Functional Optical Coherence Tomography of Rat Cortical Neurovascular Activation during Monopulse Electrical Stimulation with the Microelectrode Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. OISI-OCT System and Data Acquisition
2.3. Data Analysis
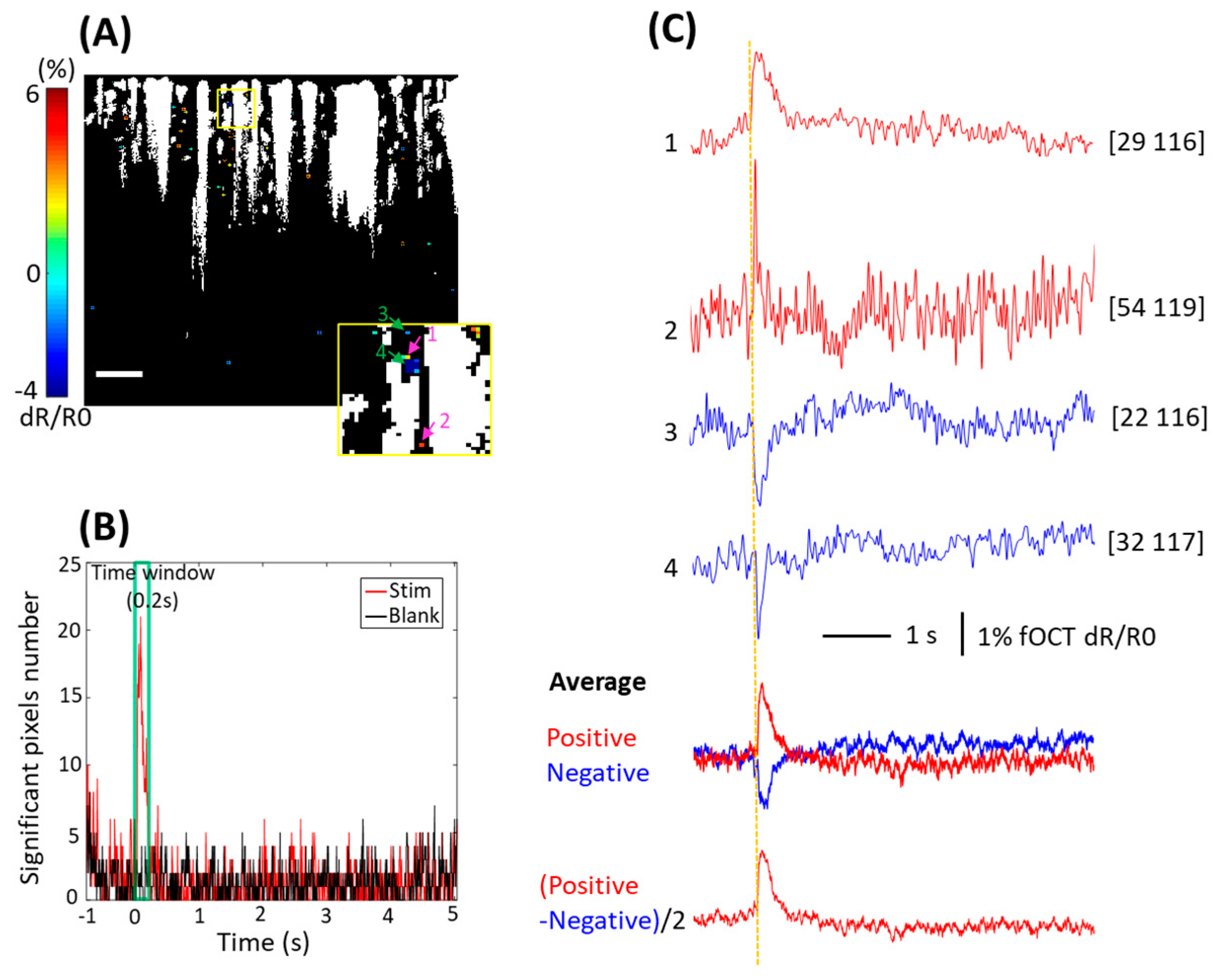
3. Results
3.1. OISI Hemodynamic Response
3.2. fOCT Depth-Resolved Hemodynamic Response
3.3. fOCT Depth-Resolved Neural Tissue Response
3.4. fOCT Neurovascular Coupling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, M.S.; Kincaid, J.; Ojemann, G.A.; Lettich, E. Brain Mapping Techniques to Maximize Resection, Safety, and Seizure Control in Children with Brain Tumors. Neurosurgery 1989, 25, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.S.; Ojemann, G.A. Intraoperative Brain Mapping Techniques in Neuro-Oncology. Stereotact. Funct. Neurosurg. 1992, 58, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.N.; King-Stephens, D.; Massey, A.D.; Nair, D.R.; Jobst, B.C.; Barkley, G.L.; Salanova, V.; Cole, A.J.; Smith, M.C.; Gwinn, R.P.; et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System Pivotal trial. Epilepsia 2014, 55, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011, 77, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Zubair, M.N.; Marquez, Y.D.; Lee, D.M.; Kalayjian, L.A.; Heck, C.N.; Liu, C.Y. A Single-Center Experience with the NeuroPace RNS System: A Review of Techniques and Potential Problems. World Neurosurg. 2015, 84, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Kahane, P.; Tassi, L.; Francione, S.; Hoffmann, D.; Russo, G.L.; Benabid, A.L. Intracerebral Low Frequency Electrical Stimulation: A New Tool for the Definition of the “Epileptogenic Area”? In Advances in Stereotactic and Functional Neurosurgery 10; Springer: Vienna, Austria, 1993; pp. 181–185. [Google Scholar]
- Balossier, A.; Etard, O.; Descat, C.; Vivien, D.; Emery, E. Epidural Cortical Stimulation as a Treatment for Poststroke Aphasia:A Systematic Review of the Literature and Underlying Neurophysiological Mechanisms. Neurorehabilit. Neural Repair 2016, 30, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.J.; Ojemann, J.G.; Rao, R.P.N. Direct Electrical Stimulation in Electrocorticographic Brain-Computer Interfaces: Enabling Technologies for Input to Cortex. Front. Neurosci. 2019, 13, 804. [Google Scholar] [CrossRef]
- Hiremath, S.V.; Tyler-Kabara, E.C.; Wheeler, J.J.; Moran, D.W.; Gaunt, R.A.; Collinger, J.L.; Foldes, S.T.; Weber, D.J.; Chen, W.; Boninger, M.L.; et al. Human perception of electrical stimulation on the surface of somatosensory cortex. PLoS ONE 2017, 12, e0176020. [Google Scholar] [CrossRef]
- Kirin, S.C.; Yanagisawa, T.; Oshino, S.; Edakawa, K.; Tanaka, M.; Kishima, H.; Nishimura, Y. Somatosensation Evoked by Cortical Surface Stimulation of the Human Primary Somatosensory Cortex. Front. Neurosci. 2019, 13, 1019. [Google Scholar] [CrossRef]
- Histed, M.H.; Bonin, V.; Reid, R.C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 2009, 63, 508–522. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nomoto, T.; Shiki, T.; Sakata, Y.; Shimada, Y.; Hayashida, Y.; Yagi, T. Focal activation of neuronal circuits induced by microstimulation in the visual cortex. J. Neural Eng. 2019, 16, 036007. [Google Scholar] [CrossRef] [PubMed]
- Kunori, N.; Takashima, I. A transparent epidural electrode array for use in conjunction with optical imaging. J. Neurosci. Methods 2015, 251, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Chernov, M.M.; Chen, G.; Torre-Healy, L.A.; Friedman, R.M.; Roe, A.W. Microelectrode array stimulation combined with intrinsic optical imaging: A novel tool for functional brain mapping. J. Neurosci. Methods 2016, 263, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Aguirre, A.D.; Ruvinskaya, L.; Devor, A.; Boas, D.A.; Fujimoto, J.G. Optical coherence tomography (OCT) reveals depth-resolved dynamics during functional brain activation. J. Neurosci. Methods 2009, 178, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.C.; Kuo, Y.M.; Syu, J.P.; Wang, H.L.; Lai, C.M.; Chen, J.W.; Lo, Y.C.; Chen, Y.Y. The use of intensity-based Doppler variance method for single vessel response to functional neurovascular activation. J. Biophotonics 2018, 11, e201800017. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Li, Y.; Rakymzhan, A.; Xie, Z.; Wang, R.K. Measurement and visualization of stimulus-evoked tissue dynamics in mouse barrel cortex using phase-sensitive optical coherence tomography. Biomed. Opt. Express 2020, 11, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Marks, D.L.; Potgieter, K.; Gillette, R.; Boppart, S.A. Functional optical coherence tomography for detecting neural activity through scattering changes. Opt. Lett. 2003, 28, 1218–1220. [Google Scholar] [CrossRef]
- Akkin, T.; Landowne, D.; Sivaprakasam, A. Detection of neural action potentials using optical coherence tomography: Intensity and phase measurements with and without dyes. Front. Neuroenerg. 2010, 2, 1487. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Enfield, J.; Jonathan, E.; Leahy, M. In vivo imaging of the microcirculation of the volar forearm using correlation mapping optical coherence tomography (cmOCT). Biomed. Opt. Express 2011, 2, 1184–1193. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, R.; Wang, B.; Messinger, J.D.; Curcio, C.A.; Yao, X. Functional Optical Coherence Tomography Enables In Vivo Physiological Assessment of Retinal Rod and Cone Photoreceptors. Sci. Rep. 2015, 5, 9595. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, M.; Chen, X.; Pohmann, R.; Polimeni, J.R.; Scheffler, K.; Rosen, B.R.; Kleinfeld, D.; Yu, X. Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron 2018, 97, 925–939.e5. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Tian, P.; Teng, I.C.; May, L.D.; Kurz, R.; Lu, K.; Scadeng, M.; Hillman, E.M.; De Crespigny, A.J.; D’Arceuil, H.E.; Mandeville, J.B.; et al. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc. Natl. Acad. Sci. USA 2010, 107, 15246–15251. [Google Scholar] [CrossRef] [PubMed]
- Baird-Daniel, E.; Daniel, A.G.S.; Wenzel, M.; Li, D.; Liou, J.Y.; Laffont, P.; Zhao, M.; Yuste, R.; Ma, H.; Schwartz, T.H. Glial Calcium Waves are Triggered by Seizure Activity and Not Essential for Initiating Ictal Onset or Neurovascular Coupling. Cereb. Cortex 2017, 27, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Shafeghat, N.; Heidarinejad, M.; Murata, N.; Nakamura, H.; Inoue, T. Optical detection of neuron connectivity by random access two-photon microscopy. J. Neurosci. Methods 2016, 263, 48–56. [Google Scholar] [CrossRef]
- Schei, J.L.; McCluskey, M.D.; Foust, A.J.; Yao, X.C.; Rector, D.M. Action potential propagation imaged with high temporal resolution near-infrared video microscopy and polarized light. Neuroimage 2008, 40, 1034–1043. [Google Scholar] [CrossRef]
- Graf, B.W.; Ralston, T.S.; Ko, H.J.; Boppart, S.A. Detecting intrinsic scattering changes correlated to neuron action potentials using OCT coherence imaging. Opt. Express 2009, 17, 13447–13457. [Google Scholar] [CrossRef]
- Gao, Y.R.; Greene, S.E.; Drew, P.J. Mechanical restriction of intracortical vessel dilation by brain tissue sculpts the hemodynamic response. Neuroimage 2015, 115, 162–176. [Google Scholar] [CrossRef]
- Park, J.; Seok, H.S.; Kim, S.-S.; Shin, H. Photoplethysmogram Analysis and Applications: An Integrative Review. Front. Physiol. 2022, 12, 808451. [Google Scholar] [CrossRef]







| Group | Year | Stimulation | Techniques | Response Results |
|---|---|---|---|---|
| Yu Chen et al. [15] | 2009 | Rat; forepaw electrical stimulation | fOCT, OISI | Reflectivity changes in fOCT correlate well with OISI, and the fOCT-layer-specific response indicates a time delay of −1.5 s to 3.5 s in both the onset and peak with respect to the stimulation pattern. |
| Wen-Chuan Kuo et al. [16] | 2018 | Rat; forepaw electrical stimulation | fOCT | Response time of small, middle and large vessels to achieve a 5% change in vascular dilation after stimulation is 1.5 s, 2 s and 5.5 s, respectively. |
| Peijun Tang et al. [17] | 2020 | Mouse; whisker stimulation | Phase-sensitive OCT (PhS-OCT), OISI | The activated neural tissue region in PhS-OCT is consistent with that in OISI. |
| Mariya Lazebnik et al. [18] | 2003 | Nerve fibers from the abdominal ganglion of the sea slug; electrical stimulation | fOCT | Optical scattering signal changes in neural tissue are a result of propagating action potentials. |
| Taner Akkin et al. [19] | 2010 | Squid giant axon; electrical stimulation | fOCT | The back-scattered intensity changes coincides with the arrival of neural action potentials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Huang, J.; Liu, T.; Gu, H.; Li, C.; Yang, K.; Yan, H.; Huang, L.; Jiang, X.; Wang, C.; et al. Functional Optical Coherence Tomography of Rat Cortical Neurovascular Activation during Monopulse Electrical Stimulation with the Microelectrode Array. Photonics 2024, 11, 420. https://doi.org/10.3390/photonics11050420
Yao L, Huang J, Liu T, Gu H, Li C, Yang K, Yan H, Huang L, Jiang X, Wang C, et al. Functional Optical Coherence Tomography of Rat Cortical Neurovascular Activation during Monopulse Electrical Stimulation with the Microelectrode Array. Photonics. 2024; 11(5):420. https://doi.org/10.3390/photonics11050420
Chicago/Turabian StyleYao, Lin, Jin Huang, Taixiang Liu, Han Gu, Changpeng Li, Ke Yang, Hongwei Yan, Lin Huang, Xiaodong Jiang, Chengcheng Wang, and et al. 2024. "Functional Optical Coherence Tomography of Rat Cortical Neurovascular Activation during Monopulse Electrical Stimulation with the Microelectrode Array" Photonics 11, no. 5: 420. https://doi.org/10.3390/photonics11050420




