Thermodynamic Theory of Biological Evolution and Aging. Experimental Confirmation of Theory
Abstract
:"In addition to entropy there may well exist other "one-way" functions which add to the overall description of the world as temporal development."Kenneth G. Denbigh [1]
Introduction
 , which for a quasi-closed system tends to the most negative value (
, which for a quasi-closed system tends to the most negative value (  ).
).  , tends to a minimum. That tendency explains the variation of the supramolecular and chemical composition and the morphology of tissues during aging. The theory makes it possible to define the principles upon which proper diets and medications can be devised to slow down aging. Such diets and medications are also useful in preventative care and in the treatment of various pathologies and among them those attending old age. The use of the Le Chatelier-Brown principle, as well as the other laws of equilibrium thermodynamics to describe quasi-closed systems, offers broad opportunities for the study of the biological world [3,4,6].
, tends to a minimum. That tendency explains the variation of the supramolecular and chemical composition and the morphology of tissues during aging. The theory makes it possible to define the principles upon which proper diets and medications can be devised to slow down aging. Such diets and medications are also useful in preventative care and in the treatment of various pathologies and among them those attending old age. The use of the Le Chatelier-Brown principle, as well as the other laws of equilibrium thermodynamics to describe quasi-closed systems, offers broad opportunities for the study of the biological world [3,4,6].The Model of Biological Evolution
 , tends to minimum, the substances that form the most stable supramolecular structures are selected. These structures are accumulated in micro- and macrovolumes of biological systems. Individual macromolecules and superstructures reduplicate in consequence of possible matrix mechanisms. The first to be selected are nucleic acids whose composition and structure (because of the thermodynamic factors) slowly adapt to the nature of the surroundings, including the nature of proteins, which, in turn, is determined by the DNA (RNA) itself. This explains the feedback in the reading off of information between protein structure and DNA. Under our model, this feedback has a thermodynamic nature. Let me note that in my view, there is thermodynamic feedback among all hierarchical levels: ecosystems ⇒ populations ⇒ organisms ⇒ cells ⇒ supramolecular structures ⇒ proteins and some other macromolecules ⇒ DNA.
, tends to minimum, the substances that form the most stable supramolecular structures are selected. These structures are accumulated in micro- and macrovolumes of biological systems. Individual macromolecules and superstructures reduplicate in consequence of possible matrix mechanisms. The first to be selected are nucleic acids whose composition and structure (because of the thermodynamic factors) slowly adapt to the nature of the surroundings, including the nature of proteins, which, in turn, is determined by the DNA (RNA) itself. This explains the feedback in the reading off of information between protein structure and DNA. Under our model, this feedback has a thermodynamic nature. Let me note that in my view, there is thermodynamic feedback among all hierarchical levels: ecosystems ⇒ populations ⇒ organisms ⇒ cells ⇒ supramolecular structures ⇒ proteins and some other macromolecules ⇒ DNA. Thermodynamic Model of Ontogeny and the Aging of Living Organisms
 [2,3,4,5,6,10]:
[2,3,4,5,6,10]:

 [4, p. 57]); the symbol «~ » stresses that the system is heterogeneous.
[4, p. 57]); the symbol «~ » stresses that the system is heterogeneous.  ) or (
) or (  ) and thermodynamic stability of its supramolecular structures during ontogeny of living beings B (
) and thermodynamic stability of its supramolecular structures during ontogeny of living beings B (  ).
).
 ) or (
) or (  ) and thermodynamic stability of its supramolecular structures during ontogeny of living beings B (
) and thermodynamic stability of its supramolecular structures during ontogeny of living beings B (  ).
).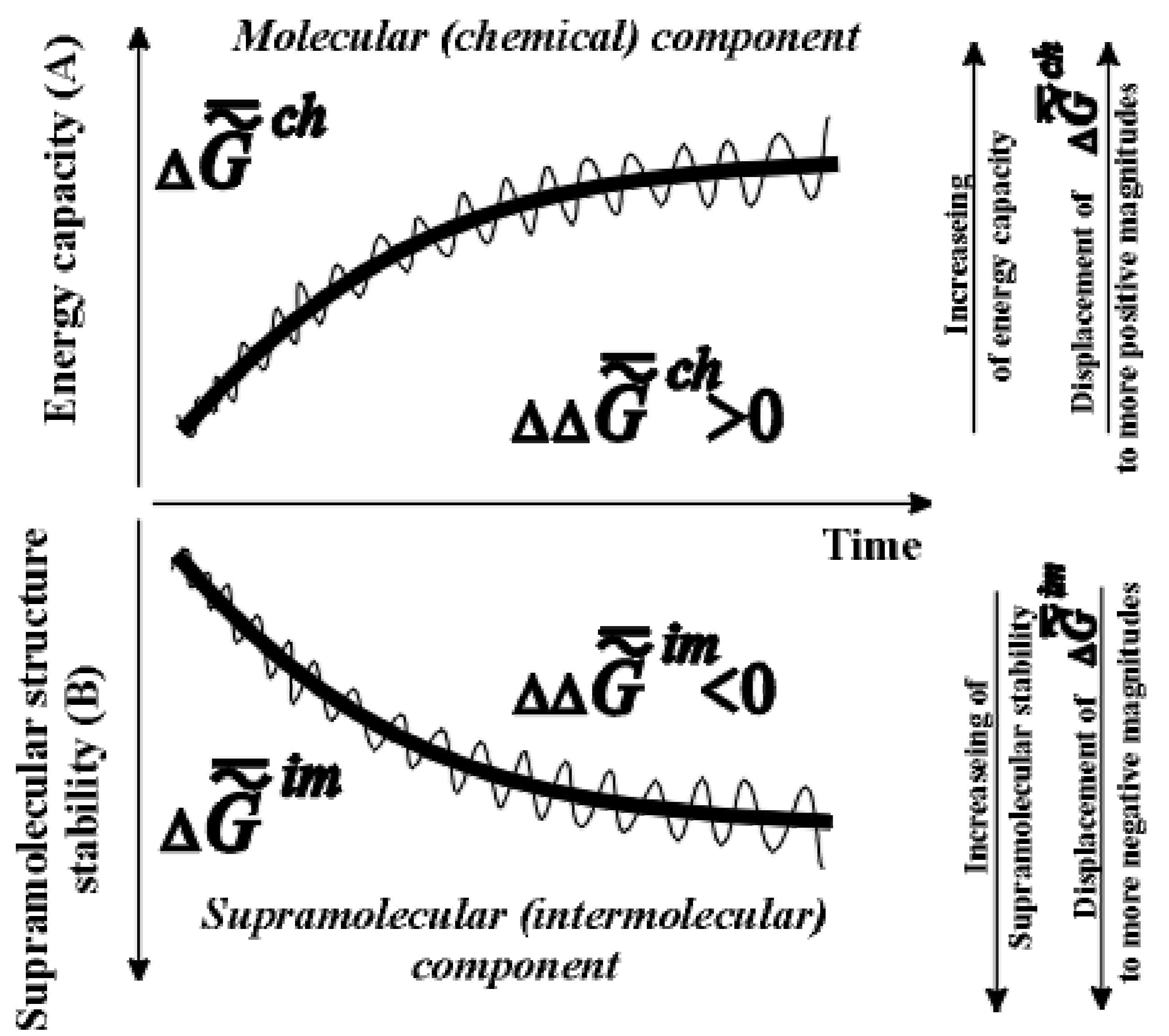
 ,
,  is much greater than
is much greater than  ). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuation of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels
). The time axis set by the second law of thermodynamics is scaleless. Jagged lines plotted onto the curves emphasize the fact that fluctuation of environmental parameters (temperature, pressure, diet, physical fields, time of day, season, etc.) change the levels  and
and  . Organisms adapt to these fluctuations only within the limits of the adaptive zone (range of tolerance).
. Organisms adapt to these fluctuations only within the limits of the adaptive zone (range of tolerance). obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics, which studies complex supramolecular structures without any detailed analysis at molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging and behavior of living systems.
obliges one to refer to a new branch of physical chemistry, supramolecular thermodynamics, which studies complex supramolecular structures without any detailed analysis at molecular level. This approach does not contradict the methods of phenomenological thermodynamics and is, perhaps, currently the only effective approach to the study of the thermodynamic aspects of evolution, aging and behavior of living systems.  or
or  (as well as
(as well as  or
or  - change of the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) «benefits» by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component,
- change of the specific enthalpy of the chemical component), which is a secondary effect. According to the second law, the thermodynamics of supramolecular interactions (or supramolecular thermodynamics) «benefits» by the accumulation in a biological system of chemical substances with a high energy capacity (the reference is to the chemical component,  ), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance [4,6]).
), which oust water from this system. This can be explained by the fact that substances with a relatively high energy capacity have a heightened capacity for participation in the formation of supramolecular structures (the principle of the stability of a chemical substance [4,6]).  is connected with the value of the Gibbs function of the formation of supramolecular structures:
is connected with the value of the Gibbs function of the formation of supramolecular structures:

 (or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value becoming more negative as biological tissue evolves (ages).
(or the specific value of the Helmholtz function, which practically coincides with it in the condensed phase) increases in absolute value becoming more negative as biological tissue evolves (ages).  can be performed with the use of the approximated Gibbs-Helmholtz equation
can be performed with the use of the approximated Gibbs-Helmholtz equation

 is the specific enthalpy variation due to melting (
is the specific enthalpy variation due to melting (  , crystallisation or self-assembly), T0 is the standard temperature (25oC) to which the calculations relate,
, crystallisation or self-assembly), T0 is the standard temperature (25oC) to which the calculations relate,  is the melting temperature of the i-th substance (or a phase consisting of i-th substances).
is the melting temperature of the i-th substance (or a phase consisting of i-th substances). 
Experimental Confirmation of Theory
 (Equation 1), characterizing the given volume of the biological mass (e.g., biological tissue) tends to a minimum in the case of quasi-closed biological systems (open systems functioning in constant conditions of the environment).
(Equation 1), characterizing the given volume of the biological mass (e.g., biological tissue) tends to a minimum in the case of quasi-closed biological systems (open systems functioning in constant conditions of the environment). 
 is the retention time of the examined substance,
is the retention time of the examined substance,  is the retention time of standard substance, are a consequence of equation (2) and have a wide practical application. I shall cite one of the hundreds of known examples is cited below.
is the retention time of standard substance, are a consequence of equation (2) and have a wide practical application. I shall cite one of the hundreds of known examples is cited below. ) and the Gibbs function (Gibbs energy) of adsorption ∆(∆G) of benzene derivatives in relation to benzene on a column of silica gel with hydroxylated surface KSS-4 (s =650 m2/g) at 32° C, the consumption of water eluent is 2.5 cm3/min [7].
) and the Gibbs function (Gibbs energy) of adsorption ∆(∆G) of benzene derivatives in relation to benzene on a column of silica gel with hydroxylated surface KSS-4 (s =650 m2/g) at 32° C, the consumption of water eluent is 2.5 cm3/min [7].
| No | Adsorbate |  , s , s | ∆(∆G), J/mol |
| 1 | Benzene | 134 | - |
| 2 | Benzoic acid | 80 | + 1312 |
| 3 | Phenol | 100 | + 746 |
| 4 | Phloroglucinol | 120 | + 264 |
| 5 | Hydroquinone | 124 | + 195 |
| 6 | Resorcinol | 127 | + 127 |
| 7 | Benzoic alcohol | 157 | - 409 |
| 8 | o-Cresol | 179 | - 746 |
| 9 | p- Cresol | 181 | - 769 |
| 10 | m - Cresol | 187 | - 850 |
| 11 | Flurobenzene | 206 | - 1096 |
| 12 | o-Xylene | 269 | - 1774 |
| 13 | m - Xylene | 287 | - 1941 |
 for different organic compounds on silica gel with hydroxylated surface.
for different organic compounds on silica gel with hydroxylated surface.
 for different organic compounds on silica gel with hydroxylated surface.
for different organic compounds on silica gel with hydroxylated surface.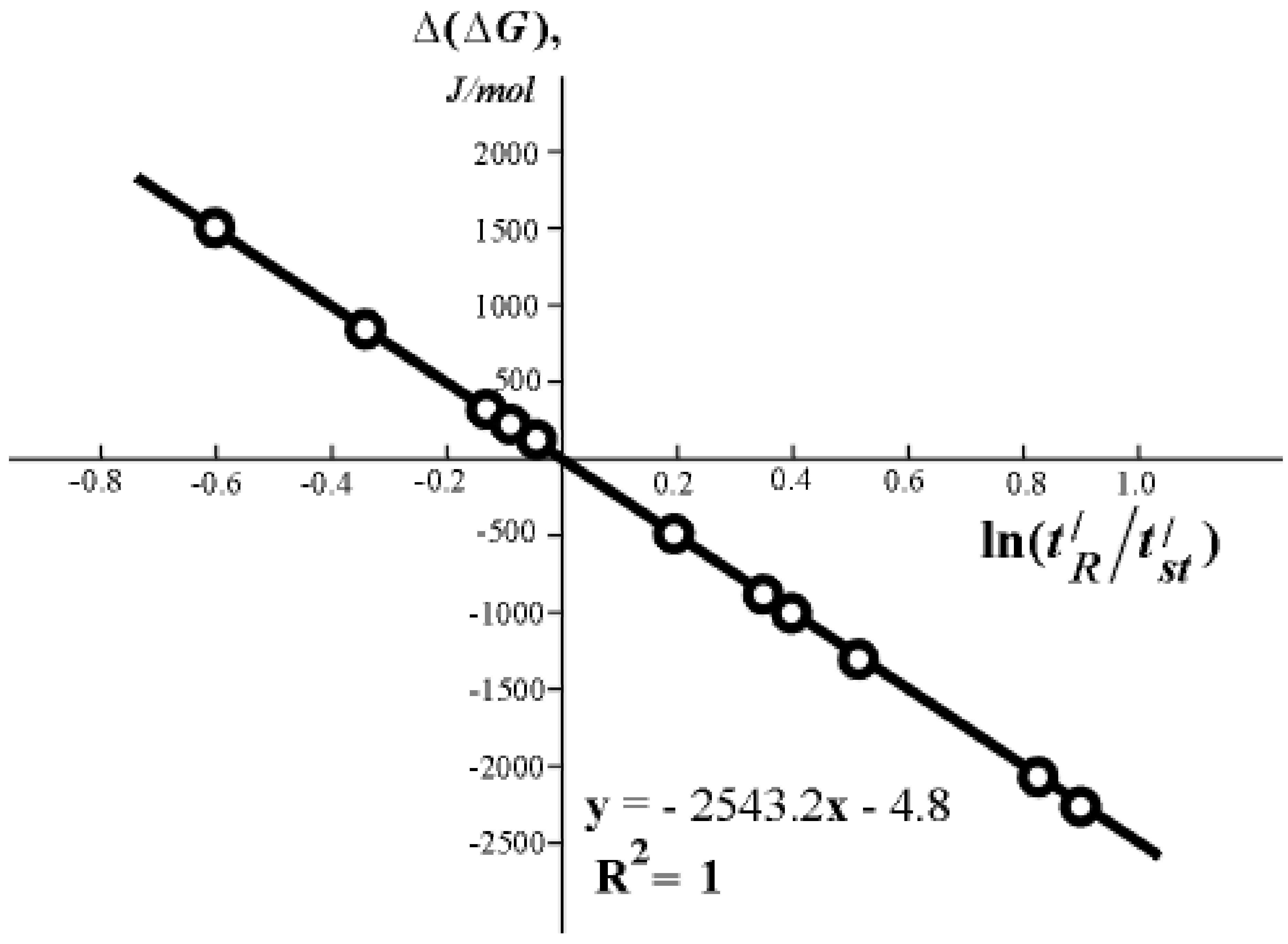
 or
or  ) of biological tissue in the process of the organism’s aging and the trend of
) of biological tissue in the process of the organism’s aging and the trend of  to the most negative value when forming supramolecular structures in ontogeny.
to the most negative value when forming supramolecular structures in ontogeny. (caloric content) on the extent of ontogeny ξ (or time) for pig tissue. The values of
(caloric content) on the extent of ontogeny ξ (or time) for pig tissue. The values of  (points) were calculated on the basis of experimental data of the chemical composition of tissues (the heat of combustion of the components: fats - 9.2 ccal/g, proteins - 5.0 ccal/g). The curve shows the results of calorimetric experiments. ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue.
(points) were calculated on the basis of experimental data of the chemical composition of tissues (the heat of combustion of the components: fats - 9.2 ccal/g, proteins - 5.0 ccal/g). The curve shows the results of calorimetric experiments. ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue.
 (caloric content) on the extent of ontogeny ξ (or time) for pig tissue. The values of
(caloric content) on the extent of ontogeny ξ (or time) for pig tissue. The values of  (points) were calculated on the basis of experimental data of the chemical composition of tissues (the heat of combustion of the components: fats - 9.2 ccal/g, proteins - 5.0 ccal/g). The curve shows the results of calorimetric experiments. ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue.
(points) were calculated on the basis of experimental data of the chemical composition of tissues (the heat of combustion of the components: fats - 9.2 ccal/g, proteins - 5.0 ccal/g). The curve shows the results of calorimetric experiments. ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue.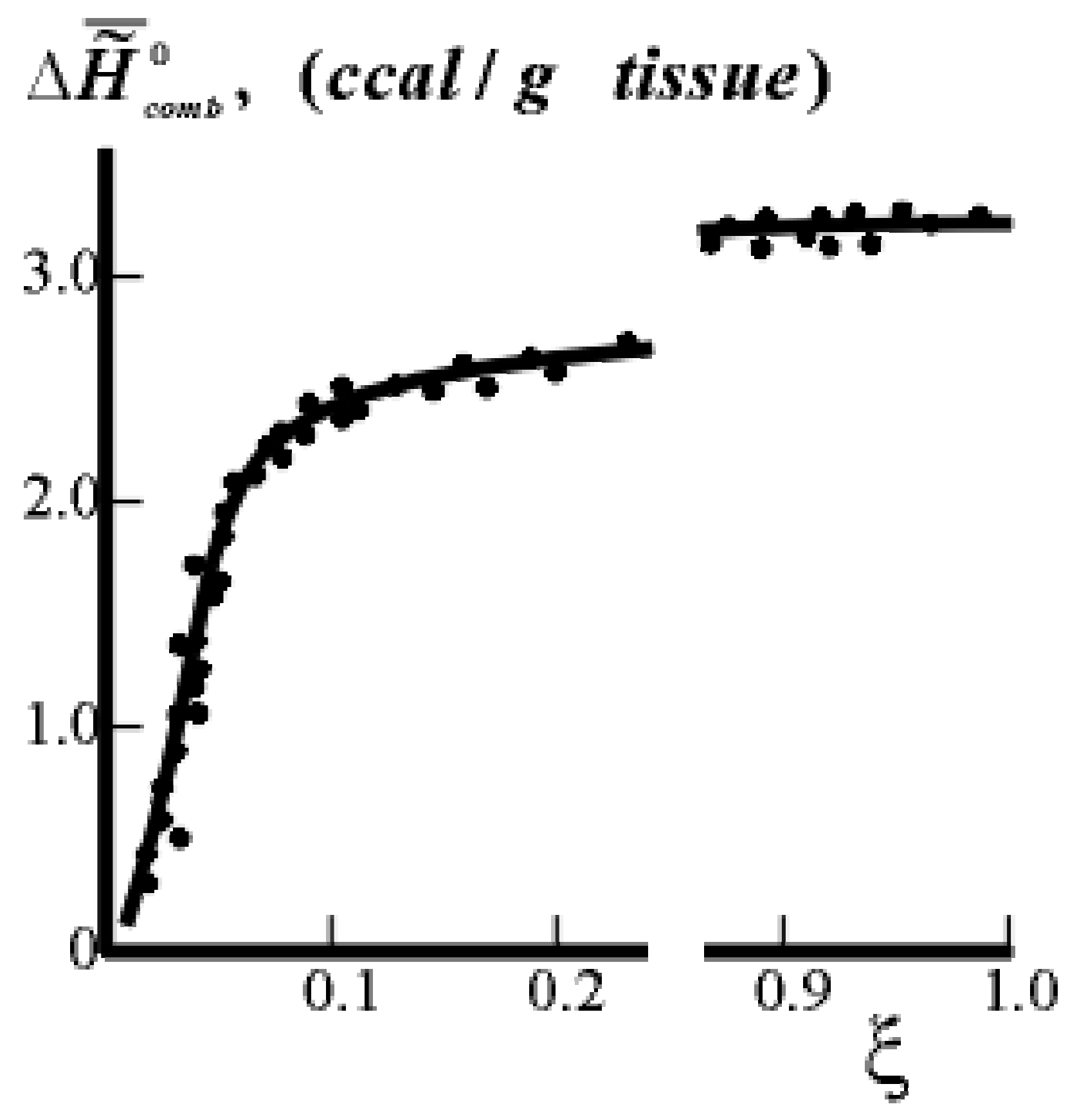
 on the extent of ontogeny, ξ (or time). ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue, for rat skin collagen. The values of
on the extent of ontogeny, ξ (or time). ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue, for rat skin collagen. The values of  (cal/g collagen) were calculated from the data of F.Flandin et al. and Gladyshev.
(cal/g collagen) were calculated from the data of F.Flandin et al. and Gladyshev.
 on the extent of ontogeny, ξ (or time). ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue, for rat skin collagen. The values of
on the extent of ontogeny, ξ (or time). ξ = 0.0 for 100% H2O in the tissue, ξ = 1.0 for 55% H2O in the tissue, for rat skin collagen. The values of  (cal/g collagen) were calculated from the data of F.Flandin et al. and Gladyshev.
(cal/g collagen) were calculated from the data of F.Flandin et al. and Gladyshev.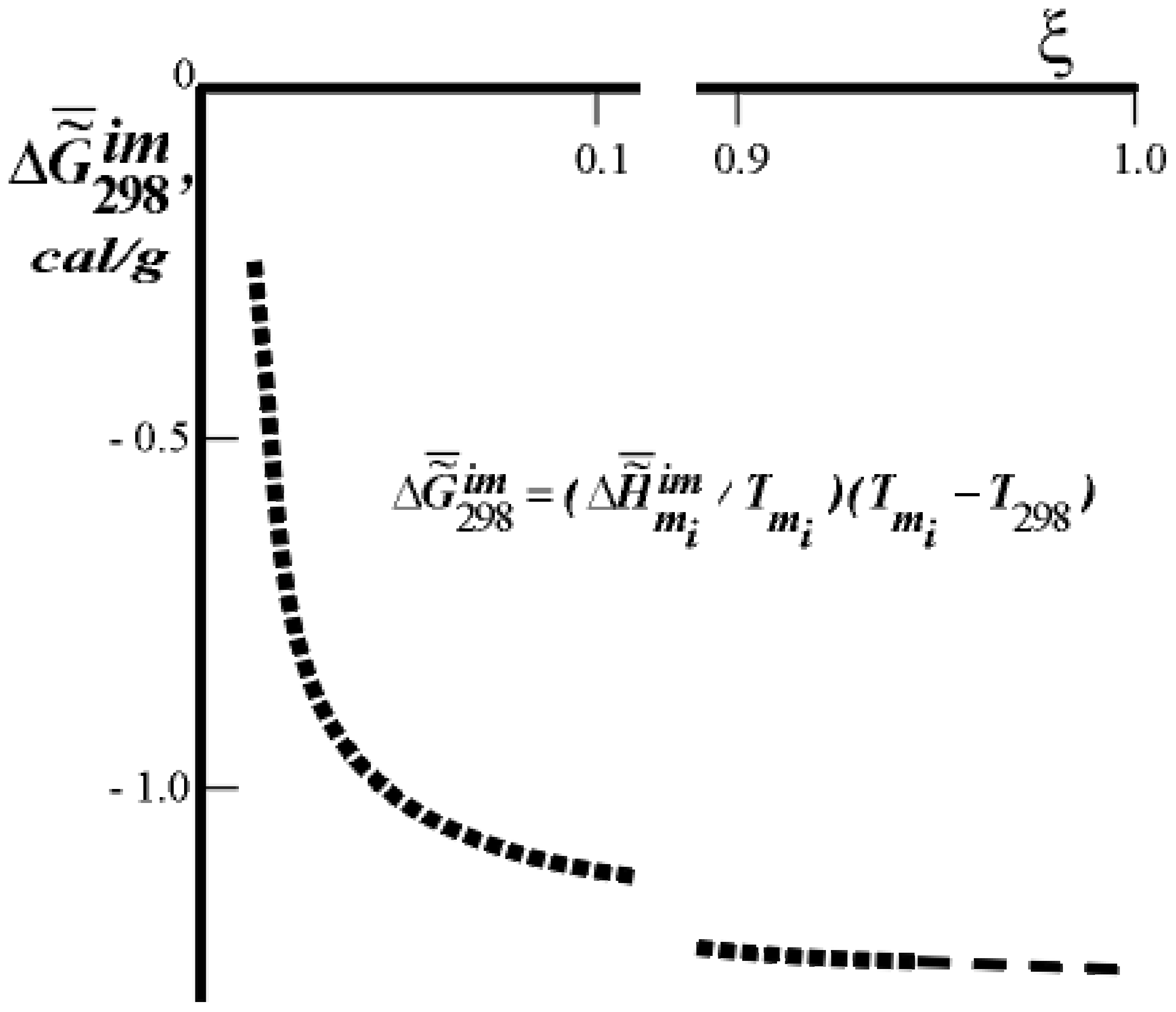
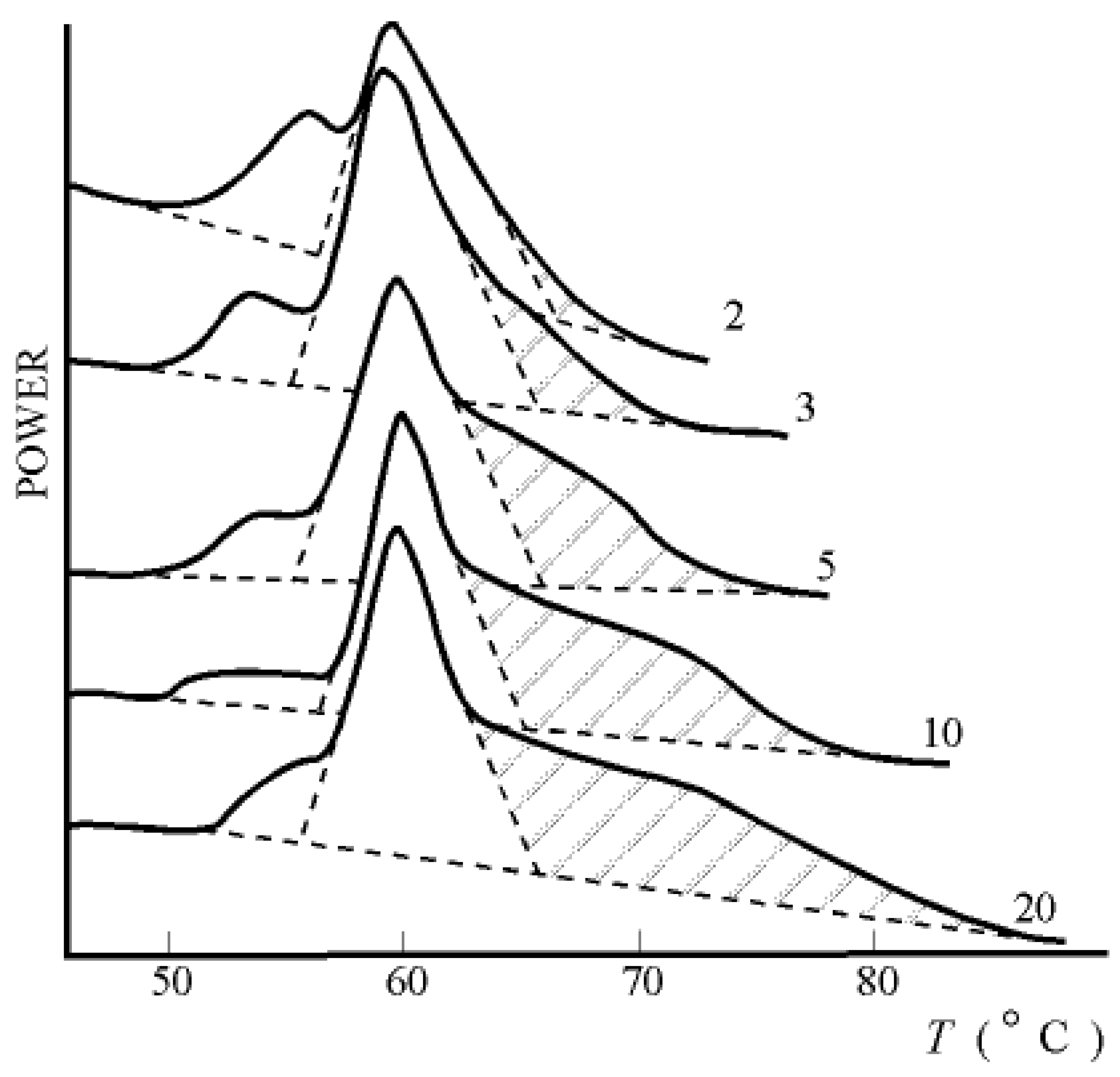
 performed with the use of the approximated Gibbs-Helmholtz equation (3) shows that, indeed, values ∆H and
performed with the use of the approximated Gibbs-Helmholtz equation (3) shows that, indeed, values ∆H and  increase during ontogeny substantially, which makes value
increase during ontogeny substantially, which makes value  significantly more negative.
significantly more negative.
 may be estimated more precisely by using the equation
may be estimated more precisely by using the equation

 is the change of heat capacity of the corresponding supramolecular structures during phase transition.
is the change of heat capacity of the corresponding supramolecular structures during phase transition.  (
(  ) and decrease of
) and decrease of  in ontogeny (as well as in phylogeny) is not likely to surprise physico-chemists, since it is qualitatively obvious that they are a consequence of the lower concentration of water in the biological mass as the latter ages.
in ontogeny (as well as in phylogeny) is not likely to surprise physico-chemists, since it is qualitatively obvious that they are a consequence of the lower concentration of water in the biological mass as the latter ages. 
References and Notes
- Denbigh, K.G. The Many Faces of Irreversibility. Brit. J. Phil. Sci. 1989, 40, 501–518. [Google Scholar] [CrossRef]
- Gladyshev, G.P. Termodinamika of the Ierarkhicheskikh Sistem (Thermodynamics of the Hierarchic Systems). In Khimicheskaya Enciklopedia (Chemical Encyclopedia); Bolshaya Rossiiskaya Enciklopedia: Moscow, 1995; Vol. 4, p. 535. [Google Scholar]
- Gladyshev, G.P. Termodinamika i Makrokinetika Prirodnykh Ierarkhicheskikh Processov (Thermodynamics and Macrokinetics of Natural Hierarchical Processes); Nauka: Moscow, 1988; p. 287. [Google Scholar]
- Gladyshev, G.P. Thermodynamic Theory of the Evolution of Living Beings; Nova Sci. Publ. Inc.: New York, 1997; p. 142. [Google Scholar]
- Gladyshev, G.P. On the Macrokinetics and Thermodynamics of Natural Hierarchic Processes. J. Phys. Chem. 1987, 61, 2289–2301. [Google Scholar]
- Gladyshev, G.P. Thermodynamic Theory of the Evolution of Living Beings; Luch: Moscow, 1996; p. 98. [Google Scholar]
- Kiselev, A.V.; Poshkus, D.P.; Yashin, Ya.I. Moleculyarnye osnovy adsorbcionnoi chromatografii; Chimiya: Moscow, 1986; p. 272. [Google Scholar]
- Gladyshev, G.P. In Thermodynamics of Aging. In AAAS Annual Meeting and Science Innovation Exhibition (150th Anniversary Celebration), Philadelphia, Pennsylvania, Track: Emerging Science: Transforming the Next Generation. 12-17 February 1998. A-30; S-26 (AAAS, Scope, 1997).
- Gladyshev, G.P. Thermodynamics of Aging. Biology Bulletin 1998, 25, 433–441. [Google Scholar]
- Gladyshev, G.P. On the Thermodynamics of Biological Evolution. J. Theoret. Biol. 1978, 75, 425–444. [Google Scholar] [CrossRef]
- Flandin, F.; Buffevant, Ch.; Herbage, D. A. Differential Scanning Calorimetry Analysis of the Age - Related Changes in the Thermal Stability of Rat Skin Collagen. Biochimica et Biophysica Acta 1984, 791, 205–211. [Google Scholar] [CrossRef]
- Gladyshev, G.P. A Motive Force of Biological Evolution. Herald of the Russian Academy of Sciences 1994, 64, 118–124. [Google Scholar]
- Aleksandrov, V.Ya. Kletki, Makromolekuly i temperatura (Cells, macromolecules, and temperature); Nauka: Leningrad, 1975; p. 330. [Google Scholar]
- Novak, L.P. Aging, Total Body Potassium, Fat-Free Mass, and Cell Mass in Males and Females Between 18 and 85 Years. Journal of Gerontology 1972, 27, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.H.S.; McWhir, J.; Ritchie, W.A.; Wilmut, I. Sheep Cloned by Nuclear Transfer a Cultured Cell Line. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Moxon, E.R.; Wills, Ch. DNA - Microsatellites: Agents of Evolution? Scientific American 1999, 72–77. [Google Scholar] [CrossRef]
- Lepock, J. Supramolecular Thermodynamics. In AAAS Annual Meeting and Science Innovation Exhibition (150th Anniversary Celebration), Philadelphia, Pennsylvania, Track: Emerging Science: Transforming the Next Generation. February 12-17, 1998. P. A-30, S-26.
- Gladyshev, G.P. The Motive Force of Evolution of Living Matter and Thermodynamic Theory of Aging. Abstract and Report. In Pan-American Congress-99, San Antonio, Texas, USA, February 21-24, 1999.
- Kurnakova, N.V.; Gazaev, M.A. The Mountains and the Theory of Aging. Abstract and Report. In Pan-American Congress-99, Aging in the Americas: Frontiers of Care, Policy and Research. San Antonio, Texas, USA, February 21-24, 1999.
- Gladyshev, G.P.; Kurnakova, N.V. Motive Force of Evolution of Living Matter and Thermodynamic Theory of Aging. Advances in Gerontology (Russian Academy of Sciences) 1999, 2, 49–58. [Google Scholar]
- Gladyshev, G.P. Thermodynamic Theory of Biological Evolution and Aging. Modern Development in Thermody-namics. In Materials for Third Gordon Research Conference. Report, II Ciocco, Tuscany, Italy, April 18-23, 1999.
- Hayflick, L. How and Why We Age; Ballantine Books: New York, 1996; p. 377. [Google Scholar]
- Gladyshev, G.P. On the Thermodynamics, Entropy and Evolution of Biological Systems: What is Life from a Physical Chemist's Viewpoint. Entropy 1999, 1, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Gladyshev, G.P. Supramolecular Thermodynamics is a Key to Understanding Phenomena of Life. Abstract and Report. In 37th IUPAC Congress. 27th GDCh General Meeting, Berlin, Germany, August 14-19, 1999; p. 873.
- Gladyshev, G.P. Thermodynamics of Aging. Abstract and Report. In “′99 International Symposium on Aging and Antiaging Science & Technology”, Beijing, China, September 8-12, 1999; p. 26.
- See the http://www.endeav.org/evolut website.
© 1999 by MDPI (http://www.mdpi.org). Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Gladyshev, G.P. Thermodynamic Theory of Biological Evolution and Aging. Experimental Confirmation of Theory. Entropy 1999, 1, 55-68. https://doi.org/10.3390/e1040055
Gladyshev GP. Thermodynamic Theory of Biological Evolution and Aging. Experimental Confirmation of Theory. Entropy. 1999; 1(4):55-68. https://doi.org/10.3390/e1040055
Chicago/Turabian StyleGladyshev, Georgi P. 1999. "Thermodynamic Theory of Biological Evolution and Aging. Experimental Confirmation of Theory" Entropy 1, no. 4: 55-68. https://doi.org/10.3390/e1040055




