A Review of Emerging Technologies for the Extraction of Bioactive Compounds from Berries (Phalsa Berries)
Abstract
:1. Introduction
2. Various Bioactive Compounds in Berries
2.1. Flavonoids
2.2. Phenols
2.3. Anthocyanins
2.4. Antioxidant
3. Effect of Solvent on Extraction Techniques
4. Characterization of Extracted Bioactive Compounds
4.1. SEM (Scanning Electron Microscopy)
4.2. XRD (X-ray Diffraction)
4.3. FTIR (Fourier Transform Infrared Spectroscopy)
4.4. NMR (Nuclear Magnetic Resonance)
5. Different Non-Thermal Methods Used for Extraction of Bioactive Compounds
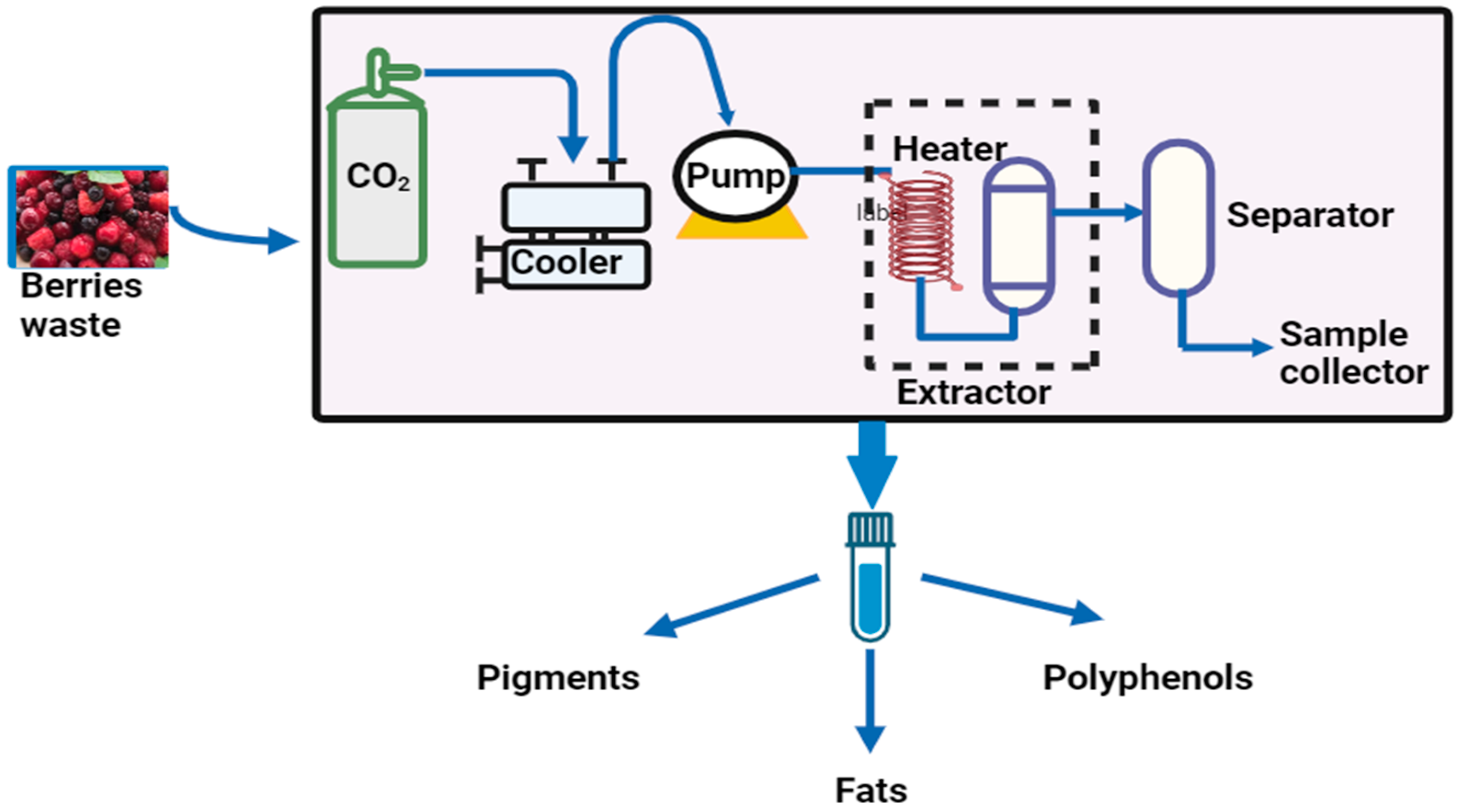
5.1. Supercritical CO2 Extraction
5.2. Microwave-Assisted Extraction (MAE)
5.3. Ultrasound-Assisted Extraction (UAE)
5.4. Pulsed Electric Field (PEF)
5.5. Pressurized Liquid Extraction (PLE)
6. Future Scope and Limitations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, B.; Bajpai, V. Traditional uses, phytochemistry, quality control and biological activities of genus Grewia. Phytomed. Plus 2022, 2, 100290. [Google Scholar] [CrossRef]
- Khan, R.S.; Asghar, W.; Khalid, N.; Nazir, W.; Farooq, M.; Ahmed, I.; Syed, Q.A. Phalsa (Grewia asiatica L.) fruit berry a promising functional food ingredient: A comprehensive review. J. Berry Res. 2019, 9, 179–193. [Google Scholar] [CrossRef]
- Sabtain, B.; Farooq, R.; Shafique, B.; Modassar, M.; Ranjha, A.N. A narrative review on the phytochemistry, nutritional profile and properties of prickly pear fruit. Open Access J. Biog. Sci. Res. 2021, 7. [Google Scholar] [CrossRef]
- Dev, R.; Sharma, G.K.; Singh, T.; Dayal, D.; Sureshkumar, M. Distribution of Grewia species in Kachchh Gujarat, India: Taxonomy, traditional knowledge and economic potentialities. Int. J. Pure Appl. Biosci. 2017, 5, 567–574. [Google Scholar]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernandez, M.D.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Kaur, S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Shaikh, A.M.; Harsányi, E.; Kovács, B. Phytochemical and pharmacological characteristics of phalsa (Grewia asiatica L.): A comprehensive review. Heliyon 2024, 10, e25046. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Ishaq, M.; Usman, M.; Zhao, L.; Ullah, A.; Wang, C. Nutraceutical perspectives and value addition of phalsa (Grewia asiatica L.): A Review. J. Food Biochem. 2020, 44, e13228. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins-a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Vatai, T.; Škerget, M.; Knez, Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Zhou, S.-H.; Fang, Z.-X.; Lü, Y.; Chen, J.-C.; Liu, D.-H.; Ye, X.-Q. Phenolics and antioxidant properties of bayberry (Myrica rubra Sieb. et Zucc.) pomace. Food Chem. 2009, 112, 394–399. [Google Scholar] [CrossRef]
- Wani, T.A.; Pandith, S.A.; Rana, S.; Bhat, W.W.; Dhar, N.; Razdan, S.; Chandra, S.; Kitchlu, S.; Sharma, N.; Lattoo, S.K. Promiscuous breeding behaviour in relation to reproductive success in Grewia asiatica L. (Malvaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 211, 62–71. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Rehman, R.; Salman, M.; Dar, A.; Anzano, J.M.; Ashraf, U.; Ashraf, S. Microwave chemistry: Effect of ions on dielectric heating in microwave ovens. Arab. J. Chem. 2015, 8, 100–104. [Google Scholar] [CrossRef]
- Ullah, F.; Bano, A.; Nosheen, A. Effects of plant growth regulators on growth and oil quality of canola (Brassica napus L.) under drought stress. Pak. J. Bot. 2012, 44, 1873–1880. [Google Scholar]
- Tiwari, D.K.; Singh, D.; Barman, K.; Patel, V.B. Bioactive compounds and processed products of phalsa (Grewia subinaequalis L.) Fruit. Pop. Kheti 2014, 2, 128–132. [Google Scholar]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Komartin, R.S.; Stroescu, M.; Chira, N.; Stan, R.; Stoica-Guzun, A. Optimization of oil extraction from Lallemantia iberica seeds using ultrasound-assisted extraction. J. Food Meas. Charact. 2021, 15, 2010–2020. [Google Scholar] [CrossRef]
- Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-assisted extraction of blackberry seed oil: Optimization and oil characterization. Molecules 2023, 28, 2486. [Google Scholar] [CrossRef]
- Dave, R.; Rao, T.V.R.; Nandane, A.S. RSM-based optimization of edible-coating formulations for preserving post-harvest quality and enhancing storability of phalsa (Grewia asiatica L.). J. Food Process. Preserv. 2016, 40, 509–520. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, S.; Amarowicz, R.; De Feo, V. Antioxidant activity of the extracts of some cowpea (Vigna unguiculata (L) Walp.) cultivars commonly consumed in Pakistan. Molecules 2013, 18, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Sinha, J.; Purwar, S.; Chuhan, S.K.; Rai, G. Nutritional and medicinal potential of Grewia subinaequalis DC. (syn. G. asiatica.) (Phalsa). J. Med. Plants Res. 2015, 9, 594–612. [Google Scholar]
- Goyal, P.K. Phytochemical and pharmacological properties of the genus Grewia: A review. Int. J. Pharm. Pharmaceut. Sci. 2012, 4, 72–78. [Google Scholar] [CrossRef]
- Chirumbolo, S. Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: Really a promising path? J. Sci. Food Agric. 2012, 92, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.; Chin, N.; Yusof, Y.; Talib, R.; Law, C. Optimization of total monomeric anthocyanin (TMA) and total phenolic content (TPC) extractions from mangosteen (Garcinia mangostana Linn.) hull using ultrasonic treatments. Ind. Crops Prod. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Gamlath, C.J.; Martin, G.J.; Hemar, Y.; Ashokkumar, M. Effect of sonication, microwaves and high-pressure processing on ACE-inhibitory activity and antioxidant potential of Cheddar cheese during ripening. Ultrason. Sonochem. 2020, 67, 105140. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mejia, L.R.; Cruz, C.; Berrone, P.; De Castro, J. The bind that ties: Socioemotional wealth preservation in family firms. Acad. Manag. Ann. 2011, 5, 653–707. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Lin, Y.; Nasiru, M.M.; Zhang, J.; Abid, M.; Murtaza, M.A.; Zhao, L. Comparative study: Thermal and non-thermal treatment on enzyme deactivation and selected quality attributes of fresh carrot juice. Int. J. Food Sci. Technol. 2022, 57, 827–841. [Google Scholar] [CrossRef]
- Nadeem, M.; Ubaid, N.; Qureshi, T.M.; Munir, M.; Mehmood, A. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochem. 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Villamiel, M.; de Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V.; Yu, L.J. Emerging preservation methods for fruit juices and beverages. Food Addit. 2012, 22, 65–82. [Google Scholar]
- Lei, Z.; Sumner, B.W.; Bhatia, A.; Sarma, S.J.; Sumner, L.W. UHPLC-MS Analyses of Plant Flavonoids. Curr. Protoc. Plant Biol. 2019, 4, e20085. [Google Scholar] [CrossRef] [PubMed]
- Delage, B. “Flavonoids”. Linus Pauling Institute, Oregon State University, Corvallis, Oregon. 2015. Retrieved 2021-01-26.
- Sharma, N.; Patni, V. In vivo and in vitro qualitative phytochemical screening of Grewia species. Int. J. Biol. Pharm. Res. 2013, 4, 634–639. [Google Scholar]
- Gupta, P.; Bhatnagar, I.; Kim, S.-K.; Verma, A.K.; Sharma, A. In-vitro cancer cell cytotoxicity and alpha amylase inhibition effect of seven tropical fruit residues. Asian Pac. J. Trop. Biomed. 2014, 4, S665–S671. [Google Scholar] [CrossRef]
- Khatune, N.A.; Rahman, B.M.; Barman, R.K.; Wahed, M.I.I. Antidiabetic, antihyperlipidemic and antioxidant properties of ethanol extract of Grewia asiatica Linn. bark in alloxan-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Mitra, A.; Datta, D.; Saha, A.; Hazra, J. Evaluation of antipyretic and analgesic activity of Parusaka (Grewia asiatica Linn.): An indigenous Indian plant. Int. J. Res. Ayurveda Pharm. 2012, 3, 519–523. [Google Scholar]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, I.U.; Sherin, L.; Ashfaq, M. Evaulation of antioxidant activity of Grewia asiatica berry using 2, 2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) and N, N-dimethyl-p-phenylenediamine radical cations decolourization assays. Asian J. Chem. 2008, 20, 5123. [Google Scholar]
- Talpur, M.K.; Talpur, F.N.; Balouch, A.; Nizamani, S.M.; Surhio, M.A.; Shah, M.R. Analysis and characterization of anthocyanin from phalsa (Grewia asiatica). MOJ Food Process. Technol. 2017, 5, 299–305. [Google Scholar] [CrossRef]
- Abou Zeid, A.H.; Mohammed, R.S.; Sleem, A.A. Biologically Active Polysaccharides from Grewia asiatica Linn. Leaves. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1080–1087. [Google Scholar]
- Akhtar, N.; Haq, I.U.; Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Islary, A.; Sarmah, J.; Basumatary, S. Proximate composition, mineral content, phytochemical analysis and in vitro antioxidant activities of a wild edible fruit (Grewia sapida Roxb. ex DC.) found in Assam of North-East India. J. Investig. Biochem. 2016, 5, 21. [Google Scholar] [CrossRef]
- Vyas, P.B.; Tadapaneni, V.R.R.; Thakkar, V.R. Chemical elicitors improve the shelf life of phalsa (Grewia asiatica L.) by inducing antioxidants and controlling microbes. Fruits 2016, 71, 307–317. [Google Scholar] [CrossRef]
- Hidalgo, G.-I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.; Naz, S.; Ahmad, S.; Sayeed, S.A. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int. J. Food Sci. Technol. 2011, 46, 250–256. [Google Scholar] [CrossRef]
- Salamone, F.; Volti, G.L.; Titta, L.; Puzzo, L.; Barbagallo, I.; La Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. WJG 2012, 18, 3862. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.V.; Sisodia, R. Evaluation of the free radical scavenging activity and radioprotective efficacy of Grewia asiatica fruit. J. Radiol. Prot. 2009, 29, 429. [Google Scholar] [CrossRef]
- Shukla, R. Estimation of phytochemicals and in vitro antioxidant activity of different solvent extracts of Grewia asiatica fruit. Research and Reviews. J. Bot. Sci. 2016, hal-03634814. [Google Scholar]
- Srivastava, J.; Kumar, S.; Vankar, P.S. Correlation of antioxidant activity and phytochemical profile in native plants. Nutr. Food Sci. 2012, 42, 71–79. [Google Scholar] [CrossRef]
- Tiwari, B.; O’Donnell, C.; Cullen, P. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng. 2009, 93, 166–171. [Google Scholar] [CrossRef]
- Sharma, C.; Malgaonkar, M.; Sangvikar, S.G.; Murthy, S.N.; Pawar, S.D. In vitro evaluation of antimicrobial and antioxidant profile of Grewia L. root extracts. J. Appl. Life Sci. Int. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of antioxidant activity of garden blackberries (Rubus fruticosus L.) extracts obtained with different extraction solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Cravino, J.A.; Manwaring, C.W.; Stathakis, J.G.; Shalliker, R.A. Extracting antioxidants from blueberries. J. Liq. Chromatogr. Relat. Technol. 2023, 46, 225–237. [Google Scholar] [CrossRef]
- Qamar, M.; Akhtar, S.; Barnard, R.T.; Sestili, P.; Ziora, Z.M.; Lazarte, C.E.; Ismail, T. Antiinflammatory and anticancer properties of Grewia asiatica crude extracts and fractions: A bioassay-guided approach. BioMed Res. Int. 2022, 2022, 2277417. [Google Scholar] [CrossRef]
- Pinelo, M.R.M.; Sineiro, J.; Nunez, M.J. Extraction of antioxidant Pomace–characterization by 13C and 1H NMR spectroscopy. Annmagn. Reson. 2004, 4, 56–63. [Google Scholar]
- Rubilar, M.; Pinelo, M.; Franco, D.; Sineiro, J.; Núnez, M.J. Agroindustrial residues as a source of antioxidants. Afinidad 2003, 60, 153–160. [Google Scholar]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J. Sci. Food Agric. 2003, 83, 496–502. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; García, O.P.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res. Int. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Baran, M.F. Green synthesis of silver nanoparticles (Agnps) using Pistacia terebinthus leaf extract: Antimicrobial effect characterization. Int. J. Math. Eng. Nat. Sci. 2018, 2602, 67–75. [Google Scholar]
- Barbi, R.C.T.; Hornung, P.S.; Ávila, S.; Alves, F.E.D.S.B.; Beta, T.; Ribani, R.H. Ripe and unripe inajá (Maximilia maripa) fruit: A new high source of added value bioactive compounds. Food Chem. 2020, 331, 127333. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Mohanty, S.K.; Sinniah, U.R. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Neiva Correia, M.J.; Mateus, M.M.; Saraiva, J.A.; Vicente, A.A.; Moldão, M. Fourier transform infrared (FT-IR) spectroscopy as a possible rapid tool to evaluate abiotic stress effects on pineapple by-products. Appl. Sci. 2019, 9, 4141. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Characteristics and properties of hydroxypropylated rice starch based biodegradable films. Food Hydrocoll. 2015, 50, 54–64. [Google Scholar] [CrossRef]
- Todasca, M.C.; Fotescu, L.; Chira, N.A.; Deleanu, C.; Rosca, S. Composition changes in wines produced by different growing techniques examined through 1H-NMR spectroscopy. Rev. Chim. 2011, 62, 131–134. [Google Scholar]
- Beveridge, T.H.; Girard, B.; Kopp, T.; Drover, J.C. Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: Varietal effects. J. Agric. Food Chem. 2005, 53, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D. Supercritical carbon dioxide technology: A promising technique for the non-thermal processing of freshly fruit and vegetable juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Junaid, P.M.; Dar, A.H.; Dar, I.H.; Khan, S.A.; Manzoor, A.; Ganaie, T.A.; Shams, R. Extraction of antioxidants from agro-industrial waste. In Extraction of Natural Products from Agro-Industrial Wastes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 143–156. [Google Scholar]
- Leusink, G.J.; Kitts, D.D.; Yaghmaee, P.; Durance, T. Retention of antioxidant capacity of vacuum microwave dried cranberry. J. Food Sci. 2010, 75, C311–C316. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Toepfl, S.; Siemer, C.; Heinz, V. Effect of high-intensity electric field pulses on solid foods. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 147–154. [Google Scholar]
- Li, X.; Chen, F.; Li, S.; Jia, J.; Gu, H.; Yang, L. An efficient homogenate-microwave-assisted extraction of flavonols and anthocyanins from blackcurrant marc: Optimization using combination of Plackett-Burman design and Box-Behnken design. Ind. Crops Prod. 2016, 94, 834–847. [Google Scholar] [CrossRef]
- Altuntas, J.; Evrendilek, G.A.; Sangun, M.K.; Zhang, H.Q. Effects of pulsed electric field processing on the quality and microbial inactivation of sour cherry juice. Int. J. Food Sci. Technol. 2010, 45, 899–905. [Google Scholar] [CrossRef]
- Tripodo, G.; Ibáñez, E.; Cifuentes, A.; Gilbert-López, B.; Fanali, C. Optimization of pressurized liquid extraction by response surface methodology of Goji berry (Lycium barbarum L.) phenolic bioactive compounds. Electrophoresis 2018, 39, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.A.; Sachin, B.S.; Wakte, P.S.; Shinde, D.B. Optimization of supercritical fluid extraction and HPLC identification of wedelolactone from Wedelia calendulacea by orthogonal array design. J. Adv. Res. 2014, 5, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kagliwal, L.D.; Pol, A.S.; Patil, S.C.; Singhal, R.S.; Patravale, V.B. Antioxidant-rich extract from dehydrated seabuckthorn berries by supercritical carbon dioxide extraction. Food Bioprocess Technol. 2012, 5, 2768–2776. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction methods of citrus peel phenolic compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Jäger, H.; Meneses, N.; Esteve, M.J.; Frígola, A.; Knorr, D. Evaluation of quality changes of blueberry juice during refrigerated storage after high-pressure and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2012, 14, 18–24. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M. Color characteristics of carrots: Effect of drying and rehydration. Int. J. Food Prop. 2012, 15, 450–466. [Google Scholar] [CrossRef]
- Varray, F.; Cachard, C.; Kybic, J.; Novell, A.; Bouakaz, A.; Basset, O. A multi-frequency approach to increase the native resolution of ultrasound images. In Proceedings of the 20th European Signal Processing Conference (EUSIPCO), Bucharest, Romania, 27–31 August 2012. [Google Scholar]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Ind. Crops Prod. 2014, 53, 274–281. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef]
- Yang, N.; Huang, K.; Lyu, C.; Wang, J. Pulsed electric field technology in the manufacturing processes of wine, beer, and rice wine: A review. Food Control 2016, 61, 28–38. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]


| S. No. | Search Term | Descriptor |
|---|---|---|
| 1. | Berries | “Berries” OR “Phalsa” OR “Fruits” |
| 2. | Bioactive compounds | “Phenol” OR “Flavonoid” OR “Antioxidant” OR “Nutraceuticals” |
| 3. | Extraction | “Thermal Processing” OR “Non-thermal Processing” |
| 4. | Combination | 1 AND 2 AND 3 |
| Assays | Fruit | Leaves | Root | Reference |
|---|---|---|---|---|
| Flavonoid (mg QE/g DW) | 116.95 | 9.90 | 16.37 | [34] |
| Phenols (mg GAE/g DW) | 294.353 | 30.20 | 11.00 | [39,40] |
| Anthocyanin (μg/g) | 1193.8 | - | - | [41] |
| Antioxidant IC50 (μg/mL) | 4.88 | 23.9 | - | [42] |
| DPPH IC50 (μg/mL) | 257.66 | 16.3 | 82.5 | [43] |
| FRAP IC50 (μg/mL) | 4.14 | 18.3 | 82.53 | [43,44] |
| ABTS IC50 (μg/mL) | 134.22 | - | 96.41 | [45] |
| Characterization Techniques | Key Findings | References |
|---|---|---|
| SEM | The morphological structure of nanoparticles at different resolutions at 0.5, 1, and 5 μm scales was observed in berry extract prepared using powder under different concentrations and temperatures. | [68] |
| XRD | Crystallinity of fiber in raw and defatted powder under different temperatures, conditions, and concentrations shows a broad peak at an angle of 18°. | [69] |
| FTIR | FTIR was used for identifying the functional groups in different spectra of 4000–400 cm−1 with a resolution of 2 cm−1. The starch film in berries created a hydrogen bond and was displayed under an absorption band. | [70] |
| NMR | NMR revealed the proton environment and produced a graph of the correlation between intensity and absorption frequency. Different acids were observed in fruit berries (caffeic acid, kaempferol, 5-hydroxyferuluc acid, 3,4,5-trihydroxycinnamic). | [71] |
| Non-Thermal Techniques | Bioactive Component Techniques | Mode | Advantages | Reference |
|---|---|---|---|---|
| Supercritical CO2 extraction | Extraction of phenolic compounds and fatty acid from berries using CO2 as a solvent and ethanol as a co-solvent | The extraction process produces value-added products with low critical temperature and pressure (31.1 °C, 7.39 MPa). Volatile secondary metabolites are used to extract polyphenol compounds. | Environmentally safe Inexpensive Low toxicity Low polarity | [75,76] |
| Microwave assisted extraction | Polysaccharides, methoxyl group of pectin, extraction of polyphenolic compounds using acetone and ethanol as solvents. | It employs electromagnetic waves with frequencies between 300 MHz and 300 GHz with wavelengths of 1 cm to 1 m to dehydrate berries with strong antioxidant capacity. | Homogenous Less solvent consumed Improved extraction yield | [77,78,79] |
| Ultrasound-assisted extraction | Extraction of polysaccharides and phenolic compounds from fruit peel using acetone and ethanol as solvents by the UAE method | The ability to extract solvents using ultrasonic waves (20–100 kHz) is considered a rapid process for the extraction, crystallization, emulsification, homogenization, and enzyme inactivation of berries with higher phenolic and sugar levels | Improved efficiency Versatile Environmentally friendly Increased extraction yield | [80,81] |
| Pulsed electric field | Phenolic compounds and antioxidants extracted using aqueous extract from fruit parts | It involves two electrodes, and an electric field is applied in a pulsed manner with pulse amplitudes ranging from 10 to 80 kV/cm. PEF treatments resulted in an increase in anthocyanin content due to enhanced extraction, improved quality and microbial inactivation in sour cherry juice. | Less energy required Non-toxic Less solvent required Improved productivity Environmentally friendly | [82,83,84] |
| Pressurized liquid extraction | Polyphenolic compounds extracted from berries using ethanol and water a s solvent | To remove the biological components, high pressure (3.3–20.3 MPa) and high temperature (40 °C–200 °C) requires low quantity of solvent. | High yield Less solvent consumed Reliable | [77,85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shams, R.; Kaur, S.; Dash, K.K.; Czipa, N.; Kovács, B.; Shaikh, A.M. A Review of Emerging Technologies for the Extraction of Bioactive Compounds from Berries (Phalsa Berries). Horticulturae 2024, 10, 455. https://doi.org/10.3390/horticulturae10050455
Shams R, Kaur S, Dash KK, Czipa N, Kovács B, Shaikh AM. A Review of Emerging Technologies for the Extraction of Bioactive Compounds from Berries (Phalsa Berries). Horticulturae. 2024; 10(5):455. https://doi.org/10.3390/horticulturae10050455
Chicago/Turabian StyleShams, Rafeeya, Simrat Kaur, Kshirod Kumar Dash, Nikolett Czipa, Béla Kovács, and Ayaz Mukarram Shaikh. 2024. "A Review of Emerging Technologies for the Extraction of Bioactive Compounds from Berries (Phalsa Berries)" Horticulturae 10, no. 5: 455. https://doi.org/10.3390/horticulturae10050455








