Development and Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations Containing Flurbiprofen Solid Dispersions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Solid Dispersions
2.1.1. Solubility Studies
2.1.2. Determination of Drug Content
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Differential Scanning Calorimetry (DSC)
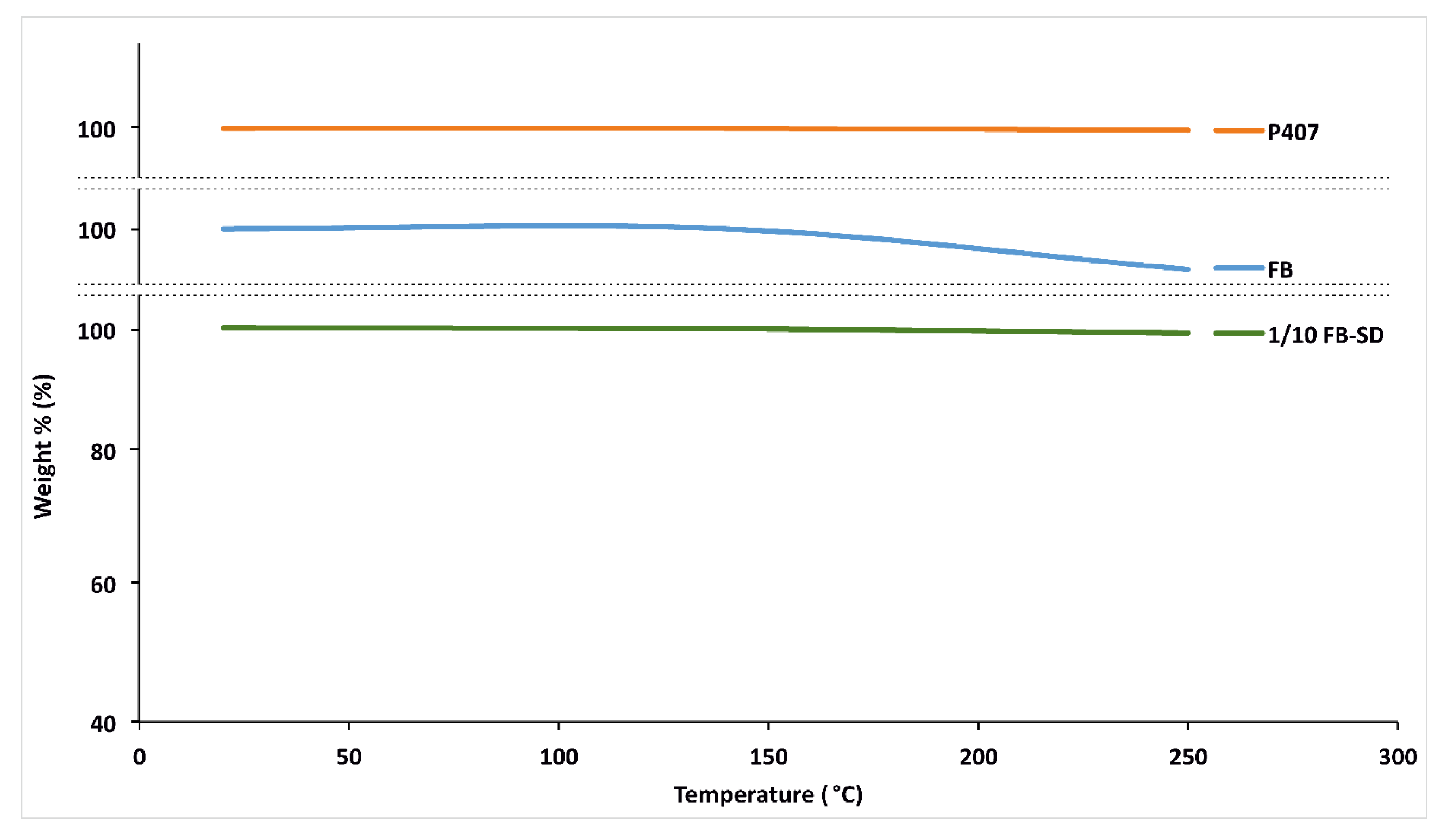
2.1.5. Thermogravimetric Analysis (TGA)
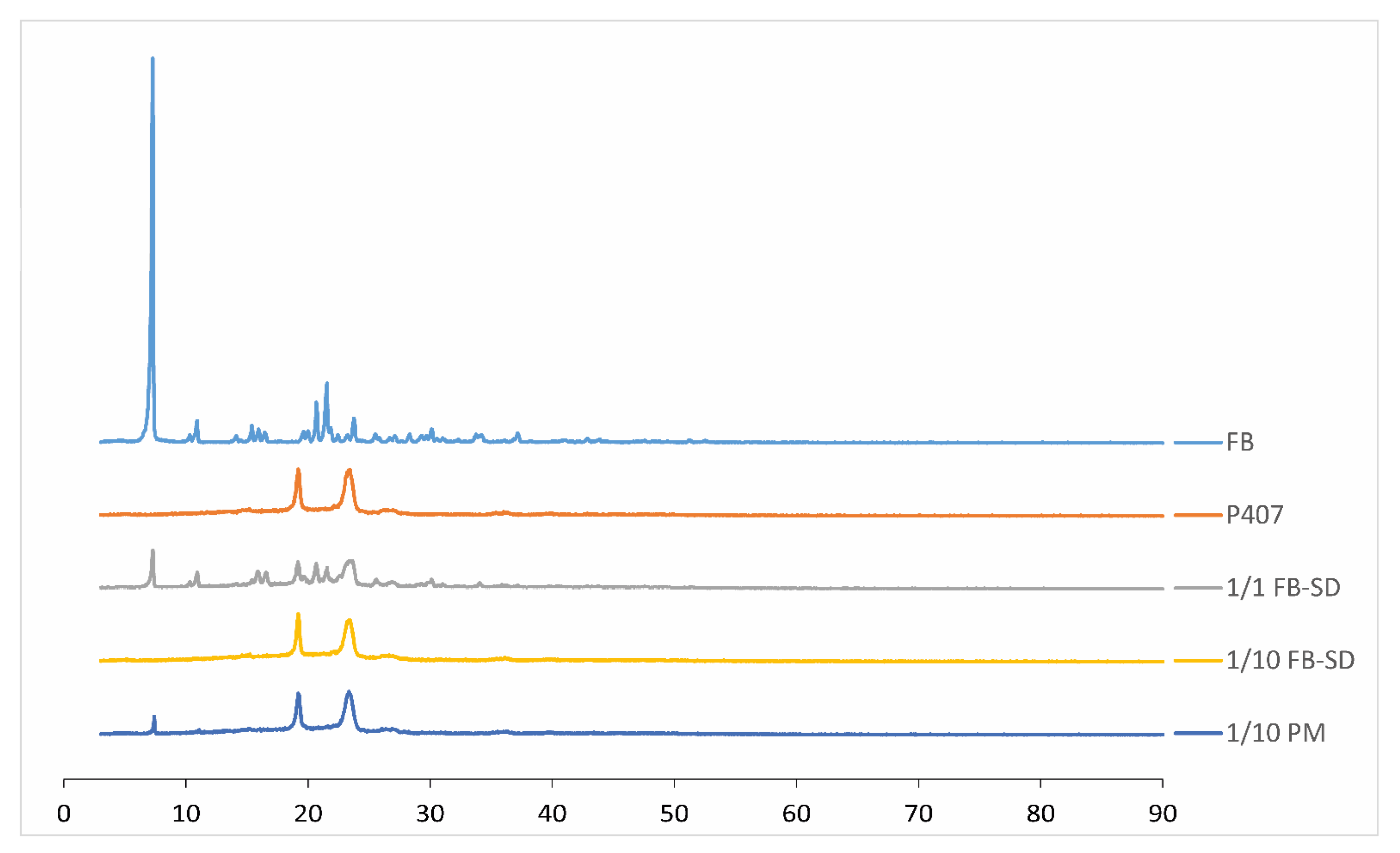
2.1.6. X-ray Powder Diffraction Studies
2.1.7. Stability Studies
2.2. Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations
2.2.1. Gelation Temperature and Gelation Time
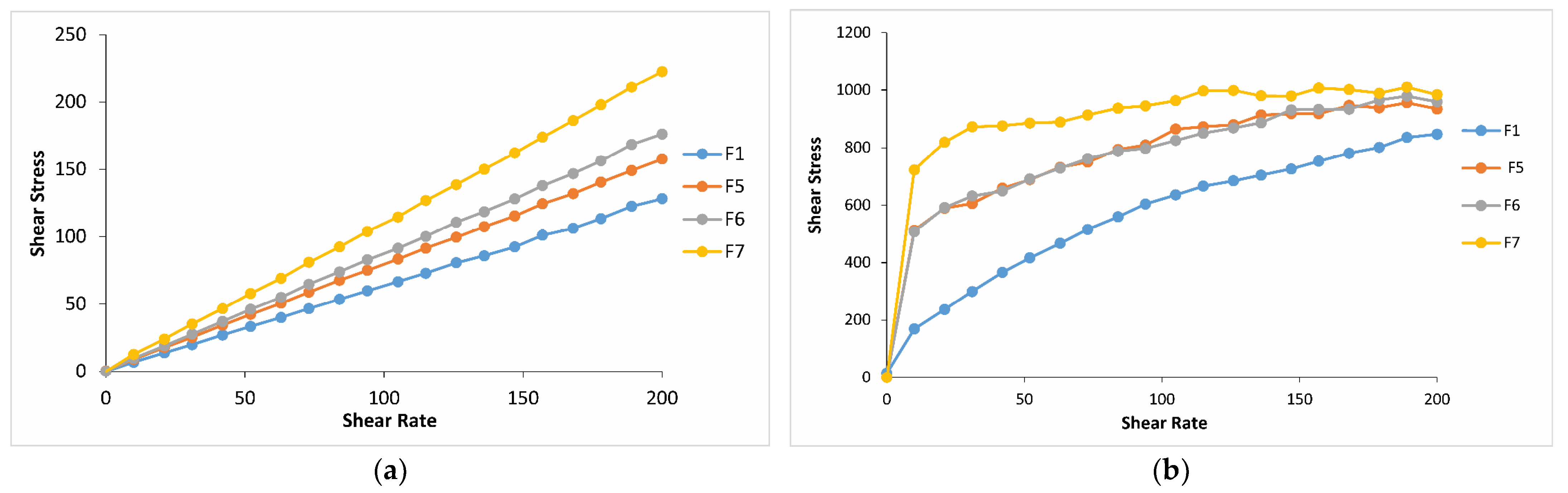
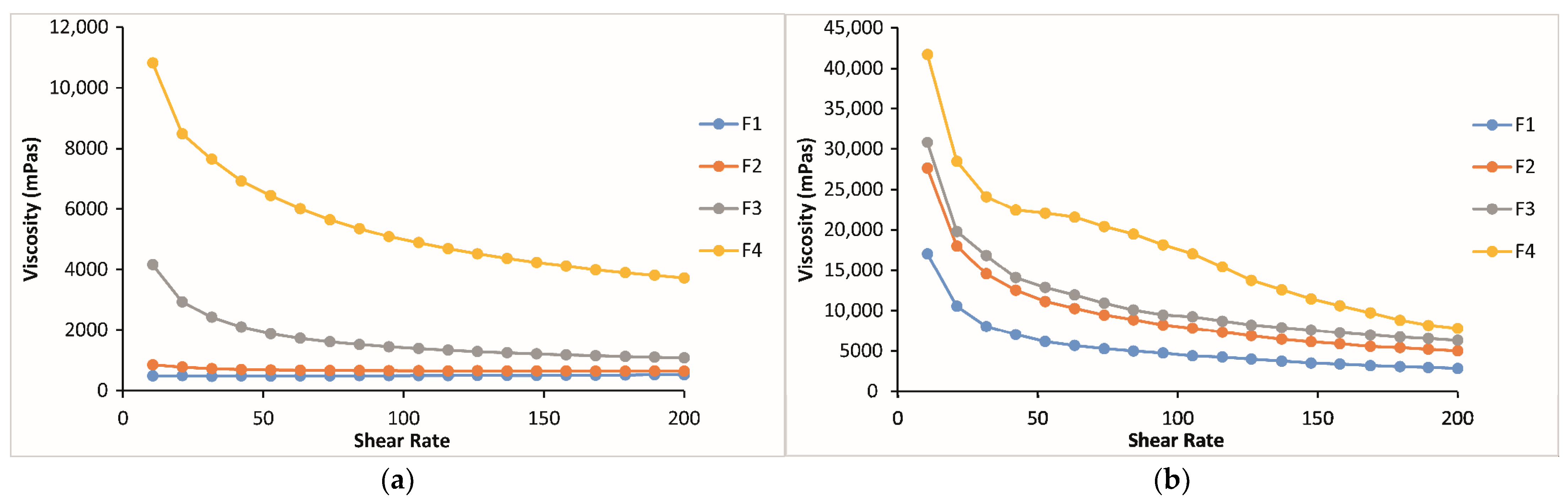
2.2.2. Rheological Evaluation of Thermosensitive and Bioadhesive Ophthalmic Formulations
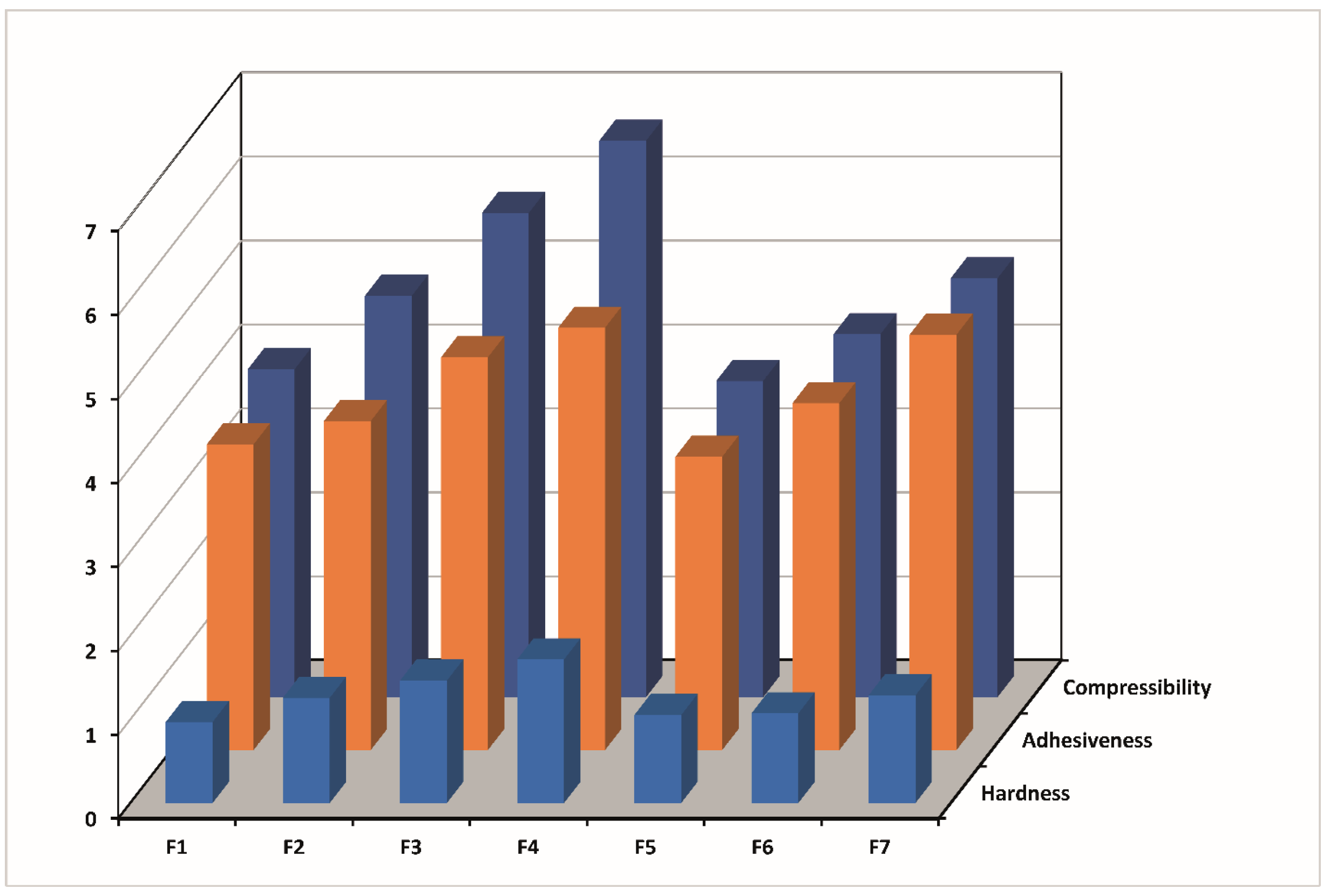
2.3. Mechanical Properties of Thermosensitive and Bioadhesive Ophthalmic Formulations
2.4. In Vitro Drug Release from Thermosensitive and Bioadhesive Ophthalmic Formulations
2.5. Kinetic Evaluations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Solid Dispersions
4.3. Characterization of Solid Dispersions
4.3.1. Solubility Studies
4.3.2. Determination of Drug Content
4.3.3. Scanning Electron Microscopy (SEM)
4.3.4. Differential Scanning Calorimetry (DSC)
4.3.5. Thermogravimetric Analysis (TGA)
4.3.6. X-ray Powder Diffraction Studies
4.3.7. Stability Studies
4.4. Preformulation Studies of Thermosensitive Ophthalmic Formulations
4.5. Preparation of Thermosensitive and Bioadhesive Ophthalmic Formulations
4.6. Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations
4.6.1. Measurement of Gelation Temperature
4.6.2. Rheological Evaluation of Thermosensitive and Bioadhesive Ophthalmic Formulations
- -
- At a constant frequency value (1 Hz) and at temperature values ranging between 25 and 40 °C to evaluate the gelation temperature of the samples (Method 2).
- -
- At constant temperature (34 °C) and frequency (1 Hz) values and at increasing times to determine the gelation time of the sample at the physiological temperature.
4.6.3. Mechanical Properties of Thermosensitive and Bioadhesive Ophthalmic Formulations
4.6.4. In Vitro Drug Release from Thermosensitive and Bioadhesive Ophthalmic Formulations
4.6.5. Kinetic Evaluations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological Evaluation of Poloxamer as an in Situ Gel for Ophthalmic Use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Zaman, M.; Hameed, H.; Sarwar, H.S.; Khan, M.A.; Irfan, A.; Shazly, G.A.; Paiva-Santos, A.C.; Jardan, Y.A.B. Lamotrigine-Loaded Poloxamer-Based Thermo-Responsive Sol–Gel: Formulation, In Vitro Assessment, Ex Vivo Permeation, and Toxicology Study. Gels 2023, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research Progress of In-Situ Gelling Ophthalmic Drug Delivery System. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Ichikawa, M.; Kawakami, S.; Yamamura, K.; Nishida, K.; Nakamura, J. In Situ Ocular Absorption of Tilisolol through Ocular Membranes in Albino Rabbits. J. Pharm. Sci. 1996, 85, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.L.; Ping, Q. Poly(N-Isopropylacrylamide)–Chitosan as Thermosensitive in Situ Gel-Forming System for Ocular Drug Delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Xu, H.; Ding, P.T.; Li, S.M.; Zheng, J.M. Thermosetting Gels with Modulated Gelation Temperature for Ophthalmic Use: The Rheological and Gamma Scintigraphic Studies. J. Control. Release 2002, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of Loteprednol Etabonate-Loaded Cationic Nanoemulsified in-Situ Ophthalmic Gel for Sustained Delivery and Enhanced Ocular Bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.F.; Robinson, J.R. Quantitative Precorneal Disposition of Topically Applied Pilocarpine Nitrate in Rabbit Eyes. J. Pharm. Sci. 1976, 65, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Makoid, M.C.; Sieg, J.W.; Robinson, J.R. Corneal Drug Absorption: An Illustration of Parallel First-Order Absorption and Rapid Loss of Drug from Absorption Depot. J. Pharm. Sci. 1976, 65, 150–153. [Google Scholar] [CrossRef]
- Patton, T.F.; Robinson, J.R. Influence of Topical Anesthesia on Tear Dynamics and Ocular Drug Bioavailability in Albino Rabbits. J. Pharm. Sci. 1975, 64, 267–271. [Google Scholar] [CrossRef]
- Sieg, J.W.; Robinson, J.R. Vehicle Effects on Ocular Drug Bioavailability i: Evaluation of Fluorometholone. J. Pharm. Sci. 1975, 64, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Sieg, J.W.; Robinson, J.R. Vehicle Effects on Ocular Drug Bioavailability II: Evaluation of Pilocarpine. J. Pharm. Sci. 1977, 66, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, M.; Li, K.; Hong, W.; Lv, Q.; Li, Y.; Xie, S.; Han, J.; Tian, B. Cell Penetrating Peptide TAT-Functionalized Liposomes for Efficient Ophthalmic Delivery of Flurbiprofen: Penetration and Its Underlying Mechanism, Retention, Anti-Inflammation and Biocompatibility. Int. J. Pharm. 2021, 598, 120405. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chen, W.; Huang, C.; Li, L.; Chen, C.; Li, W.; Wu, C. Development of a Poloxamer Analogs/Carbopol-Based in Situ Gelling and Mucoadhesive Ophthalmic Delivery System for Puerarin. Int. J. Pharm. 2007, 337, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Lobel, E.; Trevgoda, A.; Peled, Y. A Novel in Situ-Forming Ophthalmic Drug Delivery System from Alginates Undergoing Gelation in the Eye. J. Control. Release 1997, 44, 201–208. [Google Scholar] [CrossRef]
- Bochot, A.; Fattal, E.; Gulik, A.; Couarraze, G.; Couvreur, P. Liposomes Dispersed within a Thermosensitive Gel: A New Dosage Form for Ocular Delivery of Oligonucleotides. Pharm. Res. 1998, 15, 1364–1369. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic Gels: Past, Present and Future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-D.; Xu, H.; Wang, C.; Nie, S.-F.; Pan, W.-S. Pluronic F127-g-Poly(Acrylic Acid) Copolymers as in Situ Gelling Vehicle for Ophthalmic Drug Delivery System. Int. J. Pharm. 2008, 350, 247–256. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Mehta, K.A.; Kislalioglu, M.S.; Phuapradit, W.; Malick, A.W.; Shah, N.H. Multi-Unit Controlled Release Systems of Nifedipine and Nifedipine:Pluronic F-68 Solid Dispersions: Characterization of Release Mechanisms. Drug Dev. Ind. Pharm. 2002, 28, 275–285. [Google Scholar] [CrossRef]
- Ali, A.; Sharma, S.N. Preparation and Evaluation of Solid Dispersions of Ibuprofen. Highly Accessed Article. Indian J. Pharm. Sci. 1991, 53, 233–236. [Google Scholar]
- Newa, M.; Bhandari, K.H.; Li, D.X.; Kwon, T.-H.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Lyoo, W.S.; Yong, C.S.; Choi, H.G. Preparation, Characterization and in Vivo Evaluation of Ibuprofen Binary Solid Dispersions with Poloxamer 188. Int. J. Pharm. 2007, 343, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, S.M.; Sunada, H. Preparation and Evaluation of Ibuprofen Solid Dispersion Systems with Kollidon Particles Using a Pulse Combustion Dryer System. Chem. Pharm. Bull. 2007, 55, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced Drug Delivery and Targeting Technologies for the Ocular Diseases. BioImpacts BI 2016, 6, 49–67. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, P.; Powell, R.J. The Eye in Systemic Inflammatory Diseases. Lancet Lond. Engl. 2004, 364, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Wenk, M.R.; Tong, L. Lipid-Based Therapy for Ocular Surface Inflammation and Disease. Trends Mol. Med. 2015, 21, 736–748. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular Delivery of Macromolecules. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 172–181. [Google Scholar] [CrossRef]
- Newa, M.; Bhandari, K.H.; Oh, D.H.; Kim, Y.R.; Sung, J.H.; Kim, J.O.; Woo, J.S.; Choi, H.G.; Yong, C.S. Enhanced Dissolution of Ibuprofen Using Solid Dispersion with Poloxamer 407. Arch. Pharm. Res. 2008, 31, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A.; Maheshwari, M.; Tyagi, A.K.; Chauhan, B.; Kadam, S.S. Preparation and Characterization of Flurbiprofen Beads by Melt Solidification Technique. AAPS PharmSciTech 2003, 4, 514–522. [Google Scholar] [CrossRef]
- Schilling, S.U.; Bruce, C.D.; Shah, N.H.; Malick, A.W.; McGinity, J.W. Citric Acid Monohydrate as a Release-Modifying Agent in Melt Extruded Matrix Tablets. Int. J. Pharm. 2008, 361, 158–168. [Google Scholar] [CrossRef]
- Okur, N.Ü.; Yozgatlı, V.; Şenyiğit, Z. Formulation and Detailed Characterization of Voriconazole Loaded in Situ Gels for Ocular Application. Oküler Uygul. Için Vorikonazol Yüklü Situ Jellerin Formülasyonu Ve Detaylı Karakterizasyonu 2020, 44, 33–49. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Krtalić, I.; Radošević, S.; Hafner, A.; Grassi, M.; Nenadić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. D-Optimal Design in the Development of Rheologically Improved In Situ Forming Ophthalmic Gel. J. Pharm. Sci. 2018, 107, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.H. In Vitro and in Vivo Evaluation of Pluronic F127-Based Ocular Delivery System for Timolol Maleate. Int. J. Pharm. 2002, 241, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Blanchard, J. In Vitro Evaluation of Pluronic F127-Based Controlled-Release Ocular Delivery Systems for Pilocarpine. J. Pharm. Sci. 1998, 87, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A Poloxamer/Chitosan in Situ Forming Gel with Prolonged Retention Time for Ocular Delivery. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ozgüney, I.; Kardhiqi, A. Properties of Bioadhesive Ketoprofen Liquid Suppositories: Preparation, Determination of Gelation Temperature, Viscosity Studies and Evaluation of Mechanical Properties Using Texture Analyzer by 4 × 4 Factorial Design. Pharm. Dev. Technol. 2014, 19, 968–975. [Google Scholar] [CrossRef]
- Schick, M. Configuration of the Polyoxyethylene Chain in Bulk, Nonionic Surfactant. Surfactant Sci. 1966, 1, 753–793. [Google Scholar]
- Kramaric, A.; Resman, A.; Kofler, B.; Zmitek, J. Thermoreversible gel as a liquid pharmaceutical carrier for a galenic formulation. Eur. Patent No. 0551626 (A1), 1992. [Google Scholar]
- Choi, H.-G.; Jung, J.-H.; Ryu, J.-M.; Yoon, S.-J.; Oh, Y.-K.; Kim, C.-K. Development of in Situ-Gelling and Mucoadhesive Acetaminophen Liquid Suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Vadnere, M.; Amidon, G.; Lindenbaum, S.; Haslam, J.L. Thermodynamic Studies on the Gel-Sol Transition of Some Pluronic Polyols. Int. J. Pharm. 1984, 22, 207–218. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, J.; Lenaerts, V.; Raymond, P.; Ong, H. Diffusion of Rat Atrial Natriuretic Factor in Thermoreversible Poloxamer Gels. Biomaterials 1989, 10, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Drabik, B.R. Rheological Properties of Poloxamer Vehicles. Int. J. Pharm. 1984, 18, 269–276. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Washington, C.; Davies, M.C.; Hadgraft, J. The Behaviour of Pluronic F127 in Aqueous Solution Studied Using Fluorescent Probes. Int. J. Pharm. 1987, 40, 93–99. [Google Scholar] [CrossRef]
- Mortensen, K.; Brown, W. Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Triblock Copolymers in Aqueous Solution. The Influence of Relative Block Size. Macromolecules 1993, 26, 4128–4135. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.-P.; Guo, Y.-S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.-H.; Chen, L. Thermosensitive and Mucoadhesive in Situ Gel Based on Poloxamer as New Carrier for Rectal Administration of Nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Chang, J.Y.; Oh, Y.-K.; Kong, H.S.; Kim, E.J.; Jang, D.D.; Nam, K.T.; Kim, C.-K. Prolonged Antifungal Effects of Clotrimazole-Containing Mucoadhesive Thermosensitive Gels on Vaginitis. J. Control. Release Off. J. Control. Release Soc. 2002, 82, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Chung, S.J.; Lee, M.H.; Kim, C.K.; Shim, C.K. Increased Bioavailability of Propranolol in Rats by Retaining Thermally Gelling Liquid Suppositories in the Rectum. J. Control. Release Off. J. Control. Release Soc. 1999, 59, 163–172. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Richardson, J.L.; Davies, M.C.; Palin, K.J.; Hadgraft, J. The Effect of Solutes and Polymers on the Gelation Properties of Pluronic F-127 Solutions for Controlled Drug Delivery. J. Control. Release 1987, 5, 113–118. [Google Scholar] [CrossRef]
- ElHady, S.S.; Mortada, N.; Awad, G.A.S.; Zaki, N.; Taha, R.A. Development of in Situ Gelling and Mucoadhesive Mebeverine Hydrochloride Solution for Rectal Administration. Saudi Pharm. J. 2003, 11, 159–171. [Google Scholar]
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef]
- Benedetto, D.A.; Shah, D.O.; Kaufman, H.E. The Instilled Fluid Dynamics and Surface Chemistry of Polymers in the Preocular Tear Film. Investig. Ophthalmol. 1975, 14, 887–902. [Google Scholar]
- Calvo, P.; Alonso, M.J.; Vila-Jato, J.L.; Robinson, J.R. Improved Ocular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef]
- Ünlü, N.; Selek, H.; İrkeç, M.; Şumnu, M.M.; Hıncal, A.A. Kuru Göz Hastalığı ve Yapay Gözyaşı Formülasyonları. Hacettep Üniversitesi Yayın. 1995, 16, 7–8. [Google Scholar]
- Kim, E.Y.; Gao, Z.G.; Park, J.S.; Li, H.; Han, K. rhEGF/HP-Beta-CD Complex in Poloxamer Gel for Ophthalmic Delivery. Int. J. Pharm. 2002, 233, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.R.; Sung, K.C. Carbopol/Pluronic Phase Change Solutions for Ophthalmic Drug Delivery. J. Control. Release Off. J. Control. Release Soc. 2000, 69, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.F.; Robinson, J.R. Ocular Evaluation of Polyvinyl Alcohol Vehicle in Rabbits. J. Pharm. Sci. 1975, 64, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, K. Thermoreversible Networks: Viscoelastic Properties and Structure of Gels, Softcover reprint of the original, 1st ed.; 1997 edition; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-662-14795-5. [Google Scholar]
- Zhou, Z.; Chu, B. Anomalous Association Behavior of an Ethylene Oxide/Propylene Oxide ABA Block Copolymer in Water. Macromolecules 1987, 20, 3089–3091. [Google Scholar] [CrossRef]
- Zhou, Z.; Chu, B. Anomalous Micellization Behavior and Composition Heterogeneity of a Triblock ABA Copolymer of (A) Ethylene Oxide and (B) Propylene Oxide in Aqueous Solution. Macromolecules 1988, 21, 2548–2554. [Google Scholar] [CrossRef]
- Glatter, O.; Scherf, G.; Schillen, K.; Brown, W. Characterization of a Poly(Ethylene Oxide)-Poly(Propylene Oxide) Triblock Copolymer (EO27-PO39-EO27) in Aqueous Solution. Macromolecules 1994, 27, 6046–6054. [Google Scholar] [CrossRef]
- Hvidt, S.; Joergensen, E.B.; Brown, W.; Schillen, K. Micellization and Gelation of Aqueous Solutions of a Triblock Copolymer Studied by Rheological Techniques and Scanning Calorimetry. J. Phys. Chem. 1994, 98, 12320–12328. [Google Scholar] [CrossRef]
- Ceulemans, J.; Ludwig, A. Recent Advances on Bioadhesive Ocular Dosage Forms. FABAD J. Pharm. Sci. 2002, 27, 211–230. [Google Scholar]
- Dudinski, O.; Finnin, B.; Reed, B. Acceptability of Thickened Eye Drops to Human Subjects. Cur. Ther. Res. 1983, 33, 322–338. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, Viscoelastic and Mucoadhesive Properties of Pharmaceutical Gels Composed of Cellulose Polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Interpretation of Mucoadhesive Properties of Polymer Gel Preparations Using a Tensile Strength Method. J. Pharm. Pharmacol. 2001, 53, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Djokic, J.; Coulter, W.A. Development and Mechanical Characterization of Bioadhesive Semi-Solid, Polymeric Systems Containing Tetracycline for the Treatment of Periodontal Diseases. Pharm. Res. 1996, 13, 1734–1738. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Hilmioglu-Polat, S.; Metin, D.Y.; Zekioglu, O.; Guneri, T.; Jones, D.S. In-Situ Gel Formulations of Econazole Nitrate: Preparation and in-Vitro and in-Vivo Evaluation. J. Pharm. Pharmacol. 2011, 63, 1274–1282. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A.M.; Araman, A. Effect of Thiolated Polymers to Textural and Mucoadhesive Properties of Vaginal Gel Formulations Prepared with Polycarbophil and Chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef]
- Woolfson, A.D.; McCafferty, D.F.; Gorman, S.P.; McCarron, P.A.; Price, J.H. Design of an Apparatus Incorporating a Linear Variable Differential Transformer for the Measurement of Type III Bioadhesion to Cervical Tissue. Int. J. Pharm. 1992, 84, 69–76. [Google Scholar] [CrossRef]
- Efentakis, M.; Koutlis, A.; Vlachou, M. Development and Evaluation of Oral Multiple-Unit and Single-Unit Hydrophilic Controlled-Release Systems. AAPS PharmSciTech 2000, 1, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Okudaira, A.; Tsutusmi, Y.; Takahashi, I.; Nakanishi, T.; Kiyono, H.; Mayumi, T. Characterization of Mucoadhesive Microspheres for the Induction of Mucosal and Systemic Immune Responses. Vaccine 2000, 19, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-G.; Oh, Y.-K.; Kim, C.-K. In Situ Gelling and Mucoadhesive Liquid Suppository Containing Acetaminophen: Enhanced Bioavailability. Int. J. Pharm. 1998, 165, 23–32. [Google Scholar] [CrossRef]
- Karasulu, E.; Yeşim Karasulu, H.; Ertan, G.; Kirilmaz, L.; Güneri, T. Extended Release Lipophilic Indomethacin Microspheres: Formulation Factors and Mathematical Equations Fitted Drug Release Rates. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2003, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and That of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef]
- Eloy, J.O.; Marchetti, J.M. Solid Dispersions Containing Ursolic Acid in Poloxamer 407 and PEG 6000: A Comparative Study of Fusion and Solvent Methods. Powder Technol. 2014, 253, 98–106. [Google Scholar] [CrossRef]
- ICH, Q2 (R1) Validation of Analytical Procedure: Text and Methodology—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=37c045b9-2557-4ac3-9b64-926486abd588 (accessed on 18 February 2024).
- ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products-Scientific Guideline|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-and-drug-products-scientific-guideline (accessed on 18 February 2024).
- Cho, K.Y.; Chung, T.W.; Kim, B.C.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of Ciprofloxacin from Poloxamer-Graft-Hyaluronic Acid Hydrogels in Vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Artificial Skin. I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Mansour, M.; Mansour, S.; Mortada, N.D.; Abd Elhady, S.S. Ocular Poloxamer-Based Ciprofloxacin Hydrochloride in Situ Forming Gels. Drug Dev. Ind. Pharm. 2008, 34, 744–752. [Google Scholar] [CrossRef]
- El-Kamel, A.; El-Khatib, M. Thermally Reversible in Situ Gelling Carbamazepine Liquid Suppository. Drug Deliv. 2006, 13, 143–148. [Google Scholar] [CrossRef]
- Rossi, S.; Marciello, M.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Dacarro, C.; Grisoli, P.; Caramella, C. Thermally Sensitive Gels Based on Chitosan Derivatives for the Treatment of Oral Mucositis. Eur. J. Pharm. Biopharm. 2010, 74, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; Coulter, W.A.; McClelland, C.; Irwin, C.R. Design, Characterisation and Preliminary Clinical Evaluation of a Novel Mucoadhesive Topical Formulation Containing Tetracycline for the Treatment of Periodontal Disease. J. Control. Release Off. J. Control. Release Soc. 2000, 67, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and Mechanical Properties of Poloxamer Mixtures as a Mucoadhesive Gel Base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.F.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and Biodegradable Polymeric Micelles for Paclitaxel Delivery. J. Control. Release Off. J. Control. Release Soc. 2005, 103, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Ege, M.A.; Karasulu, H.Y.; Karasulu, E.; Ertan, G. A Computer Program Designed for In Vitro Dissolution Kinetics in in Vitro-In Vivo Kinetic Correlations and Routine Application. Sci. Pharm. 2001, 69, S127–S128. [Google Scholar]
- Peppas, N.A. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]








| Measurement | Observed Absorbance Values | ||
|---|---|---|---|
| Deep Freezer (−20 ± 5 °C) | Refrigerator (5 ± 3 °C) | Room Temperature (25 ± 2 °C) | |
| Initially | 0.483 | 0.483 | 0.483 |
| 15th day | 0.474 | 0.476 | 0.482 |
| 30th day | 0.479 | 0.478 | 0.474 |
| 60th day | 0.467 | 0.467 | 0.469 |
| Mean | 0.475 | 0.476 | 0.477 |
| SD | 0.007 | 0.007 | 0.007 |
| CV% | 1.45 | 1.4 | 1.4 |
| P407/P188% | Gelation Temperature (°C) |
|---|---|
| 15/18 | 40.4 ± 0.07 |
| 15/20 | 39.1 ± 0.07 |
| 15/26 | 34.4 ± 0.07 |
| 15/26.5 (F1) | 33.9 ± 0.71 |
| 15/27 | 33 ± 0.07 |
| 16/27 | 34.8 ± 0.14 |
| 24/5 | 33.2 ± 0.15 |
| 23/5 | 36 ± 0.8 |
| F | P407/P188 %15/%26.5 | CP (%) | CMC (%) | Gelation Time (s) | Gelation Temperature (°C) | |
|---|---|---|---|---|---|---|
| Method 1 | Method 2 | |||||
| F1 | + | − | − | 298 | 33.9 | 34.0 |
| F2 | + | 0.2 | − | 120 | 33.3 | 32.5 |
| F3 | + | 0.4 | − | 71 | 32.6 | 32.0 |
| F4 | + | 0.6 | − | 73 | 32.4 | 32.0 |
| F5 | + | − | 0.2 | 235 | 33.2 | 33.7 |
| F6 | + | − | 0.4 | 207 | 33 | 33.7 |
| F7 | + | − | 0.6 | 150 | 32.9 | 33.0 |
| F | H ± SD(N) | C ± SD(N mm) | A ± SD(N mm) | H ± SD(N) | C ± SD(N mm) | A ± SD(N mm) |
|---|---|---|---|---|---|---|
| 25 °C | 34 °C | |||||
| F1 | 0.011 ± 0.00 | 0.043 ± 0.00 | 0.039 ± 0.00 | 0.965 ± 0.48 | 3.906 ± 0.04 | 3.642 ± 0.13 |
| F2 | 0.015 ± 0.05 | 0.050 ± 0.00 | 0.044 ± 0.00 | 1.253 ± 0.18 | 4.781 ± 0.20 | 3.920 ± 0.26 |
| F3 | 0.027 ± 0.11 | 0.066 ± 0.00 | 0.105 ± 0.00 | 1.458 ± 0.49 | 5.770 ± 0.09 | 4.679 ± 0.13 |
| F4 | 0.034 ± 0.30 | 0.084 ± 0.01 | 0.145 ± 0.00 | 1.710 ± 0.98 | 6.628 ± 0.78 | 5.031 ± 0.43 |
| F5 | 0.010 ± 0.05 | 0.035 ± 0.00 | 0.032 ± 0.00 | 1.048 ± 0.00 | 3.768 ± 0.26 | 3.494 ± 0.54 |
| F6 | 0.011 ± 0.02 | 0.036 ± 0.00 | 0.029 ± 0.00 | 1.068 ± 0.50 | 4.324 ± 0.16 | 4.133 ± 0.27 |
| F7 | 0.010 ± 0.03 | 0.033 ± 0.01 | 0.029 ± 0.00 | 1.283 ± 0.50 | 4.990 ± 0.08 | 4.949 ± 0.25 |
| Formulation | Zero-Order | First-Order | Higuchi | Hixson-Crowell | ||||
|---|---|---|---|---|---|---|---|---|
| r2 | ∑(Resid)2/n−2 | r2 | ∑(Resid)2/n−2 | r2 | ∑(Resid)2/n−2 | r2 | ∑(Resid)2/n−2 | |
| F1 | 0.9763 | 120.4020 | 0.9896 | 107.7413 | 0.9813 | 83.0534 | 0.9907 | 334.9105 |
| F2 | 0.9785 | 154.6521 | 0.9979 | 15.0035 | 0.9936 | 21.9354 | 0.9962 | 74.4396 |
| F3 | 0.9805 | 95.1133 | 0.9955 | 34.5983 | 0.9893 | 39.0485 | 0.9950 | 159.5296 |
| F4 | 0.9848 | 45.9916 | 0.9780 | 43.8904 | 0.9665 | 66.5108 | 0.9821 | 154.6797 |
| F5 | 0.9772 | 311.5803 | 0.9967 | 24.6281 | 0.9931 | 25.8022 | 0.9964 | 55.7461 |
| F6 | 0.9731 | 131.8692 | 0.9937 | 46.0530 | 0.9856 | 55.7539 | 0.9911 | 184.4683 |
| F7 | 0.9891 | 28.7591 | 0.9932 | 20.2110 | 0.9870 | 33.2258 | 0.9941 | 101.2407 |
| Formulation | n | k | R2 |
|---|---|---|---|
| F1 | 1.0148 | 1.009 | 0.9836 |
| F2 | 1.0044 | 0.9829 | 0.9882 |
| F3 | 1.0281 | 0.9644 | 0.9899 |
| F4 | 1.0506 | 0.7744 | 0.986 |
| F5 | 0.9323 | 1.072 | 0.9911 |
| F6 | 1.0351 | 0.9713 | 0.9873 |
| F7 | 1.0404 | 0.8657 | 0.9957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adısanoğlu, P.; Özgüney, I. Development and Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations Containing Flurbiprofen Solid Dispersions. Gels 2024, 10, 267. https://doi.org/10.3390/gels10040267
Adısanoğlu P, Özgüney I. Development and Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations Containing Flurbiprofen Solid Dispersions. Gels. 2024; 10(4):267. https://doi.org/10.3390/gels10040267
Chicago/Turabian StyleAdısanoğlu, Pınar, and Işık Özgüney. 2024. "Development and Characterization of Thermosensitive and Bioadhesive Ophthalmic Formulations Containing Flurbiprofen Solid Dispersions" Gels 10, no. 4: 267. https://doi.org/10.3390/gels10040267






