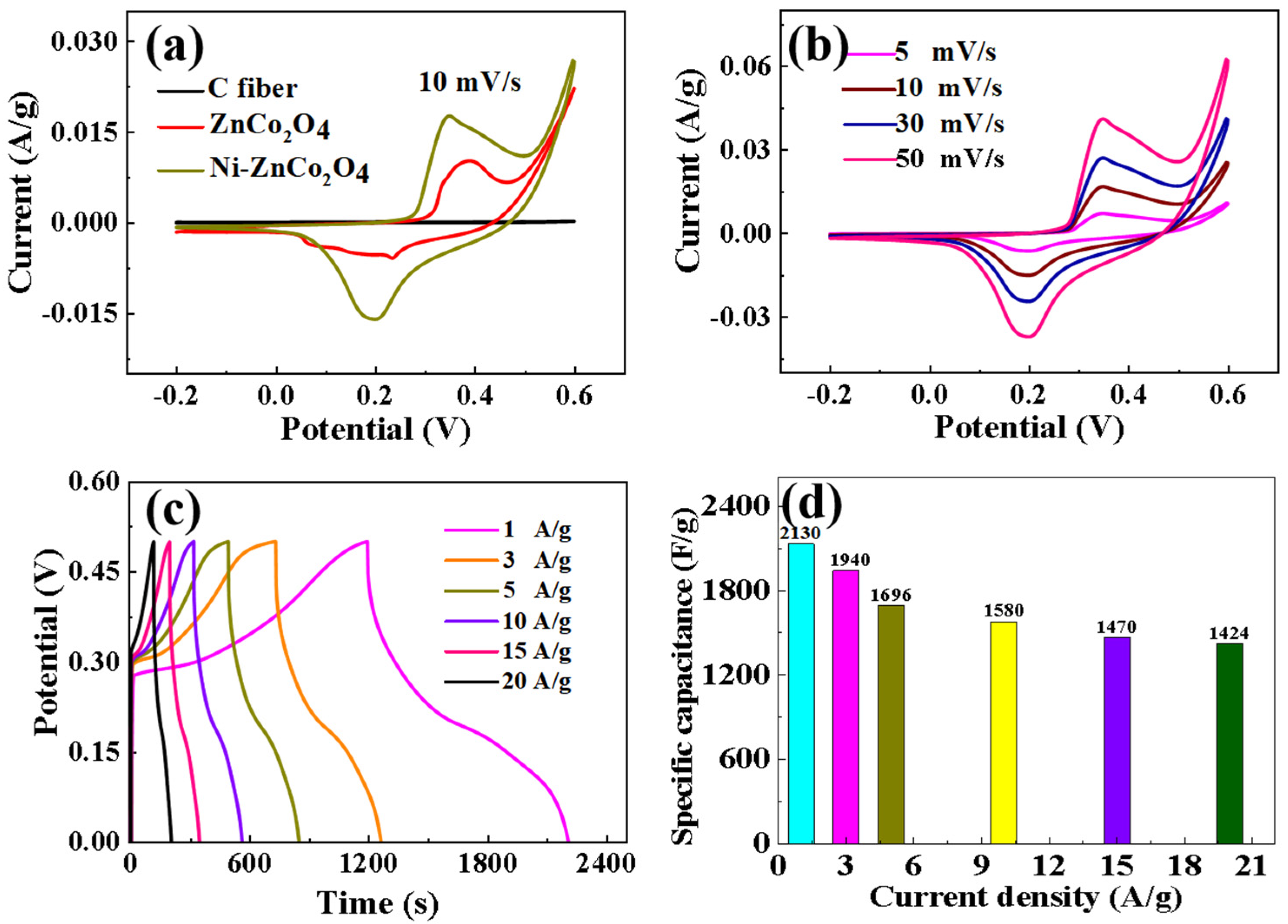
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, A.; Farrok, O.; Haque, M.M. Environmental impact of renewable energy source based electrical power plants: Solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic. Renew. Sustain. Energy Rev. 2022, 161, 112279. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Kar, K.K. Introduction to supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials II: Performance; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Li, M.; Guo, Q.; Chen, L.; Li, L.; Hou, H.; Zhao, Y. Microstructure and properties of graphene nanoplatelets reinforced AZ91D matrix composites prepared by electromagnetic stirring casting. J. Mater. Res. Technol. 2022, 21, 4138–4150. [Google Scholar] [CrossRef]
- Zhao, Y. Stability of phase boundary between L12-Ni3Al phases: A phase field study. Intermetallics 2022, 144, 107528. [Google Scholar] [CrossRef]
- Balaji, T.E.; Tanaya Das, H.; Maiyalagan, T. Recent trends in bimetallic oxides and their composites as electrode materials for supercapacitor applications. ChemElectroChem 2021, 8, 1723–1746. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Ahmad, A.; Siyal, S.H.; Akram, S.; Zhao, G.; Bahajjaj, A.A.A.; Ouladsmane, M.; Alfakeer, M. Design and fabrication of bimetallic oxide nanonest-like structure/carbon cloth composite electrode for supercapacitors. Ceram. Int. 2021, 47, 30747–30755. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, J.; Chen, Y.; Mei, L.; Duan, X.; Zhang, G.; Chen, L.; Wang, T.; Lu, B. Simple method for the preparation of highly porous ZnCo2O4 nanotubes with enhanced electrochemical property for supercapacitor. Electrochim. Acta 2014, 123, 450–455. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, X.; An, H.; Ding, S.; Zhang, H.; Zeng, X.; Fan, M.; Yang, X. Hollow cotton carbon based NiCo2S4/NiMoO4 hybrid arrays for high performance supercapacitor. J. Energy Storage 2023, 59, 106553. [Google Scholar] [CrossRef]
- Xiang, K.; Wu, D.; Fan, Y.; You, W.; Zhang, D.; Luo, J.L.; Fu, X.Z. Enhancing bifunctional electrodes of oxygen vacancy abundant ZnCo2O4 nanosheets for supercapacitor and oxygen evolution. Chem. Eng. J. 2021, 425, 130583. [Google Scholar] [CrossRef]
- Nti, F.; Anang, D.A.; Han, J.I. Facilely synthesized NiMoO4/CoMoO4 nanorods as electrode material for high performance supercapacitor. J. Alloys Compd. 2018, 742, 342–350. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Gao, H.; Shen, H.; Wu, H.; Xia, X.; Wu, X.; Lei, W.; Yang, J.; Hao, Q. Controllable synthesis of ZnCo2O4@NiCo2O4 heterostructures on Ni foam for hybrid supercapacitors with superior performance. J. Alloys Compd. 2022, 891, 162053. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Wu, Q.; He, T.; Zhang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Zhao, L.; Wu, Y.-Z.; Ran, F. Cyclic stability of supercapacitors: Materials, energy storage mechanism, test methods, and device. J. Mater. Chem. A 2021, 9, 24094–24147. [Google Scholar] [CrossRef]
- Wu, L.; Sun, L.; Li, X.; Zhang, Q.; Si, H.; Zhang, Y.; Wang, K.; Zhang, Y. Mesoporous ZnCo2O4-CNT microflowers as bifunctional material for supercapacitive and lithium energy storage. Appl. Surf. Sci. 2020, 506, 144964. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Chen, M.; Wu, Y.; Liu, G.; Qi, P.; Fu, M.; Wu, H.; Tang, Y. Rationally designed hierarchical ZnCo2O4/C core-shell nanowire arrays for high performance and stable supercapacitors. J. Alloys Compd. 2021, 876, 160037. [Google Scholar] [CrossRef]
- Bhagwan, J.; Hussain, S.K.; Yu, J.S. Aqueous asymmetric supercapacitors based on ZnCo2O4 nanoparticles via facile combustion method. J. Alloys Compd. 2020, 815, 152456. [Google Scholar] [CrossRef]









| Drug Names | Molecular Formula | Manufacturer |
|---|---|---|
| Zinc Nitrate Hexahydrate | Zn(NO3)2·6H2O | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Cobaltous nitrate hexahydrate | Co(NO3)2·6H2O | Tianjin Hiens Opdex Technology Co., Ltd. (Tianjin, China) |
| Ammonium fluoride | NH4F | Langfang Qianyao Chemical Reagent Co., Ltd. (Langfang, China) |
| Urea | CH4N2O | Anhui Haoyuan Chemical Group Co., Ltd. (Fuyang, China) |
| Carbon cloth | C | Jiangsu Sutong Carbon Fiber Co., Ltd. (Nantong, China) |
| carbon nanotube | C | Jiangsu Cnano Technology Co., Ltd. (Zhenjiang, China) |
| Nickel nitrate hexahydrate | Ni(NO3)2·6H2O | Nanjing Chemical Reagent Co., Ltd. (Nanjing, China) |
| Electrode Material | Current Density/(A/g) | Specific Capacitance/(F/g) | Cycle Number | Capacitance Retention/% | Ref. |
|---|---|---|---|---|---|
| Hollow cotton carbon-based NiCo2S4/NiMoO4 hybrid arrays | 5.0 | 2323 | 10,000 | 90.0 | [9] |
| OV-ZnCo2O4 | 1.0 | 211.6 | [10] | ||
| NiMoO4/CoMoO4 nanorods | 1.0 | 1445 | 3000 | 78.8 | [11] |
| ZnCo2O4@NiCo2O4 | 1.0 | 1728.1 | 10,000 | 91.3 | [12] |
| Ni-ZnCo2O4 | 1.0 | 2130 | 10,000 | 91.8 | This paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Liu, Y.; Wang, J.; Ma, T.; Zhi, H.; Xiao, W.; Wang, Y.; Wang, J. Preparation and Capacitive Properties of Ni-Doped Zinc Cobaltate/Carbon Fiber Composite Porous Mesh Materials. Coatings 2024, 14, 584. https://doi.org/10.3390/coatings14050584
Chen D, Liu Y, Wang J, Ma T, Zhi H, Xiao W, Wang Y, Wang J. Preparation and Capacitive Properties of Ni-Doped Zinc Cobaltate/Carbon Fiber Composite Porous Mesh Materials. Coatings. 2024; 14(5):584. https://doi.org/10.3390/coatings14050584
Chicago/Turabian StyleChen, Donghua, Yang Liu, Jun Wang, Tenghao Ma, Hui Zhi, Wei Xiao, Yabin Wang, and Jing Wang. 2024. "Preparation and Capacitive Properties of Ni-Doped Zinc Cobaltate/Carbon Fiber Composite Porous Mesh Materials" Coatings 14, no. 5: 584. https://doi.org/10.3390/coatings14050584






