Dual-Function Hybrid Coatings Based on Polytetrafluoroethylene and Cu2O for Anti-Biocorrosion and Anti-Wear Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. SRB Cultivation
2.2. Materials and Electrode Preparation
2.3. Electrochemical Experiments
2.4. Tribological Experiments
3. Results and Discussion
3.1. Electrochemical Measurements
3.1.1. EIS
3.1.2. Potentiodynamic Polarization Curves
3.2. Characterization of Corrosion Morphologies
3.3. Tribological Behaviors
4. Conclusions
- (1)
- The presence of SRB in the medium aggravates the corrosion damage of the carbon steel and coatings. After immersion for 18 days, the Rct of the steel immersed in the SRB medium was lower than that of the steel immersed in the sterile medium by up to 2.09 × 102 Ω·cm2, and the icorr of the steel was 1.62 × 10−7 Amp/cm2 higher than that of the steel immersed for the same period in the sterile medium.
- (2)
- The epoxy-based coatings filled with PTFE or/and Cu2O significantly mitigate the corrosion of the carbon steel. After being immersed in the SRB medium for 18 days, the EP/1.5Cu2O/15PTFE exhibited the lowest icorr and the highest Rct and no obvious defects and failures were noticed on the corroded surface. The hydrophobic feature of PTFE can benefit the electrochemical corrosion of the coating, whereas Cu2O nanoparticles are more effective than PTFE particles for enhancing the biocorrosion resistance of the epoxy coating. The synergetic anti-biocorrosion role of PTFE and Cu2O was identified.
- (3)
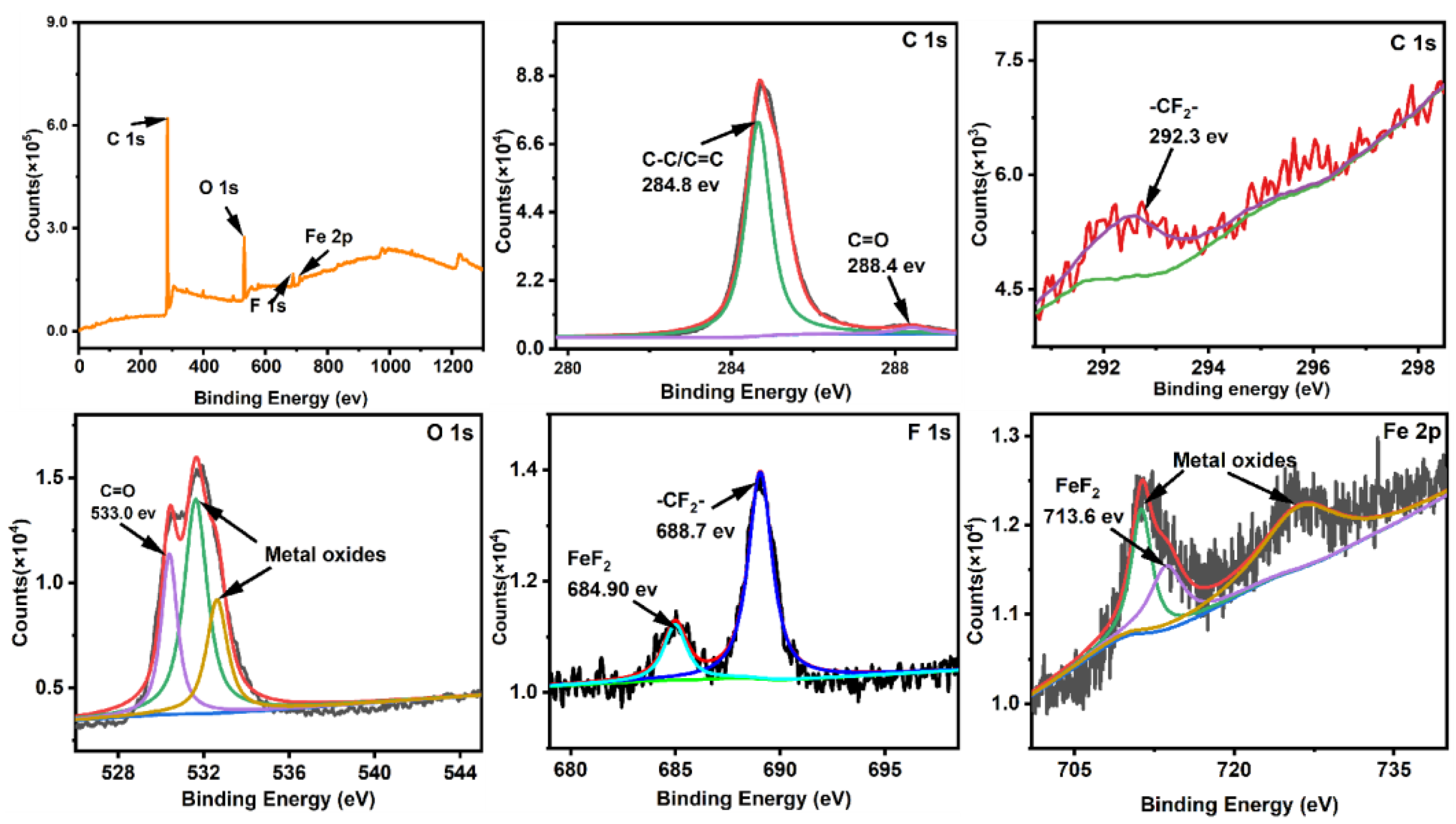
- In comparison to neat EP and EP/1.5Cu2O coatings, the EP-based coatings filled with PTFE exhibit a much improved tribological performance under water lubrication conditions. FeF2 fluoride and the -CF2- group are identified on the steel counterface sliding against EP/1.5Cu2O/15PTFE. It is surmised that tribofilm growth as a result of the tribo-chemical actions of PTFE molecules plays an important role in friction and wear reduction.
- (4)
- The addition of PTFE and Cu2O significantly improves the anti-corrosion and anti-wear performance of the epoxy resin coating. The present work paves the way for formulating dual-function anti-biocorrosion and anti-wear coatings for motion component applications exposed to water-based corrosion mediums.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.H.F.; Mohamed El Amine Ben, S.; Tarek, Z. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2022, 143, 106885. [Google Scholar]
- Gudze, M.T.; Melchers, R.E. Operational based corrosion analysis in naval ships. Corros. Sci. 2008, 50, 3296–3367. [Google Scholar] [CrossRef]
- Makarenko, V.D.; Makarenko, I.O.; Chernov, V.Y.; Petrovskii, V.A. Corrosion and mechanical resistance in oil borehole equipment. Chem. Pet. Eng. 2003, 39, 557–562. [Google Scholar] [CrossRef]
- Jia, X.; Yuan, S.; Li, B.; Miu, H.; Yuan, J.; Wang, C.; Zhu, Z.; Zhang, Y. Carbon Nanomaterials: Application and prospects of urban and industrial wastewater pollution treatment based on abrasion and corrosion resistance. Front. Chem. 2020, 8, 600594. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Cheng, X.; Chen, S.; Liu, G. Constructing zwitterionic nanofiber film for anti-adhesion of marine corrosive microorganisms. J. Mater. Sci. Technol. 2020, 70, 145–155. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Chambers, B.; Lomans, B.P.; Head, I.M.; Tsesmetzis, N. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl. Environ. Microbiol. 2016, 80, 2545–2554. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Yang, C.-T.; Yang, C.-G.; Xu, D.-K.; Li, Q.; Yin, L.; Qiu, C.-S.; Liu, D.; Yang, K. Stern–geary constant for X80 pipeline steel in the presence of different corrosive microorganisms. Acta Metall. Sin. Engl. Lett. 2019, 32, 1483–1489. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T.; Lovley, D.R. Microbially mediated metal corrosion. Nat. Rev. Microbiol. 2023, 21, 705–718. [Google Scholar] [CrossRef]
- Zhang, Z.; Ouyang, W.; Liang, X.; Yan, X.; Yuan, C.; Zhou, X.; Guo, Z.; Dong, C.; Liu, Z.; Jin, Y.; et al. Review of the evolution and prevention of friction, wear, and noise for water-lubricated bearings used in ships. Friction 2023, 12, 1–38. [Google Scholar] [CrossRef]
- Roy, M.; Stack, M.M. Tribo-corrosion V 2016—A return to hyderabad. Tribol. Int. 2020, 141, 105945. [Google Scholar] [CrossRef]
- Soltanahmadi, S.; Morina, A.; van Eijk, M.C.P.; Nedelcu, I.; Neville, A. Tribochemical study of micropitting in tribocorrosive lubricated contacts: The influence of water and relative humidity. Tribol. Int. 2017, 107, 184–198. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, H.; Li, G.; Guo, X.; Wan, Y.; Zhang, G. Significance of an in-situ generated boundary film on tribocorrosion behavior of polymer-metal sliding pair. J. Colloid Interface Sci. 2018, 518, 263–276. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, L.; Li, G.; Zhao, F.; Che, Q.; Wang, C.; Zhang, G. Tuning the tribofilm nanostructures of polymer-on-metal joint replacements for simultaneously enhancing anti-wear performance and corrosion resistance. Acta Biomater. 2019, 87, 285–295. [Google Scholar] [CrossRef]
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Parvathavarthini, N.; Mudali, U.K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: An overview. Corros. Rev. 2016, 34, 41–63. [Google Scholar] [CrossRef]
- Senthilmurugan, B.; Radhakrishnan, J.S.; Poulsen, M.; Arana, V.H.; Al-Qahtani, M.; Jamsheer, A.F. Microbially induced corrosion in oilfield: Microbial quantification and optimization of biocide application. J. Chem. Technol. Biotechnol. 2019, 22, 023132. [Google Scholar] [CrossRef]
- Piotr, W.; Paweł, Ł.; Elżbieta, S.; Grzegorz, A.; Karol, C.; Szymon, S. Concrete corrosion in a wastewater treatment plant—A comprehensive case study. Constr. Build. Mater. 2021, 303, 124338. [Google Scholar]
- Shi, X.; Zhang, R.; Sand, W.; Mathivanan, K.; Zhang, Y.; Wang, N.; Duan, J.; Hou, B. Comprehensive review on the use of biocides in microbiologically influenced corrosion. Microorganisms 2023, 11, 2194. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-Y.; Xu, L.-C.; Fang, H.H.P. Anaerobic electrochemical corrosion of mild steel in the presence of extracellular polymeric substances produced by a culture enriched in sulfate-reducing bacteria. Environ. Sci. Technol. 2002, 36, 1720–1727. [Google Scholar] [CrossRef]
- Wang, J.; Yin, S.; Lu, L.; Zhou, J.; Fu, Q. Characterization of microbial-induced concrete corrosion by combining morphology observation and fluorescence staining. Case Stud. Constr. Mater. 2022, 17, e01586. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Thakur, P.; Saxena, P.; Rauniyar, S.; Gopalakrishnan, V.; Singh, R.N.; Gadhamshetty, V.; Gnimpieba, E.Z.; Jasthi, B.K.; Sani, R.K. Gene Sets and Mechanisms of sulfate-reducing bacteria biofilm formation and quorum sensing with impact on corrosion. Front. Microbiol. 2021, 12, 754140. [Google Scholar] [CrossRef] [PubMed]
- Ranlei, Z.; Bo, W.; Dongbo, L.; Yiming, C.; Qiushi, Z. Effect of sulfate-reducing bacteria from salt scale of water flooding pipeline on corrosion behavior of X80 steel. Eng. Fail. Anal. 2022, 142, 106788. [Google Scholar]
- Rajbongshi, A.; Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World. J. Microbiol. Biotechnol. 2021, 37, 111. [Google Scholar] [CrossRef] [PubMed]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2018, 35, 631–636. [Google Scholar] [CrossRef]
- King, R.A.; Miller, J.D.A. Corrosion by the Sulphate-reducing Bacteria. Nature 1971, 233, 491–492. [Google Scholar] [CrossRef]
- Iverson, W.P. Corrosion of iron and formation of iron phosphide by desulfovibrio desulfuricans. Nature 1968, 217, 1265–1267. [Google Scholar] [CrossRef]
- Gu, T.; Wang, D.; Lekbach, Y.; Xu, D. Extracellular electron transfer in microbial biocorrosion. Curr. Opin. Electrochem. 2021, 29, 100763. [Google Scholar] [CrossRef]
- Anguita, J.; Pizarro, G.; Vargas, I.T. Mathematical modelling of microbial corrosion in carbon steel due to early-biofilm formation of sulfate-reducing bacteria via extracellular electron transfer. Bioelectrochemistry 2022, 145, 108058. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Liu, J.; Cai, W.; Wang, D.; Jia, R.; Chen, S.; Gu, T. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 2019, 150, 258–267. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y. A review on conducting polymers and nanopolymer composite coatings for steel corrosion protection. Coatings 2019, 9, 807. [Google Scholar] [CrossRef]
- Kausar, A. Corrosion prevention prospects of polymeric nanocomposites: A review. J. Plast. Film Sheeting 2018, 35, 181–202. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/inorganic nanocomposite coatings with superior corrosion protection performance: A Review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym. Plast. Tech. Mater. 2019, 59, 658–673. [Google Scholar] [CrossRef]
- Ying, L.; Wu, Y.; Nie, C.; Wu, C.; Wang, G. Improvement of the tribological properties and corrosion resistance of epoxy–PTFE composite coating by nanoparticle modification. Coatings 2020, 11, 10. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Y.; Cao, S.; Jiang, J.; Wu, C.; Zhao, K. Enhanced anti-microbial corrosion of nano-CuO-loaded Ni coatings on pipeline steels in simulation environment of natural gas transportation pipeline. Ceram. Int. 2023, 49, 5543–5549. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Lv, C.; Chen, X.; Zhao, Z.; Qu, Y.; Zhu, Y. Effects of CeO2 geometry on corrosion resistance of epoxy coatings. Surf. Eng. 2019, 36, 175–183. [Google Scholar] [CrossRef]
- Guo, R.; Li, W.; Wang, X.; Lv, Y.; Chen, M.; Chen, Z.; Liu, Z.; Han, G.-C. Antimicrobial corrosion study of the epoxy coating with the graphene oxide supported Schiff base quaternary ammonium salt additives. Mater. Today Commun. 2023, 35, 105517. [Google Scholar] [CrossRef]
- Yang, G.; Chen, T.; Feng, B.; Weng, J.; Duan, K.; Wang, J.; Lu, X. Improved corrosion resistance and biocompatibility of biodegradable magnesium alloy by coating graphite carbon nitride (g-C3N4). J. Alloys Compd. 2019, 770, 823–830. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, W.; Zhao, W.; Ci, X.; Li, W. Tribological and anti-corrosion performance of epoxy resin composite coatings reinforced with differently sized cubic boron nitride (CBN) particles. Friction 2020, 9, 104–118. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Qiu, S.; Zhao, H.; Wang, L.; Xue, Q. Non-covalent functionalized multi-wall carbon nanotubes filled epoxy composites: Effect on corrosion protection and tribological performance. Surf. Coat. Technol. 2018, 340, 74–85. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, F.; Qiang, Y.; Zhao, W. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021, 176, 39–51. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Liu, G. Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings. Corros. Sci. 2021, 194, 109942. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Ferraris, S.; Miola, M.; Fucale, G.; Manfredotti, C.; Battiato, A.; Santella, D.; Vernè, E.; Vittone, E.; et al. Antibacterial coating on polymer for space application. Mater. Chem. Phys. 2012, 135, 714–722. [Google Scholar] [CrossRef]
- Zhu, P.-Y.; Feng, D.-Q.; Yasir, M.; Song, W.-L.; Hafeez, M.A.; Zhang, C.; Liu, L. Enhanced antifouling capability of PDMS/Cu2O-anchored Fe-based amorphous coatings. Surf. Coat. Technol. 2023, 475, 130–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, S.; Lin, D.; Zhang, Z.; Li, G. Functional anti-corrosive and anti-bacterial surface coatings based on cuprous oxide/polyaniline microcomposites. Mater. Des. 2022, 216, 110589. [Google Scholar] [CrossRef]
- Lancaster, J.K. A review of the influence of environmental humidity and water on friction, lubrication and wear. Tribol. Int. 1990, 23, 371–389. [Google Scholar] [CrossRef]
- Yu, P.; Li, G.; Zhang, L.; Zhao, F.; Guo, Y.; Pei, X.-Q.; Zhang, G. Role of SiC submicron-particles on tribofilm growth at water-lubricated interface of polyurethane/epoxy interpenetrating network (PU/EP IPN) composites and steel. Tribol. Int. 2021, 153, 106611. [Google Scholar] [CrossRef]
- Qi, H.; Hu, C.; Zhang, G.; Yu, J.; Zhang, Y.; He, H. Comparative study of tribological properties of carbon fibers and aramid particles reinforced polyimide composites under dry and sea water lubricated conditions. Wear 2019, 436, 203001. [Google Scholar] [CrossRef]
- Bian, D.; Li, J.; Zhao, Y.; Wang, Y. Investigation of tribological and corrosion properties in ceramic/PTFE coating. Surf. Eng. 2022, 3, 2–6. [Google Scholar]
- Li, H.; Luo, S.; Zhang, L.; Zhao, Z.; Wu, M.; Li, W.; Liu, F.-Q. Water- and acid-Sensitive Cu2O@Cu-MOF nano sustained-release capsules with superior antifouling behaviors. ACS Appl. Mater. Interfaces 2021, 14, 1910–1920. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Wang, D.; Sun, D.; Zhou, Y.; Wang, Y. Influence of anion and sulfate-reducing bacteria on the stress corrosion behavior and mechanism of X70 steel in a marine mud environment. Eng. Fail. Anal. 2023, 143, 106834. [Google Scholar] [CrossRef]
- Du, D.; Ye, G.; Pu, Z.; Zhang, W. High Quality Carbon Structural Steel Hot Rolled Steel Plate and Steel Strip: GB/T 711-2017 Standard; China Standard Press: Beijing, China, 2017. [Google Scholar]
- Shen, J.; Lei, J.; Lua, Y. High Carbon Chromium Bearing Steel: GB/T 18254– 2002 Standard; China Standard Press: Beijing, China, 2002. [Google Scholar]
- Zhao, F.; Zhang, L.; Li, G.; Guo, Y.; Qi, H.; Zhang, G. Significantly enhancing tribological performance of epoxy by filling with ionic liquid functionalized graphene oxide. Carbon 2018, 136, 309–319. [Google Scholar] [CrossRef]
- Cetin, D.; Aksu, M.L. Corrosion behavior of low-alloy steel in the presence of desulfotomaculum sp. Corros. Sci. 2009, 51, 1584–1588. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, C.; Zhang, Y.; Liu, H.; Liu, H.; Liu, H. Early corrosion behavior of X80 pipeline steel in a simulated soil solution containing Desulfovibrio desulfuricans. Bioelectrochemistry 2021, 141, 107880. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Li, P.; Li, X.; Wang, Y. Effect of cerium on the microstructure and anti-corrosion performance of Al-Zn coatings. Surf. Coat. Technol. 2023, 473, 130046. [Google Scholar] [CrossRef]
- Sachie, W.; Saad, A.-S.; Will, P.G.; Christopher, P.; Raman, R.K.S. Sulphate reducing bacteria (SRB) biofilm development and its role in microbial corrosion of carbon steel. Front. Mater. 2024, 11, 1360869. [Google Scholar]
- Yang, Y.; Xiao, C.; Yang, Y.; Liang, B. Research on the reliability of X70 steel gas pipelines under SRB main control factors. Mater. Corros. 2022, 73, 687–702. [Google Scholar] [CrossRef]
- El Saeed, A.M.; Abd El-Fattah, M.; Azzam, A.M.; Dardir, M.M.; Bader, M.M. Synthesis of cuprous oxide epoxy nanocomposite as an environmentally antimicrobial coating. Int. J. Biol. Macromol. 2016, 89, 190–197. [Google Scholar] [CrossRef]
- Wan, H.; Zhang, T.; Wang, J.; Rao, Z.; Zhang, Y.; Li, G.; Gu, T.; Liu, H. Effect of alloying element content on anaerobic microbiologically influenced corrosion sensitivity of stainless steels in enriched artificial seawater. Bioelectrochemistry 2023, 150, 108367. [Google Scholar] [CrossRef]
- Noor, N.M.; Yahaya, N.; Abdullah, A.; Tahir, M.M.; Sing, L.K. Microbiologically influenced corrosion of X-70 carbon steel by desulfovibrio vulgaris. Adv. Sci. Lett. 2012, 13, 312–316. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Zhang, D.; Zhuo, L.; Xiang, Q. Electrophoretic deposition of polytetrafluoroethylene (PTFE) as anti-corrosion coatings. Mater. Lett. 2023, 346, 134524. [Google Scholar] [CrossRef]
- Gurianov, Y.; Nakonechny, F.; Albo, Y.; Nisnevitch, M. Antibacterial composites of cuprous oxide nanoparticles and polyethylene. Int. J. Mol. Sci. 2019, 20, 439. [Google Scholar] [CrossRef]
- Cong, L.; Yefei, L.; Jing, S.; Bo, L.; Yuzhou, D.; Ronn, G.; Yimin, G.; Intizar Ali, S.; Siyong, Z.; Alfred Iing Yoong, T. Interfacial characteristics and wear performances of iron matrix composites reinforced with zirconia-toughened alumina ceramic particles. Ceram. Int. 2021, 48, 1293–1305. [Google Scholar]
- Ren, Y.; Gao, K.; Ying, S.; Zhao, Y.; Zhang, L.; Guo, D.; Xie, G. Significant enhancement of tribological properties of microcapsule/epoxy composites in the presence of high loads by incorporating PTFE. Wear 2023, 204, 514–515. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Liu, K.; Wang, W.; Ye, J. Ultralow wear PTFE composites filled with beryllia and germania particles. Wear 2020, 450–451, 203270. [Google Scholar] [CrossRef]








| Corrosion Time (Days) | Rs (Ω·cm2) | Qf (×10−8) (μF·cm2) | Qf-n | Rf (×10) (Ω·cm2) | Qct (×10−4) (μF·cm2) | Qct-n (10−1) | Rct (×102) (Ω·cm2) | O-Yo (10−3) | O-B | |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 0 | 6.75 | 6.78 | 0.94 | 2.30 | 7.25 | 0.94 | 7.52 | 2.90 | 2.79 |
| Sterile medium | 4 | 5.76 | 7.25 | 0.88 | 2.09 | 1.69 | 0.88 | 7.0 | 1.87 | 9.00 |
| 11 | 5.31 | 1.69 | 0.44 | 2.61 | 1.78 | 0.44 | 5.62 | 1.15 | 8.66 | |
| 18 | 5.78 | 1.78 | 0.62 | 2.85 | 2.89 | 0.62 | 4.99 | 4.00 | 6.00 | |
| SRB medium | 4 | 5.87 | 3.52 | 5.48 | 2.36 | 7.22 | 0.77 | 7.15 | 6.26 | 4.22 |
| 11 | 6.02 | 2.21 | 3.15 | 2.83 | 1.41 | 1.00 | 4.71 | 2.58 | 7.73 | |
| 18 | 6.00 | 1.08 | 5.27 | 2.92 | 2.56 | 0.89 | 2.90 | 1.04 | 9.92 |
| Coatings | Rs (×10−2) (Ω·cm2) | Qf (×10−10) (μF·cm2) | Qf-n | Rc (×102) (Ω·cm2) | Qct (×10−5) (μF·cm2) | Qct-n (10−1) | Rf (×103) (Ω·cm2) | Q-Yo (×10−4) | Q-n (×10−1) | Rct (×108) (Ω·cm2) | O-Yo (×10−1) | O-B (×10−5) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterile medium | EP | 3.67 | 1.87 | 1.00 | 0.48 | 3.39 | 7.61 | 7.81 | 3.75 | 4.64 | 0.15 | 9.29 | 1.39 |
| EP/1.5Cu2O | 1.01 | 6.40 | 1.00 | 5.24 | 1.01 | 2.28 | 2.38 | 1.20 | 2.37 | 2.11 | 1.02 | 1.21 | |
| EP/15PTFE | 4.99 | 6.62 | 1.00 | 6.25 | 1.13 | 4.09 | 3.43 | 1.63 | 5.20 | 4.99 | 5.01 | 2.28 | |
| EP/15PTFE/1.5Cu2O | 2.40 | 3.52 | 1.00 | 9.21 | 1.46 | 3.45 | 3.55 | 1.08 | 8.44 | 6.29 | 4.57 | 3.60 | |
| SRB medium | EP | 5.60 | 1.31 | 1.42 | 0.66 | 1.87 | 1.00 | 3.87 | 4.04 | 6.65 | 0.01 | 2.44 | 8.85 |
| EP/1.5Cu2O | 9.99 | 6.91 | 1.00 | 6.01 | 2.14 | 7.87 | 4.34 | 1.42 | 4.37 | 2.94 | 9.82 | 2.58 | |
| EP/15PTFE | 1.89 | 1.00 | 1.20 | 7.04 | 4.09 | 8.08 | 1.05 | 7.18 | 1.38 | 2.28 | 1.22 | 1.89 | |
| EP/15PTFE/1.5Cu2O | 2.33 | 5.35 | 1.00 | 9.33 | 6.95 | 2.50 | 1.04 | 4.19 | 3.84 | 5.35 | 8.45 | 8.26 |
| Corrosion Time (Days) | Io × 10−7 (Amp/cm2) | Eo (Volts) | Ba (mv) | Bc (mv) | |
|---|---|---|---|---|---|
| Initial | 0 | 1.47 | −0.54 | 18.93 | −21.63 |
| Sterile medium | 4 | 1.97 | −0.62 | 14.23 | −18.18 |
| 11 | 2.32 | −0.65 | 13.56 | −13.56 | |
| 18 | 2.55 | −0.62 | 17.73 | −23.32 | |
| SRB medium | 4 | 1.32 | −0.52 | 13.56 | −13.56 |
| 11 | 2.95 | −0.67 | 11.14 | −7.43 | |
| 18 | 4.17 | −0.63 | 7.80 | −7.80 |
| Coatings | Io × 10−7 (Amp/cm2) | Eo (Volts) | Ba (mv) | Bc (mv) | |
|---|---|---|---|---|---|
| Sterile medium | EP | 0.83 | −0.62 | 30.82 | −58.45 |
| EP/1.5Cu2O | 0.62 | −0.51 | 53.57 | −45.93 | |
| EP/15PTFE | 0.24 | −0.46 | 53.58 | −70.48 | |
| EP/15PTFE/1.5Cu2O | 0.20 | −0.45 | 64.32 | −86.42 | |
| SRB medium | EP | 1.10 | −0.61 | 16.90 | −14.54 |
| EP/1.5Cu2O | 0.75 | −0.57 | 40.99 | −52.43 | |
| EP/15PTFE | 0.35 | −0.54 | 44.45 | −79.27 | |
| EP/15PTFE/1.5Cu2O | 0.30 | −0.55 | 72.18 | −75.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, H.; Xu, Y.; He, R.; Zhang, G.; Liu, Z. Dual-Function Hybrid Coatings Based on Polytetrafluoroethylene and Cu2O for Anti-Biocorrosion and Anti-Wear Applications. Coatings 2024, 14, 592. https://doi.org/10.3390/coatings14050592
Li G, Li H, Xu Y, He R, Zhang G, Liu Z. Dual-Function Hybrid Coatings Based on Polytetrafluoroethylene and Cu2O for Anti-Biocorrosion and Anti-Wear Applications. Coatings. 2024; 14(5):592. https://doi.org/10.3390/coatings14050592
Chicago/Turabian StyleLi, Guohui, Huan Li, Yongkun Xu, Ren He, Ga Zhang, and Zongzhu Liu. 2024. "Dual-Function Hybrid Coatings Based on Polytetrafluoroethylene and Cu2O for Anti-Biocorrosion and Anti-Wear Applications" Coatings 14, no. 5: 592. https://doi.org/10.3390/coatings14050592




