Experimental Investigation of Phase Equilibria in the Al–Mo–Hf Ternary System at 400 °C and 600 °C
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
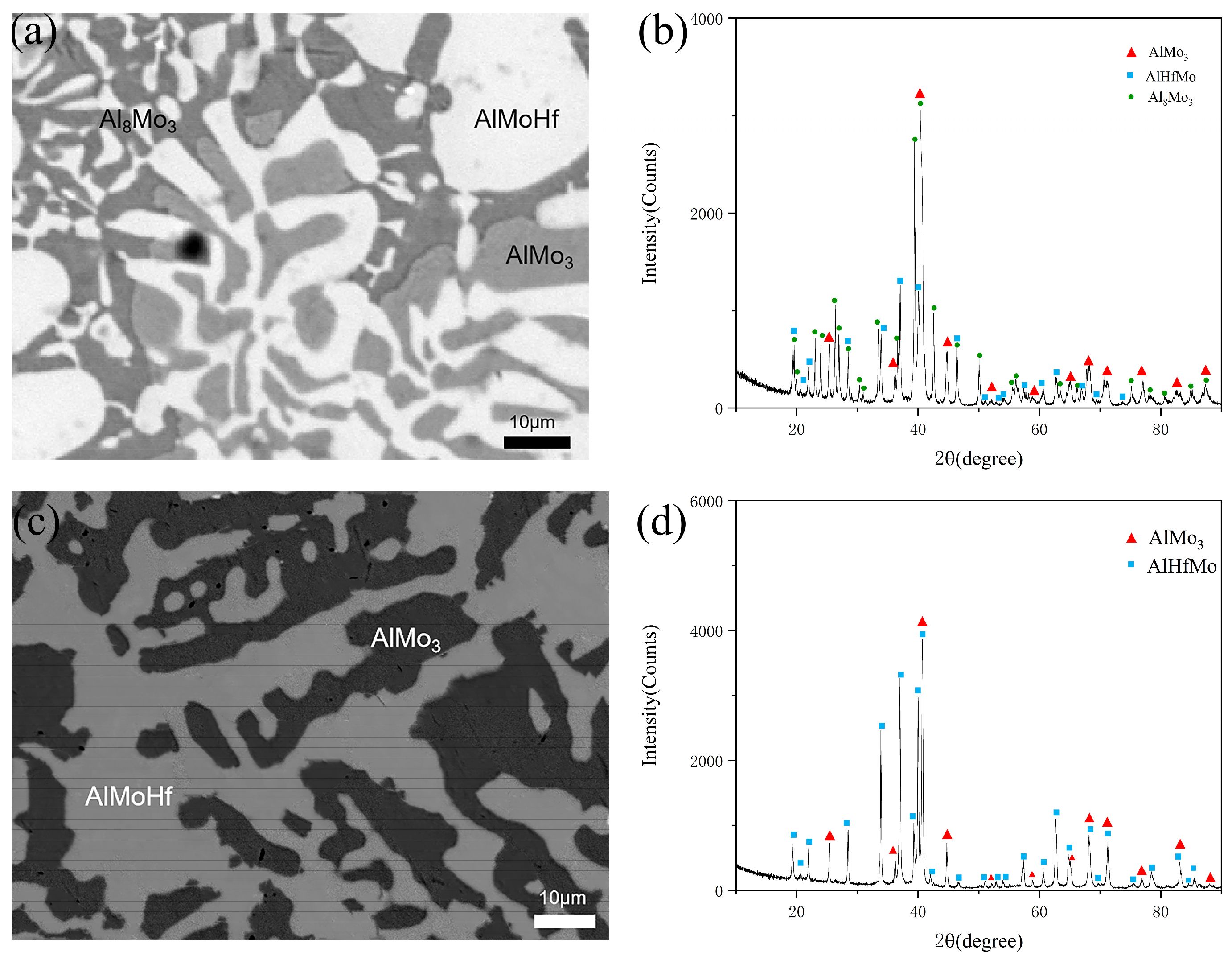
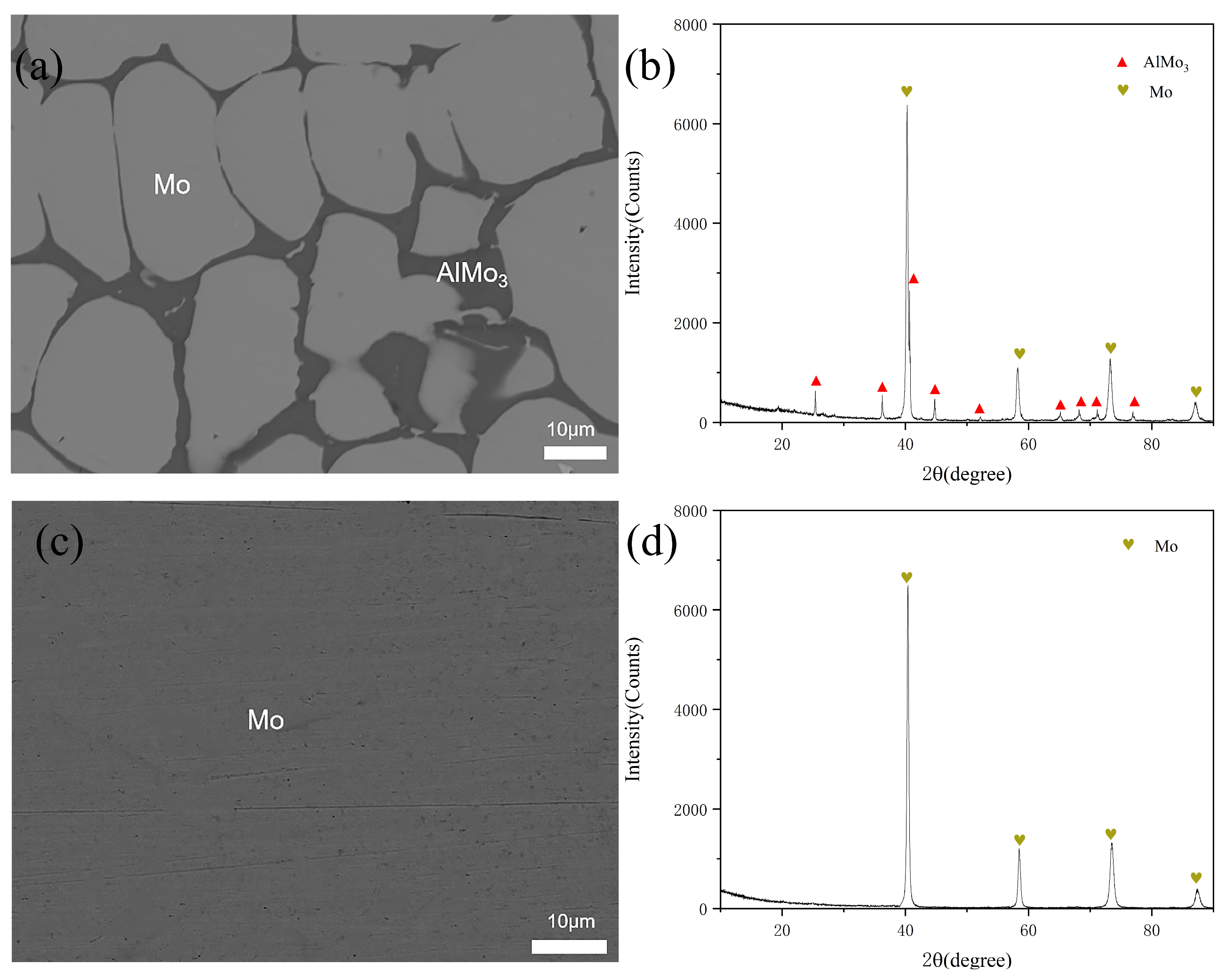
3.1. Phase Equilibrium at 400 °C
3.2. Phase Equilibrium at 600 °C
4. Conclusions
- At 400 °C, seven three-phase regions and five two-phase regions were identified, while at 600 °C, eight three-phase regions and four two-phase regions were observed. The distribution of phase zones in the isothermal sections at both temperatures was largely consistent, with some variations in the solid solubility ranges of certain compounds.
- The solid solubility range of the ternary compound AlMoHf was determined to be from Al27.32Mo35.79Hf36.89 to Al44.66Mo24.16Hf31.18 at 400 °C, and from Al25.23Mo36.88Hf37.89 to Al45.29Mo22.79Hf31.92 at 600 °C. Meanwhile the phases Al3Hf, Al3Hf2, AlHf, Al3Hf4, AlHf2, Al12Mo, Al5Mo, AlMo3, Al8Mo3, HfMo2, Hf, and Mo were determined in two isothermal sections, and the corresponding solid solubilities were determined.
- Partial solid solubility ranges of some binary compounds were also determined during the study, with the solid solubility regions of each compound increasing as the annealing temperature rose.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, Y.; Jia, Z.; Liu, Z.; Liu, M.; Roven, H.J.; Liu, Q. Effect of Zr and Er on the microstructure, mechanical and electrical properties of Al-0.4 Fe alloy. J. Alloys Compd. 2021, 857, 157611. [Google Scholar] [CrossRef]
- Atas, M.S. The relationship between reinforcement ratio and e-beam irradiation in Y2O3 reinforced Al6061 Alloys: A crystallographic assessment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2024, 548, 165252. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, Q.; Tian, Y.; Xue, R.; Zhang, L.; Liu, L. Experimental investigation and thermodynamic assessment of the Al–Ag–Sc system. J. Alloys Compd. 2023, 934, 167980. [Google Scholar] [CrossRef]
- Yildirim, M.; Atas, M.S.; Akdeniz, M.V.; Mekhrabov, A.O. Effect of Y Addition on the Structural Properties and Oxidation Behavior of Fe60Al40−nYn Alloys (n = 1, 3, and 5 at.%). Mater. High Temp. 2022, 39, 220–230. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Int. J. Mater. Res. 2022, 97, 246–265. [Google Scholar] [CrossRef]
- Wu, D.; Hao, M.; Zhang, T.; Wang, Z.; Wang, J.; Rao, G.; Zhang, L.; Ding, C.; Zhou, K.; Liu, L. Heterostructures enhance simultaneously strength and ductility of a commercial titanium alloy. Acta Mater. 2023, 257, 119182. [Google Scholar] [CrossRef]
- Xue, R.-H.; Wang, D.; Tian, Y.-Y.; Deng, Z.-X.; Liu, L.-B.; Zhang, L.-G. Effect of Sn on elastic modulus and magnetic susceptibility of Zr-16Nb-x Ti (x = 4 wt%, 6 wt%) alloys. J. Cent. South Univ. 2023, 30, 412–418. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–Zr and Al–Zr–Ti alloys during aging at 450–600 °C. Acta Mater. 2008, 56, 1182–1195. [Google Scholar] [CrossRef]
- Friedel, J. Dislocations: An introduction. Dislocations Solids 1979, 1, 1–32. [Google Scholar]
- Liu, W.; Liu, Z.; Luo, W.; Zuo, D.; Liu, H.; Zhang, R.; Wang, Q. Effect of Zr addition on coarse Laves phase stimulated nucleation of recrystallization in FeCrAl alloy. Mater. Sci. Eng. A 2022, 840, 142964. [Google Scholar] [CrossRef]
- Hallem, H.; Lefebvre, W.; Forbord, B.; Danoix, F.; Marthinsen, K. The formation of Al3 (ScxZryHf1−x−y)-dispersoids in aluminium alloys. Mater. Sci. Eng. A 2006, 421, 154–160. [Google Scholar] [CrossRef]
- Li, H.-Y.; Li, D.-W.; Zhu, Z.-X.; Chen, B.-A.; Xin, C.; Yang, C.-L.; Zhang, H.-Y.; Wei, K. Grain refinement mechanism of as-cast aluminum by hafnium. Trans. Nonferrous Met. Soc. China 2016, 26, 3059–3069. [Google Scholar] [CrossRef]
- Wu, H.; Wen, S.; Gao, K.; Huang, H.; Wang, W.; Nie, Z. Effect of Er additions on the precipitation strengthening of Al–Hf alloys. Scr. Mater. 2014, 87, 5–8. [Google Scholar] [CrossRef]
- Farkoosh, A.; Chen, X.G.; Pekguleryuz, M. Dispersoid strengthening of a high temperature Al–Si–Cu–Mg alloy via Mo addition. Mater. Sci. Eng. A 2015, 620, 181–189. [Google Scholar] [CrossRef]
- Zamani, M.; Toschi, S.; Morri, A.; Ceschini, L.; Seifeddine, S. Effect of Mo Addition on Room and High Temperature Tensile Behavior of Al-Si-Cu-Mg Alloy in As-Cast and Heat-Treated Conditions. Adv. Mater. Res. 2019, 1155, 71–79. [Google Scholar] [CrossRef]
- Atas, M.S.; Yildirim, M. Effect of Nd Addition on the Microstructure and Cyclic Oxidation Behavior of NiAl–Cr (Mo) Eutectic Alloys. Int. J. Met. 2023, 18, 1192–1203. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Wang, J.; Wu, J.; Wang, B.; Chen, X.; Moelans, N.; Wang, J.; Pan, F. Features and classification of solid solution behavior of ternary Mg alloys. J. Magnes. Alloys 2024. [Google Scholar] [CrossRef]
- Chen, T.; Gao, Q.; Yuan, Y.; Li, T.; Xi, Q.; Liu, T.; Tang, A.; Watson, A.; Pan, F. Coupling physics in machine learning to investigate the solution behavior of binary Mg alloys. J. Magnes. Alloys 2022, 10, 2817–2832. [Google Scholar] [CrossRef]
- Rath, B.; Mohanty, G.; Mondolfo, L. The aluminium-rich end of the aluminium-hafnium equilibrium diagram. J. Inst. Met. 1961, 89. [Google Scholar]
- Nowotny, H.; Schob, O.; Benesovsky, F. Die Kristallstruktur von Zr2Al und Hf2Al. Monatshefte Chem. Verwandte Teile Anderer Wiss. 1961, 92, 1300–1303. [Google Scholar] [CrossRef]
- Edshammar, L.-E. Crystal structure investigations on the Zr-Al and Hf-Al systems. Acta Chem. Scand. 1962, 16. [Google Scholar] [CrossRef]
- Kaufman, L.; Nesor, H. Calculation of the Ni-Al-W, Ni-Al-Hf and Ni-Cr-Hf systems. Can. Metall. Q. 1975, 14, 221–232. [Google Scholar] [CrossRef]
- Murray, J.; McAlister, A.; Kahan, D. The Al-Hf (aluminum-hafnium) system. J. Phase Equilibria Diffus. 1998, 19, 376. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Z.; Zhao, J.-C. Thermodynamic assessment of the Al-Hf binary system. J. Phase Equilibria 2002, 23, 416. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Hf (aluminum-hafnium). J. Phase Equilibria Diffus. 2006, 27, 538–539. [Google Scholar] [CrossRef]
- Jia, Z.-H.; Huang, H.-L.; Wang, X.-L.; Xing, Y.; Liu, Q. Hafnium in aluminum alloys: A review. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 105–119. [Google Scholar] [CrossRef]
- Taylor, A.; Doyle, N.; Kagle, B. The constitution diagram of the molybdenum-hafnium binary system. J. Less Common Met. 1961, 3, 265–280. [Google Scholar] [CrossRef]
- Rudy, E. Compendium of Phase Diagram Data; Air Force Materials Laboratory, Metals and Ceramics Division: Dayton, OH, USA, 1969. [Google Scholar]
- Garg, S.; Ackermann, R. The high temperature phase diagrams for zirconium-molybdenum and hafnium-molybdenum. Metall. Trans. A 1977, 8, 239–244. [Google Scholar] [CrossRef]
- Brewer, L.; Lamoreaux, R.H.; Ferro, R.; Marazza, R.; Girgis, K. Molybdenum: Physico-Chemical Properties of Its Compounds and Alloys; International Atomic Energy Agency: Vienna, Austria, 1980. [Google Scholar]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.; Kacprzak, L.; Scott, W.W. Binary Alloy Phase Diagrams; American Society for Metals: Materials Park, OH, USA, 1986; Volume 1. [Google Scholar]
- Shao, G. Thermodynamic assessment of the Hf–Mo and Hf–W systems. Intermetallics 2002, 10, 429–434. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Yuan, H.; Hu, B.; Du, Y.; Tan, Z. Thermodynamic reassessment of the Mo–Hf and Mo–Zr systems supported by first-principles calculations. Calphad 2020, 69, 101766. [Google Scholar] [CrossRef]
- Reimann, H. Al–Mo Alloys. Z. Metallkd. 1922, 14, 119–123. [Google Scholar]
- Roentgen, P.; Koch, W. The influence of heavy metals on Al alloys. Z. Met. 1930, 25, 182–185. [Google Scholar]
- Mondolfo, L. The Al–Mo System. In Metallography of Aluminium Alloys; John Wiley & Sons: Hoboken, NJ, USA, 1943; pp. 30–31. [Google Scholar]
- Yamaguchi, K.; Simizu, K. The equilibrium diagram of the Al–Mo system. Nippon. Kinzoku Gakkai-Shi 1940, 4, 390–392. [Google Scholar]
- Sperner, F. Das Zweistoffsystem Aluminium-Molybdän. Int. J. Mater. Res. 1959, 50, 588–591. [Google Scholar] [CrossRef]
- Schuster, J.C.; Ipser, H. The AI-Ali8Mo3 section of the binary system aluminum-molybdenum. Metall. Trans. A 1991, 22, 1729–1736. [Google Scholar] [CrossRef]
- Okamoto, H. Desk Handbook: Phase Diagrams for Binary Alloys; ASM International: Almere, The Netherlands, 2000. [Google Scholar]
- Eumann, M.; Sauthoff, G.; Palm, M. Re-evaluation of phase equilibria in the Al–Mo system. Int. J. Mater. Res. 2006, 97, 1502–1511. [Google Scholar] [CrossRef]
- Cupid, D.M.; Fabrichnaya, O.; Ebrahimi, F.; Seifert, H.J. Thermodynamic assessment of the Al–Mo system and of the Ti–Al–Mo system from 0 to 20 at.% Ti. Intermetallics 2010, 18, 1185–1196. [Google Scholar] [CrossRef]
- Peng, J.; Franke, P.; Manara, D.; Watkins, T.; Konings, R.J.; Seifert, H.J. Experimental investigation and thermodynamic re-assessment of the Al–Mo–Ni system. J. Alloys Compd. 2016, 674, 305–314. [Google Scholar] [CrossRef]
- Trojko, R.; Blažina, Ž.; Ban, Z. The effect of silicon, aluminium and germanium on the stabilization of the C14 polymorph of HfMo2. J. Less Common Met. 1983, 92, 67–74. [Google Scholar]
- Okamoto, H. Al-Mo (Aluminum-Molybdenum). J. Phase Equilibria Diffus. 2010, 31, 492–493. [Google Scholar]
- Grin, Y.N.; Ellner, M.; Peters, K.; Schuster, J. The crystal structures of Mo4Al17 and Mo5Al22. Z. Krist.-Cryst. Mater. 1995, 210, 96–99. [Google Scholar] [CrossRef]
- Leake, J. The refinement of the crystal structure of the intermetallic phase Al4Mo. Acta Crystallogr. 1964, 17, 918–924. [Google Scholar] [CrossRef]
- Forsyth, J.; Gran, G. The structure of the intermetallic phase γ (Mo–Al)–Mo3Al8. Acta Crystallogr. 1962, 15, 100–104. [Google Scholar] [CrossRef]
- Rexer, J. The phase equilibria in the aluminum-molybdenum system at temperatures above 1400 °C. Z. Met. 1971, 62, 844–848. [Google Scholar]
- Wood, E.; Compton, V.B.; Matthias, B.; Corenzwit, E. β-Wolfram structure of compounds between transition elements and aluminum, gallium and antimony. Acta Crystallogr. 1958, 11, 604–606. [Google Scholar] [CrossRef]















| Phase | Pearson Symbol | Prototype | Space Group | Lattice Parameters [nm] | Ref. | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| αAl3Hf | tI8 | A13Ti | I4/mmm | 0.3982 | … | 1.7155 | [26] |
| βAl3Hf | tI16 | A13Zr | I4/mmm | 0.3893 | … | 0.8925 | [26] |
| Al2Hf | hP12 | MgZn2 | P63/mmc | 0.6776 | … | 0.5372 | [26] |
| Al3Hf2 | oF40 | Al3Zr2 | Fdd2 | 0.5522 | 0.9523 | 1.3763 | [26] |
| AlHf | oC8 | CrB | Cmcm | 0.3253 | 1.0822 | 0.4280 | [26] |
| Al3Hf4 | hP7 | Al3Zr4 | P6 | 0.5343 | … | 0.5422 | [26] |
| Al2Hf3 | tP20 | Al2Zr3 | P42/mnm | 0.7535 | … | 0.6906 | [26] |
| AlHf2 | tI12 | Al2Cu | I4/mcm | 0.6776 | … | 0.5372 | [26] |
| αHfMo2 | cF24 | MgCu2 | Fd3m | 0.7.557 | … | … | [44] |
| βHfMo2 | hP24 | MgNi2 | P63/mmc | 0.5.366 | … | 1.7408 | [44] |
| Al12Mo | cI26 | Al12W | Im | 0.7573 | … | … | [39] |
| αAl5Mo | hP36 | ... | Rc | 0.495.1 | … | 2.623 | [39] |
| βAl5Mo | hP12 | Al5W | P6322 | 0.4912 | … | 0.886 | [39] |
| Al63M37 | ... | ... | … | … | … | … | [45] |
| Al22Mo5 | oF216 | ... | Fdd2 | 7.382 | 0.916 | 4.932 | [46] |
| Al17Mo4 | mC84 | ... | C2 | 0.9158 | 0.49323 | 2.8935 | [46] |
| Al4Mo | mC30 | Al4W | Cm | 0.5255 | 1.7768 | 0.5225 | [47] |
| Al3Mo | mC32 | ... | C2/m | 1.6396 | 0.3594 | 0.8386 | [39] |
| Al8Mo3 | mC22 | ... | Cm | 0.9207 | 0.3641 | 1.0065 | [48] |
| AlMo | cP2 | CsCl | Pmm | 0.3098 | … | … | [49] |
| AlMo3 | cP8 | Cr3Si | Pmn | 0.4950 | … | … | [50] |
| Alloy | Nominal Composition (at.%) | Experimental Results (at.%) | Phase | ||||
|---|---|---|---|---|---|---|---|
| Number | Al | Hf | Mo | Al | Hf | Mo | |
| A1 | 90 | 5 | 5 | 91.96 | 0.14 | 7.9 | Al12Mo |
| 99.78 | 0.09 | 0.13 | Al | ||||
| 75.19 | 23.71 | 1.1 | Al3Hf | ||||
| A2 | 85 | 5 | 10 | 83.37 | 0.08 | 16.55 | Al5Mo |
| 74.46 | 24.31 | 1.23 | Al3Hf | ||||
| 92.06 | 0.16 | 7.78 | Al12Mo | ||||
| A3 | 45 | 10 | 45 | 24.68 | 0.53 | 74.79 | AlMo3 |
| 40.34 | 31.41 | 28.25 | AlMoHf | ||||
| 70.55 | 0.55 | 28.9 | Al8Mo3 | ||||
| A4 | 15 | 25 | 60 | 20.13 | 5.39 | 74.48 | AlMo3 |
| 30.04 | 33.04 | 36.92 | AlMoHf | ||||
| 0.76 | 31.09 | 68.15 | HfMo2 | ||||
| A5 | 35 | 55 | 10 | 39.87 | 59.31 | 0.82 | Al2Hf3 |
| 35.4 | 36.58 | 28.02 | AlMoHf | ||||
| 33.43 | 65.84 | 0.73 | AlHf2 | ||||
| A6 | 45 | 50 | 5 | 42.41 | 56.27 | 1.32 | Al3Hf4 |
| 39.28 | 35.88 | 24.84 | AlMoHf | ||||
| 49.88 | 49.01 | 1.11 | AlHf | ||||
| A7 | 53 | 42 | 5 | 41.03 | 35.32 | 23.65 | AlMoHf |
| 50.01 | 48.93 | 1.06 | AlHf | ||||
| 59.71 | 39.41 | 0.88 | Al3Hf2 | ||||
| A8 | 40 | 55 | 5 | 40.24 | 58.62 | 1.14 | Al2Hf3 |
| 37.7 | 37.22 | 25.08 | AlMoHf | ||||
| A9 | 55 | 20 | 25 | 71.98 | 2.64 | 25.38 | Al8Mo3 |
| 44.66 | 31.18 | 24.16 | AlMoHf | ||||
| A10 | 53 | 20 | 27 | 43.26 | 30.08 | 26.66 | AlMoHf |
| 70.81 | 1.51 | 27.68 | Al8Mo3 | ||||
| A11 | 28 | 12 | 60 | 21.18 | 3.85 | 74.97 | AlMo3 |
| 31.69 | 31.24 | 37.07 | AlMoHf | ||||
| A12 | 25 | 15 | 60 | 24.03 | 2.1 | 73.87 | AlMo3 |
| 37.19 | 30.91 | 31.9 | AlMoHf | ||||
| A13 | 20 | 35 | 45 | 2.12 | 34.02 | 63.86 | HfMo2 |
| 27.79 | 34.94 | 37.27 | AlMoHf | ||||
| A14 | 15 | 37 | 48 | 1.17 | 37.04 | 61.79 | HfMo2 |
| 27.32 | 36.89 | 35.79 | AlMoHf | ||||
| A15 | 15 | 3 | 82 | 23.88 | 1.53 | 74.59 | AlMo3 |
| 3.47 | 3.45 | 93.08 | Mo | ||||
| A16 | 42 | 32 | 26 | 41.78 | 32.39 | 25.83 | AlMoHf |
| A17 | 30 | 35 | 35 | 30.83 | 34.71 | 34.46 | AlMoHf |
| A18 | 1 | 4 | 95 | 0.75 | 3.78 | 95.47 | (Mo) |
| A19 | 8 | 87 | 5 | 8.09 | 86.78 | 5.13 | (Hf) |
| A20 | 13 | 86 | 1 | 12.86 | 86.13 | 1.01 | (Hf) |
| A21 | 14 | 81 | 5 | 13.87 | 81.23 | 4.9 | (Hf) |
| A22 | 10 | 80 | 10 | 10.01 | 80.51 | 9.48 | (Hf) |
| Alloy | Nominal Composition (at.%) | Experimental Results (at.%) | Phase | ||||
|---|---|---|---|---|---|---|---|
| Number | Al | Hf | Mo | Al | Hf | Mo | |
| B1 | 90 | 5 | 5 | 42.38 | 55.94 | 1.68 | Al12Mo |
| 39.14 | 37.36 | 23.5 | Al | ||||
| 49.7 | 48.57 | 1.73 | Al3Hf | ||||
| B2 | 85 | 10 | 5 | 41.55 | 35.88 | 22.57 | Al5Mo |
| 49.87 | 48.46 | 1.67 | Al3Hf | ||||
| 59.44 | 39.27 | 1.29 | Al12Mo | ||||
| B3 | 45 | 10 | 45 | 70.45 | 3.42 | 26.13 | AlMo3 |
| 45.29 | 31.92 | 22.79 | AlMoHf | ||||
| 22.43 | 5.41 | 72.16 | Al8Mo3 | ||||
| B4 | 15 | 25 | 60 | 29.85 | 31.95 | 38.2 | AlMo3 |
| 24.36 | 3.58 | 72.06 | AlMoHf | ||||
| 39.15 | 30.41 | 30.44 | HfMo2 | ||||
| B5 | 35 | 55 | 10 | 3.92 | 32.68 | 63.4 | Al2Hf3 |
| 26.23 | 35.38 | 38.39 | AlMoHf | ||||
| 2.62 | 35.94 | 61.44 | AlHf2 | ||||
| B6 | 40 | 55 | 5 | 25.23 | 37.89 | 36.88 | Al3Hf4 |
| 4.01 | 4.29 | 91.7 | Al2Hf3 | ||||
| 22.59 | 1.48 | 75.93 | AlMoHf | ||||
| B7 | 45 | 50 | 5 | 42.38 | 55.94 | 1.68 | Al3Hf4 |
| 39.14 | 37.36 | 23.5 | AlMoHf | ||||
| 49.7 | 48.57 | 1.73 | AlHf | ||||
| B8 | 53 | 42 | 5 | 41.55 | 35.88 | 22.57 | AlMoHf |
| 49.87 | 48.46 | 1.67 | AlHf | ||||
| 59.44 | 39.27 | 1.29 | Al3Hf2 | ||||
| B9 | 55 | 20 | 25 | 70.45 | 3.42 | 26.13 | Al8Mo3 |
| 45.29 | 31.92 | 22.79 | AlMoHf | ||||
| B10 | 28 | 12 | 60 | 22.43 | 5.41 | 72.16 | AlMo3 |
| 29.85 | 31.95 | 38.2 | AlMoHf | ||||
| B11 | 25 | 15 | 60 | 24.36 | 3.58 | 72.06 | AlMo3 |
| 39.15 | 30.41 | 30.44 | AlMoHf | ||||
| B12 | 20 | 35 | 45 | 3.92 | 32.68 | 63.4 | HfMo2 |
| 26.23 | 35.38 | 38.39 | AlMoHf | ||||
| B13 | 15 | 37 | 48 | 2.62 | 35.94 | 61.44 | HfMo2 |
| 25.23 | 37.89 | 36.88 | AlMoHf | ||||
| B14 | 15 | 3 | 82 | 4.01 | 4.29 | 91.7 | (Mo) |
| 22.59 | 1.48 | 75.93 | AlMo3 | ||||
| B15 | 42 | 33 | 25 | 41.13 | 33.49 | 25.38 | AlMoHf |
| B16 | 30 | 35 | 35 | 30.03 | 35.94 | 34.03 | AlMoHf |
| B17 | 2 | 4 | 94 | 1.69 | 3.81 | 94.5 | (Mo) |
| B18 | 18 | 77 | 5 | 18.24 | 76.91 | 4.85 | (Hf) |
| B19 | 13 | 82 | 5 | 13.01 | 82.33 | 4.66 | (Hf) |
| B20 | 4 | 90 | 6 | 3.31 | 90.5 | 6.19 | (Hf) |
| B21 | 10 | 81 | 9 | 10.16 | 80.81 | 9.3 | (Hf) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yu, Z.; Liu, L.; Zhang, L. Experimental Investigation of Phase Equilibria in the Al–Mo–Hf Ternary System at 400 °C and 600 °C. Processes 2024, 12, 969. https://doi.org/10.3390/pr12050969
Liu B, Yu Z, Liu L, Zhang L. Experimental Investigation of Phase Equilibria in the Al–Mo–Hf Ternary System at 400 °C and 600 °C. Processes. 2024; 12(5):969. https://doi.org/10.3390/pr12050969
Chicago/Turabian StyleLiu, Boliang, Zhiqiang Yu, Libin Liu, and Ligang Zhang. 2024. "Experimental Investigation of Phase Equilibria in the Al–Mo–Hf Ternary System at 400 °C and 600 °C" Processes 12, no. 5: 969. https://doi.org/10.3390/pr12050969




