Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems
Abstract
:1. Introduction
2. Materials and Methods
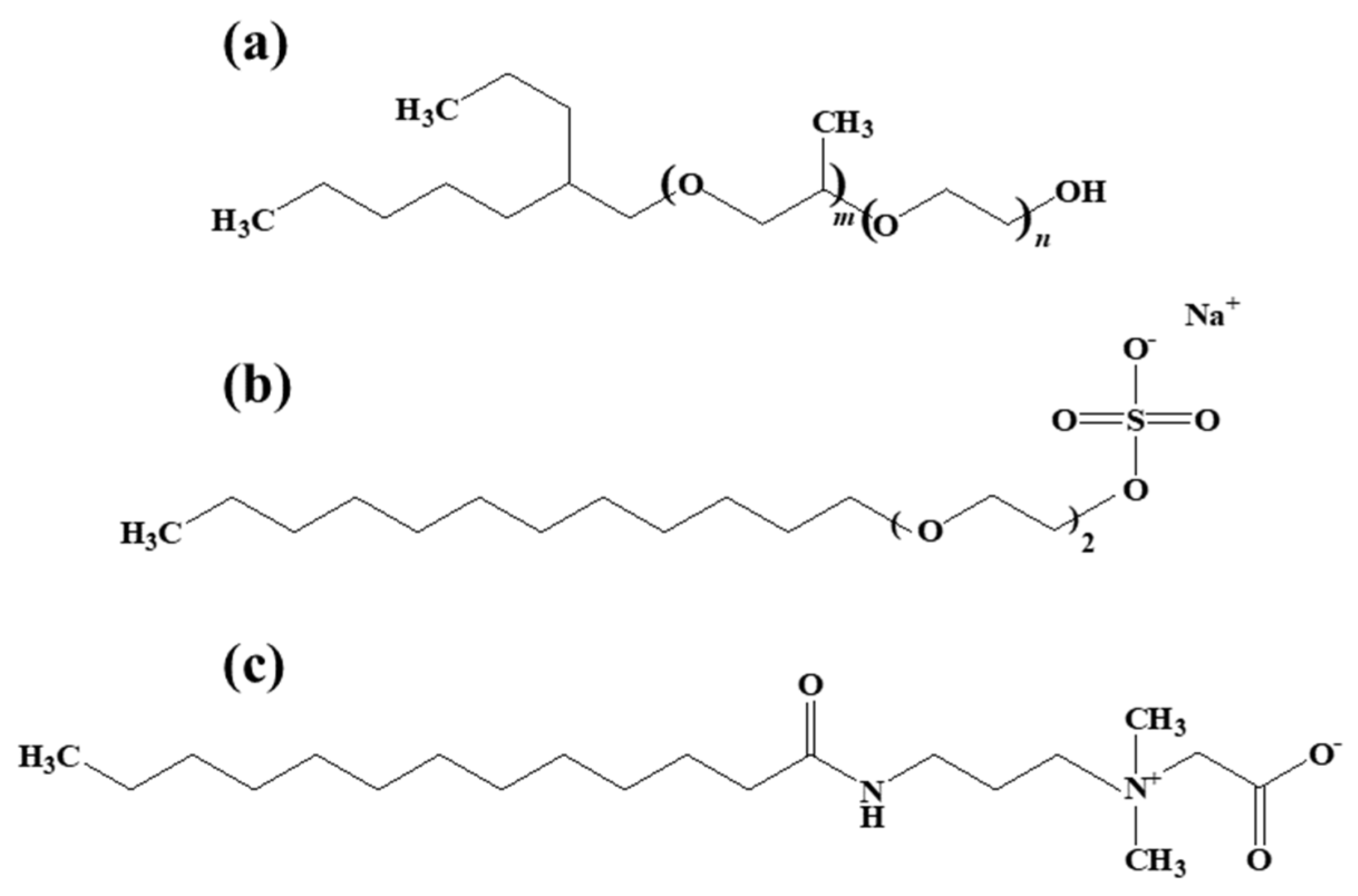
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Foaming Capacity Measurement
2.2.3. Surface Tension Measurement
2.2.4. Fat Solubilization Capacity
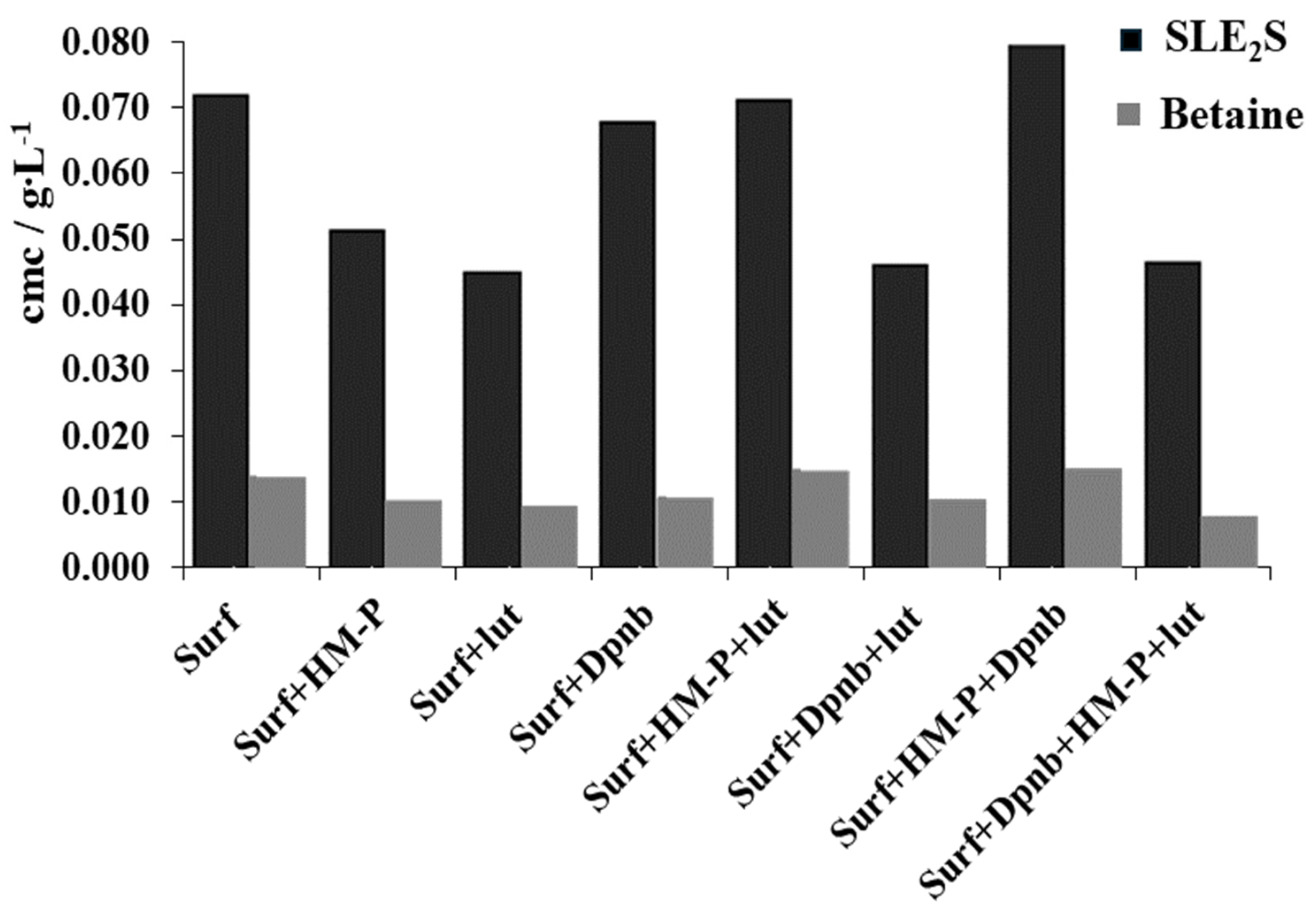
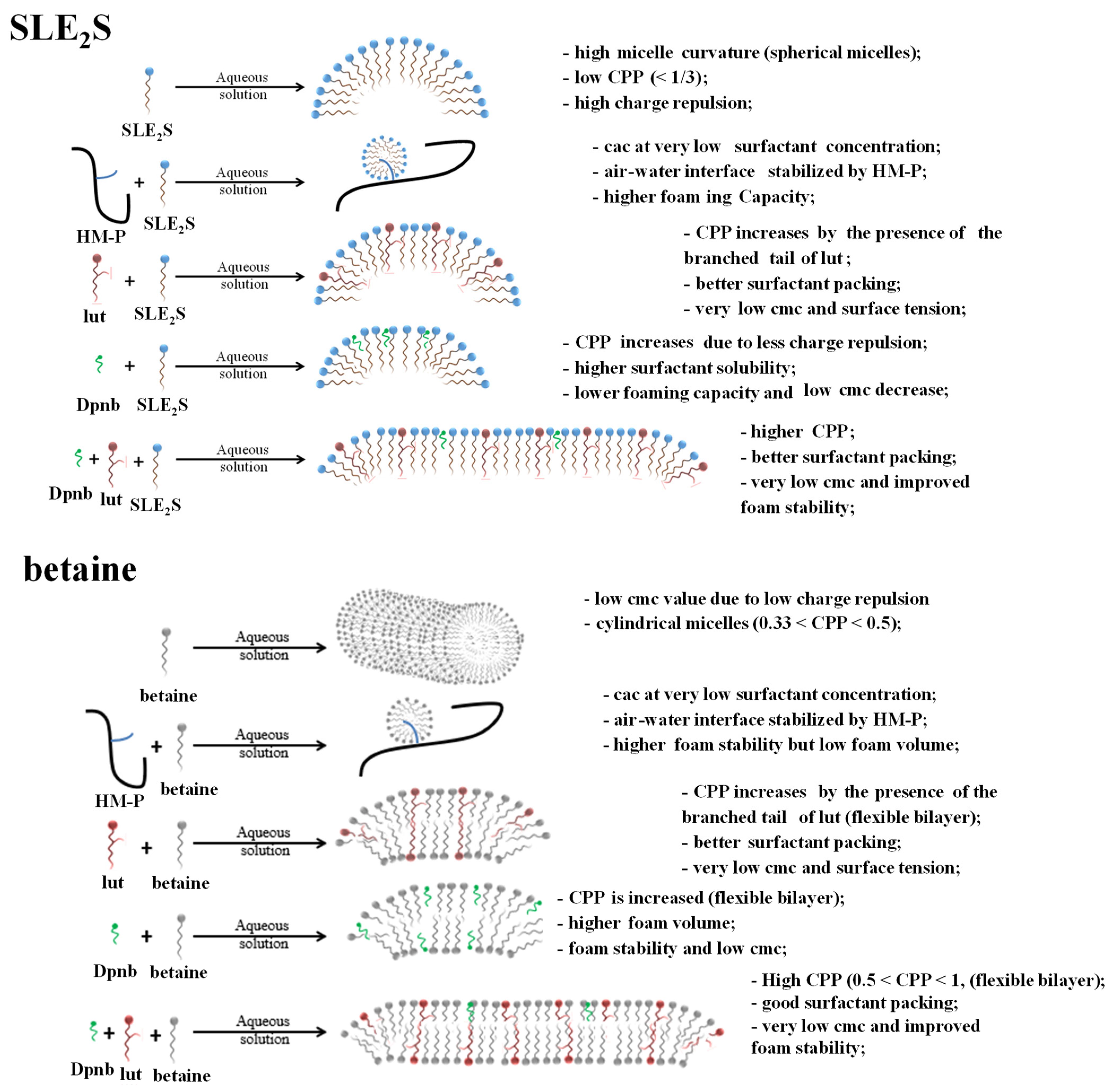
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonoda, J.; Sakai, T.; Inomata, Y. Liquid Oil That Flows in Spaces of Aqueous Foam without Defoaming. J. Phys. Chem. B 2014, 118, 9438–9444. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Foaming of Surfactant Solutions. In Surface Chemistry of Surfactants and Polymers; Wiley: Hoboken, NJ, USA, 2014; pp. 419–430. [Google Scholar] [CrossRef]
- Colloid Stability. In Emulsions, Foams, Suspensions, and Aerosols; Schramm, L.L. (Ed.) Wiley: Hoboken, NJ, USA, 2014; pp. 163–208. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Trégouët, C. Foam coarsening under a steady shear: Interplay between bubble rearrangement and film thinning dynamics. Soft Matter 2023, 19, 2090–2098. [Google Scholar] [CrossRef]
- Akbari, S.; Nour, A.H.; Yunus, R.M.; Farhan, A.H. Biosurfactants as promising multifunctional agent: A mini review. Int. J. Innov. Res. Sci. Stud. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Wang, L.; Yoon, R.-H. Effects of surface forces and film elasticity on foam stability. Int. J. Miner. Process. 2008, 85, 101–110. [Google Scholar] [CrossRef]
- Yu, W.; Kanj, M.Y. Review of foam stability in porous media: The effect of coarsening. J. Pet. Sci. Eng. 2022, 208, 109698. [Google Scholar] [CrossRef]
- Liu, H.; Shen, C.; Li, J.; Zhang, G.; Wang, Y.; Wan, H. Study on the Effect of Foam Stability on the Properties of Foamed Lightweight Soils. Materials 2023, 16, 6225. [Google Scholar] [CrossRef]
- Rusanov, A.I.; Krotov, V.V.; Nekrasov, A.G. Extremes of Some Foam Properties and Elasticity of Thin Foam Films near the Critical Micelle Concentration. Langmuir 2004, 20, 1511–1516. [Google Scholar] [CrossRef]
- Hadler, K.; Cilliers, J.J. The Effect of Particles on Surface Tension and Flotation Froth Stability. Min. Metall. Explor. 2019, 36, 63–69. [Google Scholar] [CrossRef]
- Tsekov, R.; Stöckelhuber, K.W.; Toshev, B.V. Disjoining Pressure and Surface Tension of a Small Drop. Langmuir 2000, 16, 3502–3505. [Google Scholar] [CrossRef]
- Wierenga, P.A.; Basheva, E.S.; Delahaije, R.J.B.M. Variations in foam collapse and thin film stability with constant interfacial and bulk properties. Adv. Colloid Interface Sci. 2023, 312, 102845. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Cui, Z. Synergistic Effects between Anionic and Sulfobetaine Surfactants for Stabilization of Foams Tolerant to Crude Oil in Foam Flooding. J. Surfactants Deterg. 2021, 24, 683–696. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, N.; Miao, X.; Zong, R.; Sheng, Y.; Li, C.; Lu, S. Formation of stable aqueous foams on the ethanol layer: Synergistic stabilization of fluorosurfactant and polymers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124545. [Google Scholar] [CrossRef]
- Alves, L.; Lindman, B.; Klotz, B.; Böttcher, A.; Haake, H.-M.; Antunes, F.E. Rheology of polyacrylate systems depends strongly on architecture. Colloid Polym. Sci. 2015, 293, 3285–3293. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surfactant–Polymer Systems. In Surface Chemistry of Surfactants and Polymers; Wiley: Hoboken, NJ, USA, 2014; pp. 271–293. [Google Scholar] [CrossRef]
- Pandey, S.; Bagwe, R.P.; Shah, D.O. Effect of counterions on surface and foaming properties of dodecyl sulfate. J. Colloid Interface Sci. 2003, 267, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Małysa, K.; Miller, R.; Lunkenheimer, K. Relationship between foam stability and surface elasticity forces: Fatty acid solutions. Colloids Surf. 1991, 53, 47–62. [Google Scholar] [CrossRef]
- Bergeron, V.; Jimenez-Laguna, A.I.; Radke, C.J. Hole formation and sheeting in the drainage of thin liquid films. Langmuir 1992, 8, 3027–3032. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Colloidal Stability. In Surface Chemistry of Surfactants and Polymers; John Wiley & Sons, Ltd: Chichester, UK, 2014; pp. 335–360. [Google Scholar] [CrossRef]
- Petkova, R.; Tcholakova, S.; Denkov, N.D. Foaming and Foam Stability for Mixed Polymer–Surfactant Solutions: Effects of Surfactant Type and Polymer Charge. Langmuir 2012, 28, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Bureiko, A.; Trybala, A.; Kovalchuk, N.; Starov, V. Current applications of foams formed from mixed surfactant–polymer solutions. Adv. Colloid Interface Sci. 2015, 222, 670–677. [Google Scholar] [CrossRef]
- Deng, Q.; Li, H.; Li, C.; Lv, W.; Li, Y. Enhancement of foamability and foam stability induced by interactions between a hyperbranched exopolysaccharide and a zwitterionic surfactant dodecyl sulfobetaine. RSC Adv. 2015, 5, 61868–61875. [Google Scholar] [CrossRef]
- Azdarpour, A.; Rahmani, O.; Mohammadian, E.; Parak, M.; Daud, A.R.M.; Junin, R. The effects of polymer and surfactant on polymer enhanced foam stability. In Proceedings of the 2013 IEEE Business Engineering and Industrial Applications Colloquium (BEIAC), Langkawi, Malaysia, 7–9 April 2013; pp. 97–102. [Google Scholar]
- Momin, S.A.; Yeole, P. Comparative Study of Effect of Surfactant-Polymer Interactions on Properties of Alkyl Polyglucosides and Alpha Olefin Sulphonate. J. Surfactants Deterg. 2012, 15, 291–298. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Khan, N.; Zhu, C.; Gao, Y. Effects of the surfactant, polymer, and crude oil properties on the formation and stabilization of oil-based foam liquid films: Insights from the microscale. J. Mol. Liq. 2023, 373, 121194. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, X.-Y.; Zhang, H.-Q.; Wang, Y.; Xu, Y.-F.; Peng, B.-L.; Liu, Y. Modeling of kinetic characteristics of alkaline-surfactant-polymer-strengthened foams decay under ultrasonic standing wave. Pet. Sci. 2022, 19, 1825–1839. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Gong, Q.; Xu, Z.; Liu, Z.; Zhang, L.; Zhang, L.; Zhao, S. Roles of Catanionic Surfactant Mixtures on the Stability of Foams in the Presence of Oil. Energy Fuels 2016, 30, 6355–6364. [Google Scholar] [CrossRef]
- Almobarky, M.; AlYousef, Z.; Schechter, D. Enhancing the Foam Stability Using Surfactants Mixtures. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 16 August 2018; p. 13. [Google Scholar]
- Babamahmoudi, S.; Riahi, S. Application of nano particle for enhancement of foam stability in the presence of crude oil: Experimental investigation. J. Mol. Liq. 2018, 264, 499–509. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Ozdemir, O.; Hampton, M.A.; Nguyen, A.V. Formation and stability of foams stabilized by fine particles with similar size, contact angle and different shapes. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 132–138. [Google Scholar] [CrossRef]
- Tran, T.; Gonzalez Perdomo, M.E.; Haghighi, M.; Amrouch, K. Study of the synergistic effects between different surfactant types and silica nanoparticles on the stability of liquid foams at elevated temperature. Fuel 2022, 315, 122818. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z. Highly Stable Foam Stabilized by Alumina Nanoparticles for EOR: Effects of Sodium Cumenesulfonate and Electrolyte Concentrations. Energy Fuels 2017, 31, 9016–9025. [Google Scholar] [CrossRef]
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Iqbal, H.M.N. Effective exploitation of anionic, nonionic, and nanoparticle-stabilized surfactant foams for petroleum hydrocarbon contaminated soil remediation. Sci. Total Environ. 2020, 704, 135391. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Alves, L.; Lindman, B.; Klotz, B.; Böttcher, A.; Haake, H.-M.; Antunes, F.E. On the rheology of mixed systems of hydrophobically modified polyacrylate microgels and surfactants: Role of the surfactant architecture. J. Colloid Interface Sci. 2018, 513, 489–496. [Google Scholar] [CrossRef]
- Ge, J.-J.; Zhang, T.-C.; Pan, Y.-P.; Zhang, X. The effect of betaine surfactants on the association behavior of associating polymer. Pet. Sci. 2021, 18, 1441–1449. [Google Scholar] [CrossRef]
- Jian, G.; Hou, Q.; Zhu, Y. Stability of Polymer and Surfactant Mixture Enhanced Foams in the Presence of Oil Under Static and Dynamic Conditions. J. Dispers. Sci. Technol. 2015, 36, 477–488. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Luo, H.; Peng, B.; Sun, X.; Liu, Y.; Rui, R. Foaming Properties and Foam Structure of Produced Liquid in Alkali/Surfactant/Polymer Flooding Production. J. Energy Resour. Technol. 2021, 143, 103005. [Google Scholar] [CrossRef]
- Tiso, T.; Demling, P.; Karmainski, T.; Oraby, A.; Eiken, J.; Liu, L.; Bongartz, P.; Wessling, M.; Desmond, P.; Schmitz, S.; et al. Foam control in biotechnological processes—Challenges and opportunities. Discov. Chem. Eng. 2024, 4, 2. [Google Scholar] [CrossRef]
- Varadaraj, R.; Bock, J.; Zushma, S.; Brons, N.; Colletti, T. Effect of hydrocarbon chain branching on interfacial properties of monodisperse ethoxylated alcohol surfactants. J. Colloid Interface Sci. 1991, 147, 387–395. [Google Scholar] [CrossRef]
- Aoudia, M.; Al-Haddabi, B.; Al-Harthi, Z.; Al-Rubkhi, A. Sodium Lauryl Ether Sulfate Micellization and Water Solubility Enhancement Towards Naphthalene and Pyrene: Effect of the Degree of Ethoxylation. J. Surfactants Deterg. 2010, 13, 103–111. [Google Scholar] [CrossRef]
- Jadoon, Q.; Bibi, I.; Mehmood, K.; Sajjad, S.; Nawaz, M.; Ali, F.; Bibi, S.; ur-Rehman, W.; Bano, S.; Usman, M. Interaction of surfactants with block-copolymer systems in the presence of Hofmeister anions. Mater. Res. Express 2017, 4, 035307. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Mixed Surfactant Systems. In Surface Chemistry of Surfactants and Polymers; Wiley: Hoboken, NJ, USA, 2014; pp. 251–269. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surfactant Self-Assembly. In Surface Chemistry of Surfactants and Polymers; Wiley: Hoboken, NJ, USA, 2014; pp. 113–136. [Google Scholar] [CrossRef]
- Sidim, T.; Acar, G. Alcohols Effect on Critic Micelle Concentration of Polysorbate 20 and Cetyl Trimethyl Ammonium Bromine Mixed Solutions. J. Surfactants Deterg. 2013, 16, 601–607. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface and Interfacial Tension. In Surface Chemistry of Surfactants and Polymers; Wiley: Hoboken, NJ, USA, 2014; pp. 231–249. [Google Scholar] [CrossRef]
- Becerra, N.; Toro, C.; Zanocco, A.L.; Lemp, E.; Günther, G. Characterization of micelles formed by sucrose 6-O-monoesters. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 134–139. [Google Scholar] [CrossRef]
- Godbey, W.T. Chapter 12-Gene delivery. In Biotechnology and its Applications, 2nd ed.; Godbey, W.T., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 287–325. [Google Scholar] [CrossRef]





| Surfactant System | /10−10 mol·cm−2 | A/Å Molecule−1 | CPP | |
|---|---|---|---|---|
| SLE2S | 1.30 | 127.45 | 0.16 | |
| + HM-P | 1.50 | 111.07 | 0.19 | |
| + Lut | 1.81 | 91.53 | 0.23 | |
| + Dpnb | 1.79 | 92.88 | 0.23 | |
| + HM-P + lut | 1.45 | 114.35 | 0.18 | |
| + lut + Dpnb | 2.09 | 79.30 | 0.26 | |
| + HM-P + Dpnb | 1.78 | 93.22 | 0.22 | |
| + HM-P + Dpnb + lut | 1.61 | 102.91 | 0.20 | |
| Betaine | 3.50 | 47.43 | 0.44 | |
| + HM-P | 4.28 | 38.77 | 0.54 | |
| + Lut | 4.03 | 41.25 | 0.51 | |
| + Dpnb | 4.70 | 35.30 | 0.59 | |
| + HM-P + lut | 4.26 | 38.95 | 0.54 | |
| + lut + Dpnb | 4.94 | 33.62 | 0.62 | |
| + HM-P + Dpnb | 4.01 | 41.40 | 0.51 | |
| + HM-P + Dpnb + lut | 4.29 | 38.73 | 0.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, L.; Magalhães, S.; Esteves, C.; Sebastião, M.; Antunes, F. Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems. J 2024, 7, 169-182. https://doi.org/10.3390/j7020010
Alves L, Magalhães S, Esteves C, Sebastião M, Antunes F. Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems. J. 2024; 7(2):169-182. https://doi.org/10.3390/j7020010
Chicago/Turabian StyleAlves, Luís, Solange Magalhães, Cátia Esteves, Marco Sebastião, and Filipe Antunes. 2024. "Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems" J 7, no. 2: 169-182. https://doi.org/10.3390/j7020010





