Experimental Investigation of the Effects of Grooves in Fe2O4/Water Nanofluid Pool Boiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanofluid
2.2. The Boiling System
2.3. Boiling Surface
2.4. Relationships between Surface Temperature and Heat Flux
2.5. Uncertainty Analysis
3. Results
3.1. Accuracy of Results
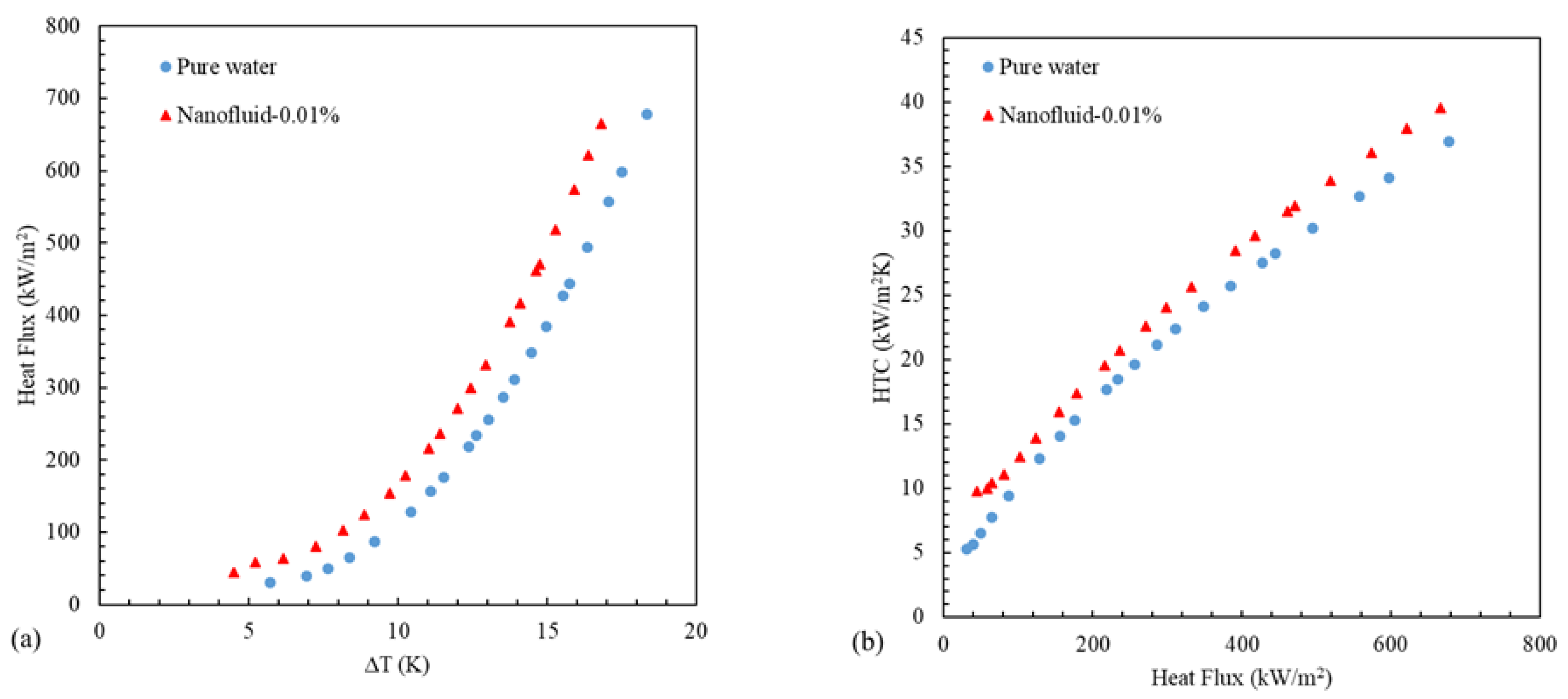
3.2. Ferrofluid Boiling of Iron Oxide/Water on the Surface of the Smooth Heater
3.3. Boiling Deionized Water on Grooved Surfaces
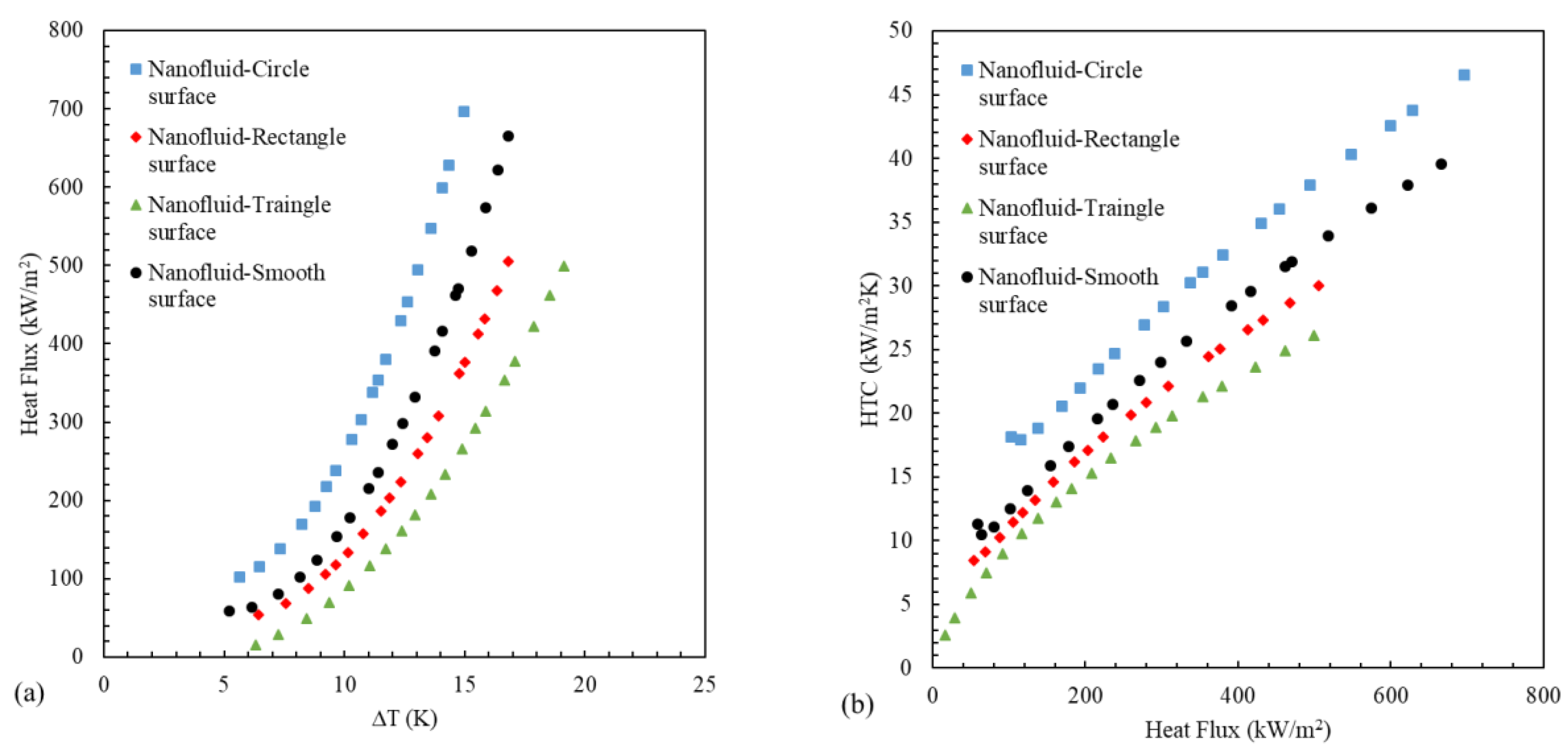
3.4. Boiling of Iron Oxide/Water Nanofluid on Grooved Surfaces
3.5. Effect of Groove Depth of Grooved Surfaces on Boiling of Water and Iron Oxide/Water Nanofluid
4. Conclusions
- In boiling deionized water on grooved surfaces, the surface with circular and rectangular grooves improves heat transfer, and the surface with triangular grooves reduces the heat transfer rate compared to the smooth surface. In a triangular groove, the thermal resistance increases due to the accumulation of bubbles in the center of the groove.
- The HTC in ferrofluid boiling increased on circular grooved surfaces and decreased on rectangular and triangular grooved surfaces due to the deposition of more nanoparticles in rectangular and triangular grooves than in circular ones due to the presence of groove corners.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Pressure correction coefficient | |
| Enthalpy () | |
| Heat transfer coefficient | |
| Current (A) | |
| Thermal conductivity () | |
| Pressure () | |
| Heat flux () | |
| Mean roughness () | |
| Temperature | |
| Uncertainty | |
| Voltage (v) | |
| Greek letters | |
| Viscosity () | |
| Density () | |
| Surface tension () | |
| Subscripts | |
| Liquid | |
| Saturation | |
| Vapor |
References
- Bergles, A.E. Enhancement of pool boiling. Int. J. Refrig. 1997, 20, 545–551. [Google Scholar] [CrossRef]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles. ASME-FED 1995, 231, 99–106. [Google Scholar]
- Kamel, M.S.; Lezsovits, F. Enhancement of pool boiling heat transfer performance using dilute cerium oxide/water nanofluid: An experimental investigation. Int. Commun. Heat Mass Transf. 2020, 114, 104587. [Google Scholar] [CrossRef]
- Tian, Z.; Etedali, S.; Afrand, M.; Abdollahi, A.; Goodarzi, M. Experimental study of the effect of various surfactants on surface sediment and pool boiling heat transfer coefficient of silica/DI water nano-fluid. Powder Technol. 2019, 356, 391–402. [Google Scholar] [CrossRef]
- Eskandari, E.; Alimoradi, H.; Pourbagian, M.; Shams, M. Numerical investigation and deep learning-based prediction of heat transfer characteristics and bubble dynamics of subcooled flow boiling in a vertical tube. Korean J. Chem. Eng. 2022, 39, 3227–3245. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Abed, A.M.; Al-Madhhachi, H.; Hadrawi, S.K.; Al-Khateeb HM, M.; Kianfar, E. RETRACTED ARTICLE: Graphene-Based Important Carbon Structures and Nanomaterials for Energy Storage Applications as Chemical Capacitors and Supercapacitor Electrodes: A Review. BioNanoScience 2023, 13, 219–248. [Google Scholar] [CrossRef]
- Roodbari, M.; Alimoradi, H.; Shams, M.; Aghanajafi, C. An experimental investigation of microstructure surface roughness on pool boiling characteristics of TiO2 nanofluid. J. Therm. Anal. Calorim. 2022, 147, 3283–3298. [Google Scholar] [CrossRef]
- Alimoradi, H.; Eskandari, E.; Pourbagian, M.; Shams, M. A parametric study of subcooled flow boiling of Al2O3/water nanofluid using numerical simulation and artificial neural networks. Nanoscale Microscale Thermophys. Eng. 2022, 26, 129–159. [Google Scholar] [CrossRef]
- Ahmad, S.; Takana, H.; Ali, K.; Akhtar, Y.; Hassan, A.M.; Ragab, A.E. Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach. Nanotechnol. Rev. 2023, 12, 20220561. [Google Scholar] [CrossRef]
- Chandra, R.; Kathiravan, R. Pool boiling characteristics of multiwalled carbon nanotube based nanofluids over a flat plate heater. Int. J. Heat Mass Transf. 2011, 54, 1289–1296. [Google Scholar]
- Hai, T.; Abidi, A.; Wang, L.; Abed, A.M.; Mahmoud, M.Z.; El Din, E.M.T.; Smaisim, G.F. Simulation of solar thermal panel systems with nanofluid flow and PCM for energy consumption management of buildings. J. Build. Eng. 2022, 58, 104981. [Google Scholar] [CrossRef]
- Shi, M.; Shuai, M.; Chen, Z. Study on pool boiling heat transfer of nanoparticle suspensions on plate surface. J. Enhanc. Heat Transf. 2007, 14, 223–231. [Google Scholar] [CrossRef]
- Shrivastav, N.; Kashyap, S.; Madan, J.; Al-Mousoi, A.K.; Mohammed, M.K.; Hossain, M.K.; Pandey, R.; Ramanujam, J. Perovskite-CIGS monolithic tandem solar cells with 29.7% efficiency: A numerical study. Energy Fuels 2023, 37, 3083–3090. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, K.; Katbar, N.M.; Akhtar, Y.; Cai, J.; Jamshed, W.; El Din, S.M.; Abd-Elmonem, A.; Abdalla, N.S.E. Vortex generation due to multiple localized magnetic fields in the hybrid nanofluid flow—A numerical investigation. Heliyon 2023, 9, e17756. [Google Scholar] [CrossRef] [PubMed]
- Norouzipour, A.; Abdollahi, A.; Afrand, M. Experimental study of the optimum size of silica nanoparticles on the pool boiling heat transfer coefficient of silicon oxide/deionized water nanofluid. Powder Technol. 2019, 345, 728–738. [Google Scholar] [CrossRef]
- Vassallo, P.; Kumar, R. Pool boiling heat transfer experiments in silica-water nanofluids. Int. J. Heat Mass Transf. 2004, 47, 407–411. [Google Scholar] [CrossRef]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Das, A.K.; Saha, P. Performance of different structured surfaces in nucleate pool boiling. Appl. Therm. Eng. 2009, 29, 3643–3653. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Enhancement in the pool boiling heat transfer of copper surface by applying electrophoretic deposited graphene oxide coatings. Int. J. Multiph. Flow 2023, 159, 104350. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M. Optimization of subcooled flow boiling in a vertical pipe by using artificial neural network and multi objective genetic algorithm. Appl. Therm. Eng. 2017, 111, 1039–1051. [Google Scholar] [CrossRef]
- Pastuszko, R.; Piasecka, M. Pool boiling on surfaces with mini-fins and micro-cavities. In Proceedings of the 6th European Thermal Sciences Conference, Poitiers, France, 4–7 September 2012. [Google Scholar]
- Dadjoo, M.; Etesami, N.; Esfahany, M.N. Influence of orientation and roughness of heater surface on critical heat flux and pool boiling heat transfer coefficient of nanofluid. Appl. Therm. Eng. 2017, 124, 353–361. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Bubble behavior and nucleation site density in subcooled flow boiling using a novel method for simulating the microstructure of surface roughness. Korean J. Chem. Eng. 2022, 39, 2945–2958. [Google Scholar] [CrossRef]
- Samawi, K.A.; Abdulrazzaq, S.J.; Zorah, M.; Al-Bahrani, M.; Mahmoud, H.M.; Abdulkareem-Alsultan, G.; Taki, A.G.; Nassar, M.F. MoS2/graphdiyne nanotube/MXene 3D-interconnected ternary aerogel: A high-performance electrocatalyst for hydrogen evolution reaction. J. Solid State Chem. 2024, 334, 124690. [Google Scholar] [CrossRef]
- Umesh, V.; Raja, B. A study on nucleate boiling heat transfer characteristics of pentane and CuO-pentane nanofluid on smooth and milled surfaces. Exp. Therm. Fluid Sci. 2015, 64, 23–29. [Google Scholar] [CrossRef]
- Narayan, P.; Baby, A.K. Survey on nucleate pool boiling of nanofluids: The effect of particle size relative to roughness. J. Nanoparticle Res. 2008, 10, 1099–1108. [Google Scholar]
- Abdollahi, A.; Salimpour, M.R.; Etesami, N. Experimental analysis of magnetic field effect on the pool boiling heat transfer of a ferrofluid. Appl. Therm. Eng. 2017, 111, 1101–1110. [Google Scholar] [CrossRef]
- Ahmad, J.; González-Lezcano, R.A.; Majdi, A.; Kahla, N.B.; Deifalla, A.F.; El-Shorbagy, M.A. Glass fibers reinforced concrete: Overview on mechanical, durability and microstructure analysis. Materials 2022, 15, 5111. [Google Scholar] [CrossRef]
- Abdollahi, A.; Salimpour, M.R.; Etesami, N. Experimental analysis of pool boiling heat transfer of ferrofluid on surfaces deposited with nanofluid. Modares Mech. Eng. 2016, 16, 19–30. [Google Scholar]
- Lee, J.H.; Lee, T.; Jeong, Y.H. Experimental study on the pool boiling CHF enhancement using magnetite-water nanofluids. Int. J. Heat Mass Transf. 2012, 55, 2656–2663. [Google Scholar] [CrossRef]
- Taleghani, M.H.; Khodadadi, S.; Maddahian, R.; Mokhtari-Dizaji, M. Enhancing the bubble collapse energy using the electrohydrodynamic force. Phys. Fluids 2023, 35, 053316. [Google Scholar] [CrossRef]
- Berger, P.; Adelman, N.B.; Beckman, K.J. Preparation and properties of an aqueous ferrofluid. J. Chem. Educ. 1999, 76, 943–948. [Google Scholar] [CrossRef]
- Jamed, M.J.; Alhathal Alanezi, A.; Alsalhy, Q.F. Effects of embedding functionalized multi-walled carbon nanotubes and alumina on the direct contact poly(vinylidene fluoride-co-hexafluoropropylene) membrane distillation performance. Chem. Eng. Commun. 2019, 206, 1035–1057. [Google Scholar] [CrossRef]
- Abdulkadhim, A.; Hamzah, H.K.; Ali, F.H.; Abed, A.M.; Abed, I.M. Natural convection among inner corrugated cylinders inside wavy enclosure filled with nanofluid superposed in porous–nanofluid layers. Int. Commun. Heat Mass Transf. 2019, 109, 104350. [Google Scholar] [CrossRef]
- Abdulkadhim, A.; Hamzah, H.K.; Ali, F.H.; Yıldız, Ç.; Abed, A.M.; Abed, E.M.; Arıcı, M. Effect of heat generation and heat absorption on natural convection of Cu-water nanofluid in a wavy enclosure under magnetic field. Int. Commun. Heat Mass Transf. 2021, 120, 105024. [Google Scholar] [CrossRef]
- Wang, H.; Habibi, M.; Marzouki, R.; Majdi, A.; Shariati, M.; Denic, N.; Ebid, A.A.K. Improving the self-healing of cementitious materials with a hydrogel system. Gels 2022, 8, 278. [Google Scholar] [CrossRef]
- Majdi, H.S.; Shubbar, A.A.; Nasr, M.S.; Al-Khafaji, Z.S.; Jafer, H.; Abdulredha, M.; Al Masoodi, Z.; Sadique, M.; Hashim, K. Experimental data on compressive strength and ultrasonic pulse velocity properties of sustainable mortar made with high content of GGBFS and CKD combinations. Data Brief 2020, 31, 105961. [Google Scholar] [CrossRef]
- Shahnazari, M.R.; Esfandiar, M. Capillary Effects on Surface Enhancement in a Non-Homogeneous Fibrous Porous Medium. Mech. Adv. Compos. Struct. 2018, 5, 83–90. [Google Scholar]
- Wu, S.; Zhu, D.; Li, X. Thermal energy storage behavior of Al2O3-H2O nanofluids. Thermochim. Acta 2009, 483, 73–77. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Jabir, M.S.; Abdulzahraa, H.G.; Mohammed, S.H.; Al-Azzawi, W.K.; Ahmed, D.S.; Singh, S.; Kumar, A.; Asaithambi, S.; Shekargoftar, M. Introduction of cadmium chloride additive to improve the performance and stability of perovskite solar cells. RSC Adv. 2022, 12, 20461–20470. [Google Scholar] [CrossRef]
- Pandey, R.; Bhattarai, S.; Sharma, K.; Madan, J.; Al-Mousoi, A.K.; Mohammed, M.K.A.; Hossain, M.K. Halide composition engineered a non-toxic Perovskite-silicon tandem solar cell with 30.7% conversion efficiency. ACS Appl. Electron. Mater. 2022, 5, 5303–5315. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Al-Mousoi, A.K.; Singh, S.; Younis, U.; Kumar, A.; Dastan, D.; Ravi, G. Ionic liquid passivator for mesoporous titanium dioxide electron transport layer to enhance the efficiency and stability of hole conductor-free perovskite solar cells. Energy Fuels 2022, 36, 12192–12200. [Google Scholar] [CrossRef]
- Alsultani, M.J.; Abed, H.H.; Ghazi, R.A.; Mohammed, M.A. Electrical Characterization of Thin Films (TiO2:ZnO)1−x (GO)x/FTO Heterojunction Prepared by Spray Pyrolysis Technique. J. Phys. Conf. Ser. 2020, 1591, 012002. [Google Scholar] [CrossRef]
- Esfandiar, M.; Nikan, F.; Shahnazari, M.R. Catalytic pyrolysis of coal particles in a fluidized bed: Experiments and modeling. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1478–1483. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Sadique, M.; Nasr, M.S.; Al-Khafaji, Z.S.; Hashim, K.S. The impact of grinding time on properties of cement mortar incorporated high volume waste paper sludge ash. Karbala Int. J. Mod. Sci. 2020, 6, 7. [Google Scholar] [CrossRef]
- Al-Aaraji NA, H.; Hashim, A.; Hadi, A.; Abduljalil, H.M. Synthesis and enhanced optical characteristics of silicon carbide/copper oxide nanostructures doped transparent polymer for optics and photonics nanodevices. Silicon 2022, 14, 10037–10044. [Google Scholar] [CrossRef]
- Khodadadi, S.; Taleghani, M.H.; Ganji, D.D.; Gorji-Bandpy, M. Heat transfer enhancement via bubble dynamics along an inclined wall. Int. Commun. Heat Mass Transf. 2023, 145, 106829. [Google Scholar] [CrossRef]
- Holman, J.P. Experimental Methods for Engineers, 8th ed.; McGraw-Hill: New York, NY, USA, 2012; pp. 63–72. [Google Scholar]
- Wen, D.; Ding, Y. Experimental investigation into the pool boiling heat transfer of aqueous based Y-alumina nanofluids. J. Nanoparticle Res. 2005, 7, 265–274. [Google Scholar] [CrossRef]
- Zhang, G.; Ali, Z.H.; Aldlemy, M.S.; Mussa, M.H.; Salih, S.Q.; Hameed, M.M.; Al-Khafaji, Z.S.; Yaseen, Z.M. Reinforced concrete deep beam shear strength capacity modelling using an integrative bio-inspired algorithm with an artificial intelligence model. Eng. Comput. 2022, 38 (Suppl. S1), 15–28. [Google Scholar] [CrossRef]
- Hu, X.; Derakhshanfard, A.H.; Patra, I.; Khalid, I.; Jalil, A.T.; Opulencia, M.J.C.; Dehkordi, R.B.; Toghraie, D.; Hekmatifar, M.; Sabetvand, R. The microchannel type effects on water-Fe3O4 nanofluid atomic behavior: Molecular dynamics approach. J. Taiwan Inst. Chem. Eng. 2022, 135, 104396. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.P.; Cundy, A.B.; Tsioulou, O.T.; Alrekabi, S. Flexural performance of reinforced concrete beams strengthened with fibre reinforced geopolymer concrete under accelerated corrosion. In Structures; Elsevier: Amsterdam, The Netherlands, 2019; Volume 19, pp. 394–410. [Google Scholar]
- Dikshit, M.K.; Singh, S.; Pathak, V.K.; Saxena, K.K.; Agrawal, M.K.; Malik, V.; Salem, K.H.; Khan, M.I. Surface characteristics optimization of biocompatible Ti6Al4V with RCCD and NSGA II using die sinking EDM. J. Mater. Res. Technol. 2023, 24, 223–235. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Talebizadehsardari, P.; Ghalambaz, M.; Majdi, H.S.; Yaïci, W.; Giddings, D. Simultaneous and consecutive charging and discharging of a PCM-based domestic air heater with metal foam. Appl. Therm. Eng. 2021, 197, 117408. [Google Scholar] [CrossRef]
- Abdulraheem, F.S.; Al-Khafaji, Z.S.; Hashim, K.S.; Muradov, M.; Kot, P.; Shubbar, A.A. Natural filtration unit for removal of heavy metals from water. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 888, p. 012034. [Google Scholar]
- Abed, A.M.; Sopian, K.; Mohammed, H.A.; Alghoul, M.A.; Ruslan, M.H.; Mat, S.; Al-Shamani, A.N. Enhance heat transfer in the channel with V-shaped wavy lower plate using liquid nanofluids. Case Stud. Therm. Eng. 2015, 5, 13–23. [Google Scholar] [CrossRef]
- Parsaee, F.; Fayzullaev, N.; Nassar, M.F.; Abd Alreda, B.; Mahmoud, H.M.; Taki, A.G.; Faraji, M. Co-Fe dual-atom isolated in N-doped graphydine as an efficient sulfur conversion catalyst in Li-S batteries. J. Alloys Compd. 2024, 988, 174136. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Abdullah, A.R.; Hasan, W.K.; Habeeb, L.J.; Nassar, M.F. Two-Way Fluid-Structure Interaction Study of Twisted Tape Insert in a Circular Tube Having Integral Fins with Nanofluid. East.-Eur. J. Enterp. Technol. 2021, 3, 111. [Google Scholar] [CrossRef]
- Mourad, A.; Aissa, A.; Abed, A.M.; Smaisim, G.F.; Toghraie, D.; Fazilati, M.A.; Younis, O.; Guedri, K.; Alizadeh, A.A. The numerical analysis of the melting process in a modified shell-and-tube phase change material heat storage system. J. Energy Storage 2022, 55, 105827. [Google Scholar] [CrossRef]
- Sangeetha, A.; Shanmugan, S.; Alrubaie, A.J.; Jaber, M.M.; Panchal, H.; Attia, M.E.H.; Elsheikh, A.H.; Mevada, D.; Essa, F.A. A review on PCM and nanofluid for various productivity enhancement methods for double slope solar still: Future challenge and current water issues. Desalination 2023, 551, 116367. [Google Scholar] [CrossRef]
- Al-Farhany, K.; Abdulkadhim, A.; Hamzah, H.K.; Ali, F.H.; Chamkha, A. MHD effects on natural convection in a U-shaped enclosure filled with nanofluid-saturated porous media with two baffles. Prog. Nucl. Energy 2022, 145, 104136. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.k.; Ali, B.M.; Zorah, M.; Al-Musawi, T.J. Experimental Investigation of the Effects of Grooves in Fe2O4/Water Nanofluid Pool Boiling. Fluids 2024, 9, 110. https://doi.org/10.3390/fluids9050110
Rashid Mk, Ali BM, Zorah M, Al-Musawi TJ. Experimental Investigation of the Effects of Grooves in Fe2O4/Water Nanofluid Pool Boiling. Fluids. 2024; 9(5):110. https://doi.org/10.3390/fluids9050110
Chicago/Turabian StyleRashid, Marwa khaleel, Bashar Mahmood Ali, Mohammed Zorah, and Tariq J. Al-Musawi. 2024. "Experimental Investigation of the Effects of Grooves in Fe2O4/Water Nanofluid Pool Boiling" Fluids 9, no. 5: 110. https://doi.org/10.3390/fluids9050110






