1. Introduction
In 2000, Sahin
et al. [
1] studied the thermoeconomic performance of an endoreversible solar-driven heat engine. In this study, they considered that the heat transfer from the hot reservoir to the working fluid is dominated by radiation because radiation heat transfer plays a key role in the collector ambient heat loss mechanism, while the mode of heat transfer from the working fluid to the cold reservoir is given by a Newtonian heat transfer law. Sahin
et al. [
1] calculated the optimum temperatures of the working fluid and the optimum efficiency of the engine operating at maximum power conditions. Later, Sahin and Kodal [
2], applied this procedure to study the thermoeconomics of an endoreversible heat engine in terms of the maximization of a profit function defined as the quotient of the power output and the annual investment cost. Recently, Barranco-Jiménez
et al. [
3], studied the optimum operation conditions of an endoreversible heat engine with different heat transfer laws at the thermal couplings but operating under maximum ecological function conditions, and more recently, Barranco-Jiménez
et al. [
4] also studied the thermoeconomic optimum operation conditions of a solar-driven heat engine. In these studies, Barranco-Jiménez
et al. considered three regimes of performance: The maximum power regime (MPR) [
5,
6,
7], the maximum efficient power [
8,
9] and the maximum ecological function regime (MER) [
10,
11]. In this work, we study the thermoeconomics of an irreversible heat engine by considering further with losses due to heat transfer across finite time temperature differences [
12,
13,
14,
15], heat leakage between thermal reservoirs [
16,
17,
18,
19,
20,
21,
22,
23,
24] and internal irreversibilities [
25,
26,
27] in terms of a parameter which comes from the Clausius inequality. In our study we use two regimes of performance: The maximum power regime, and the so-called ecological function regime. Besides, in our thermoeconomical analysis, conductive-convective and radiative terms are considered by means of a heat transfer law of the Dulong-Petit type [
28,
29]. Some of our numerical results are compared with data stemming from three power plants [
7,
30]. The article is organizes as follows: In
Section 2 we present the heat engine model; in
Section 3 the numerical results and discussion are presented; finally in
Section 4 we give the conclusions.
2. Theoretical Model
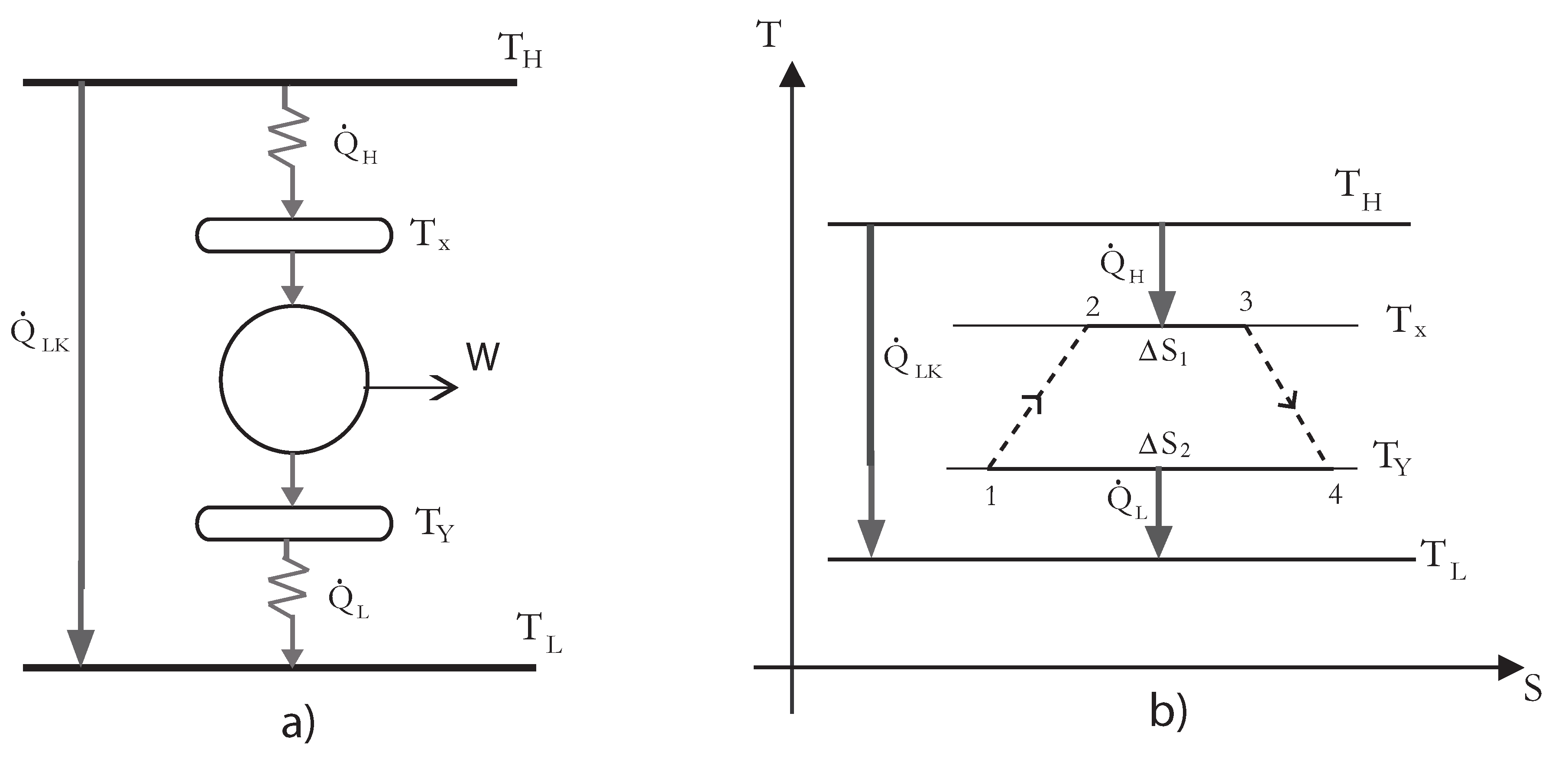
The considered irreversible solar-driven heat engine operates between a heat source of temperature
(the collector) and a heat sink of temperature
(cooling water), see
Figure 1a. The temperatures of the working fluid exchanging heat with the reservoirs at
and
are
and
, respectively. A T-S diagram of the model including heat leakage, finite time heat transfer and internal irreversibilities is also shown in
Figure 1b. The rate of heat leakage
from the hot reservoir at temperature
to the cold reservoir at temperature
with thermal conductance
γ is given by,
where
γ is the internal conductance of the heat engine and
ξ denotes the ratio of the internal conductance with respect to the hot-side convection heat transfer coefficient and heat transfer area, that is,
[
27]. The 5/4 exponent is usual in a Dulong-Petit heat transfer law [
28]. The rate of heat flow
from the hot source to the heat engine is given by,
where
is the hot side heat transfer coefficient and
is the heat transfer area of the hot side heat exchanger. Equations (1) and (2) are of the Dulong-Petit type, which include conductive-convective and radiative effects [
28], instead of only to use a Stefan-Boltzmann law, which is appropriate for space applications [
11], where the effect of atmospheric gases is not present. On the other hand, a Newtonian heat transfer is assumed as the main mode of heat transfer to the low temperature reservoir, therefore the heat flux rate
from the heat engine to the cold reservoir can be written as,
where
is the cold side heat transfer coefficient and
is the heat transfer area of the cold side heat exchanger. Then the total heat rate
transferred from the hot reservoir is,
and the total heat rate
transferred to the cold reservoir is,
Applying the first law of thermodynamics, the power output is given by,
Applying the second law of thermodynamic to the irreversible part of the model we get,
One can rewrite the inequality in Equation (7) as,
where
R is the so-called non-endoreversibility parameter [
32,
33]. Substituting Equations (2) and (3) into Equation (8), a relationship between
and
is obtained as,
where
,
and
β are the ratios of the heat transfer areas and the heat conductance parameter respectively, and are defined as,
and
These two parameters can be taken as design parameters. The thermal efficiency of the irreversible heat engine is,
Figure 1.
Schematic diagram of the irreversible heat engine and its
diagram [
31].
Figure 1.
Schematic diagram of the irreversible heat engine and its
diagram [
31].
In thermoeconomic analysis of power plant models, an objective function is defined in terms of a characteristic function (power output [
6,
31,
34], ecological function [
3,
10,
29],
etc.) and the cost involved in the performance of the power plant. In his early paper on this issue, De Vos [
6] studied the thermoeconomics of a Novikov power plant model in terms of the maximization of an objective function defined as the quotient of the power output and the performing costs of the plant. In that paper, De Vos considered a function of costs with two contributions: The cost of the investment which is assumed as proportional to the size of the plant and the cost of the fuel consumption which is assumed to be proportional to the quantity of heat input in the Novikov model. Analogously, Sahin and Kodal made a thermoeconomic analysis of a Curzon and Ahlborn [
5] model in terms of an objective function which they defined as power output per unit total cost taking into account both the investment and fuel costs [
34], but assuming that the size of the plant can be taken as proportional to the total heat transfer area, instead of the maximum heat input previously considered by De Vos [
6]. Following the Sahin
et al. procedure [
31], the objective function has been defined as the power output per unit investment cost, because a solar driven heat engine does not consume fossil fuels. In order to optimize power output per unit total cost, the objective function is given by [
31],
where
refers to annual investment cost. The investment cost of the plant is assumed to be proportional to the size of the plant. The size of the plant can be proportional to the total heat transfer area. Thus, the annual investment cost of the system can be written as [
31],
where the investment cost proportionality coefficients for the hot and cold sides
a and
b respectively are equal to the capital recovery factor times investment cost per unit heat transfer area, and their dimensions are
,
ncu being the national current unity. By using Equations (2), (6), (9), (13) and (14), we get a normalized expression for the objective function associated to the power output given by [
31],
where
and the parameter
f, is the relative investment cost of the hot size heat exchanger defined as [
31],
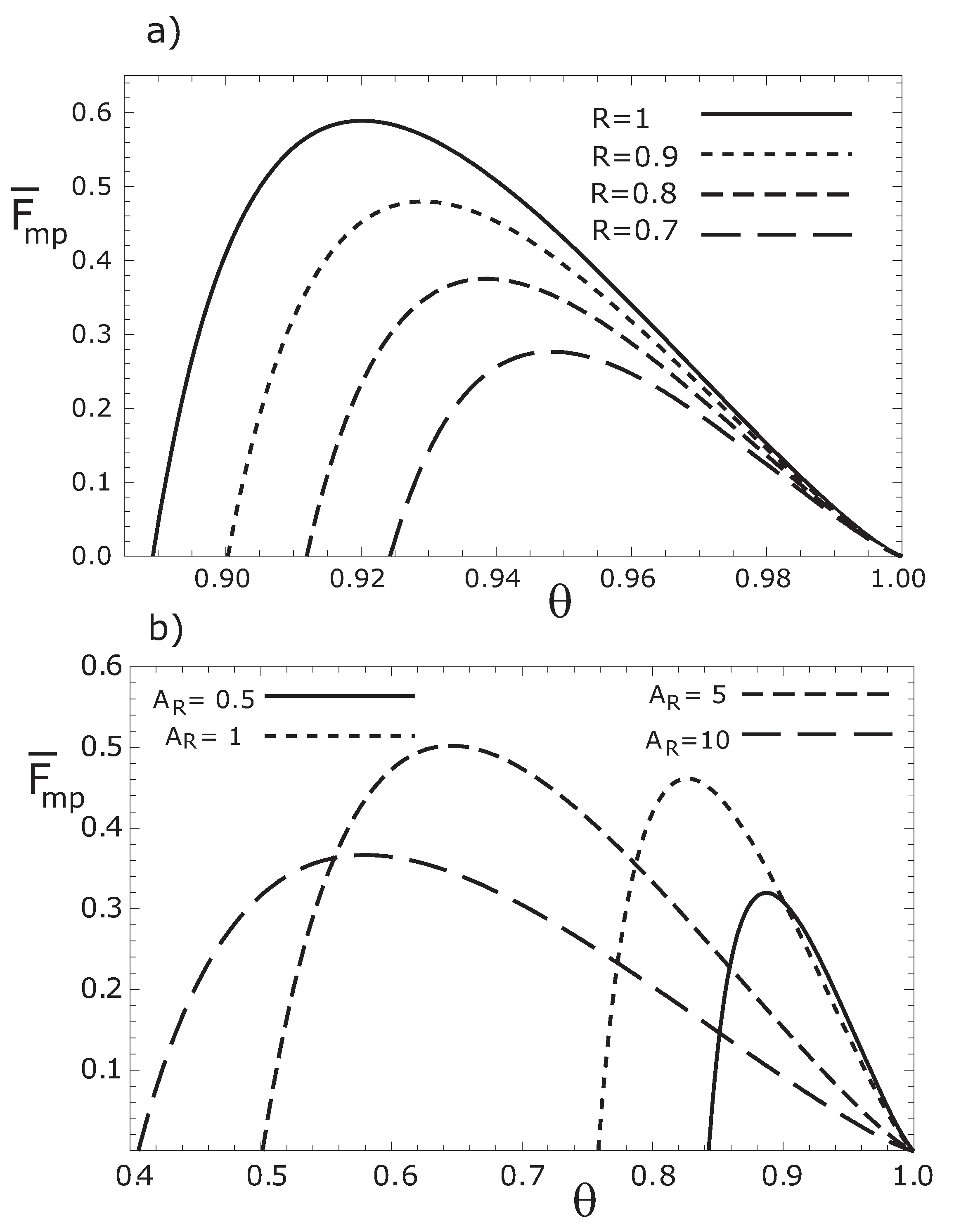
In
Figure 2a, we depict the objective function given by Equation (15)
versus θ, for several values of the parameter
R. In
Figure 2b we show the function
for several values of the parameter
.
Figure 2.
Variation of the thermoeconomic objective function respect to , for (a) different values of the parameter R with , and for (b) several values of with .
Figure 2.
Variation of the thermoeconomic objective function respect to , for (a) different values of the parameter R with , and for (b) several values of with .
For our thermoeconomic optimization approach, we define another objective function in terms of the so-called ecological function [
10,
29], divided by the annual investment cost, that is,
, where Σ is the total entropy production of the engine model. Analogously to Equation (15), the normalized objective function associated to the ecological function is given by,
And, by using Equations (1–3), the thermal efficiency,
η, of the irreversible heat engine can be expressed by,
In Equation (17) we have applied the second law of thermodynamics to calculate the total entropy production given by
(see
Figure 1). The dimensionless thermoeconomic objective functions [Equations (15) and (17)], can be plotted with respect to
, for given values of
and
f as shown in
Figure 2a and
Figure 2b, and
Figure 3a and
Figure 3b for the cases of the maximum power output and maximum ecological function conditions, respectively. In all cases we use
, as in [
31], where
K and therefore
K, this value of
τ is for comparison with [
31], however a more realistic value of
could be of the order of 431K [
7], which is the effective sky temperature stemming from the dilution of solar energy.
Figure 3.
Variation of the thermoeconomic objective function respect to , for (a) different values of the parameter R with , and for (b) several values of with .
Figure 3.
Variation of the thermoeconomic objective function respect to , for (a) different values of the parameter R with , and for (b) several values of with .
As it could be seen from
Figure 2a–
Figure 3b, there is a value of
θ that maximizes the objective functions for given
f,
and
τ values. In
Figure 4, we show the comparison of the aforementioned two objective functions. Since the two objective functions and thermal efficiency depend on the working fluid temperatures
, the objective functions given by Equations (15) and (17) can be maximized with respect to
or
, that is, we calculate
, the
values obtained give us the maximum values for
and
functions, respectively. This optimization procedure has been numerically carried out in the next section [
3,
31].
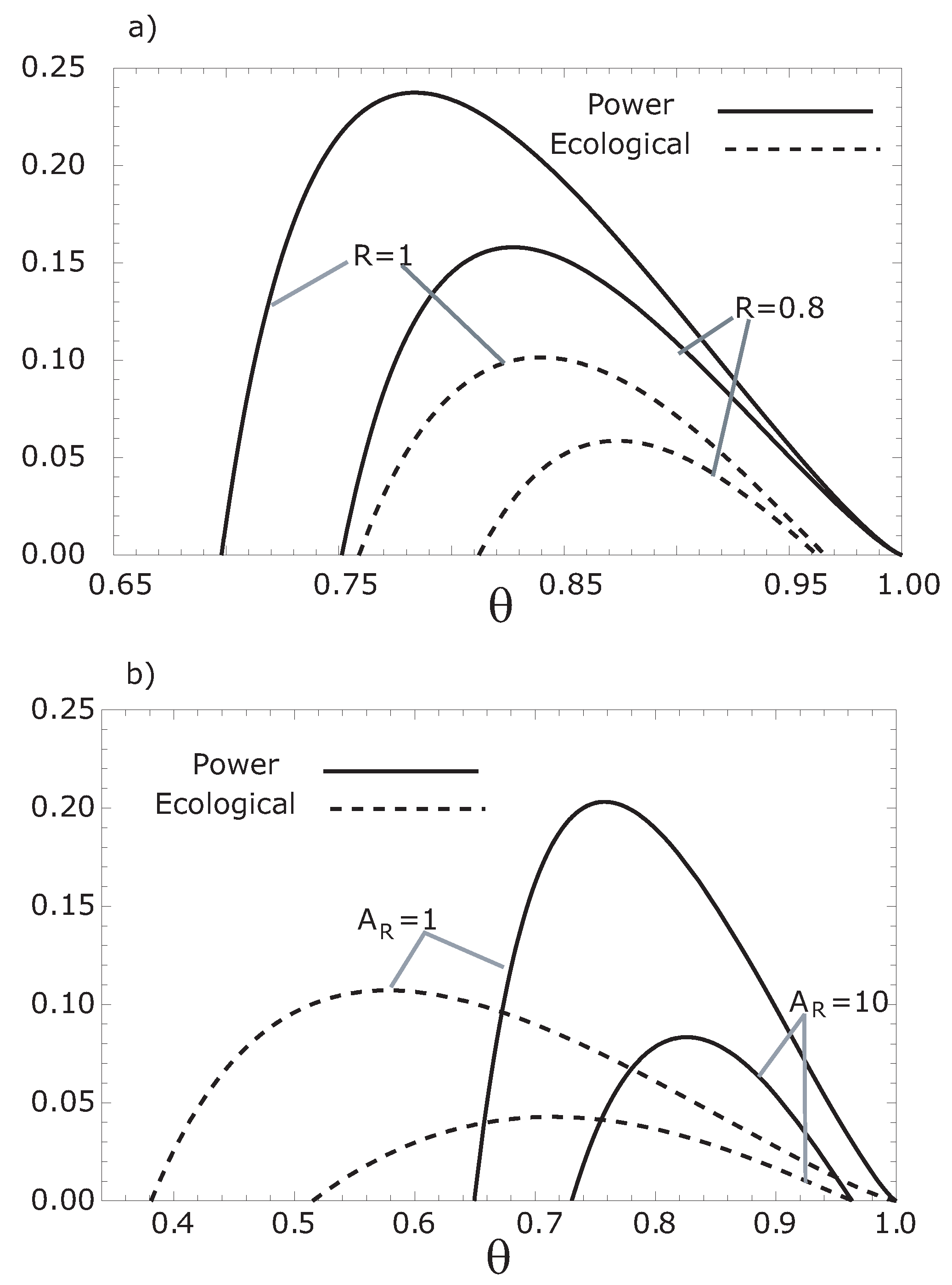
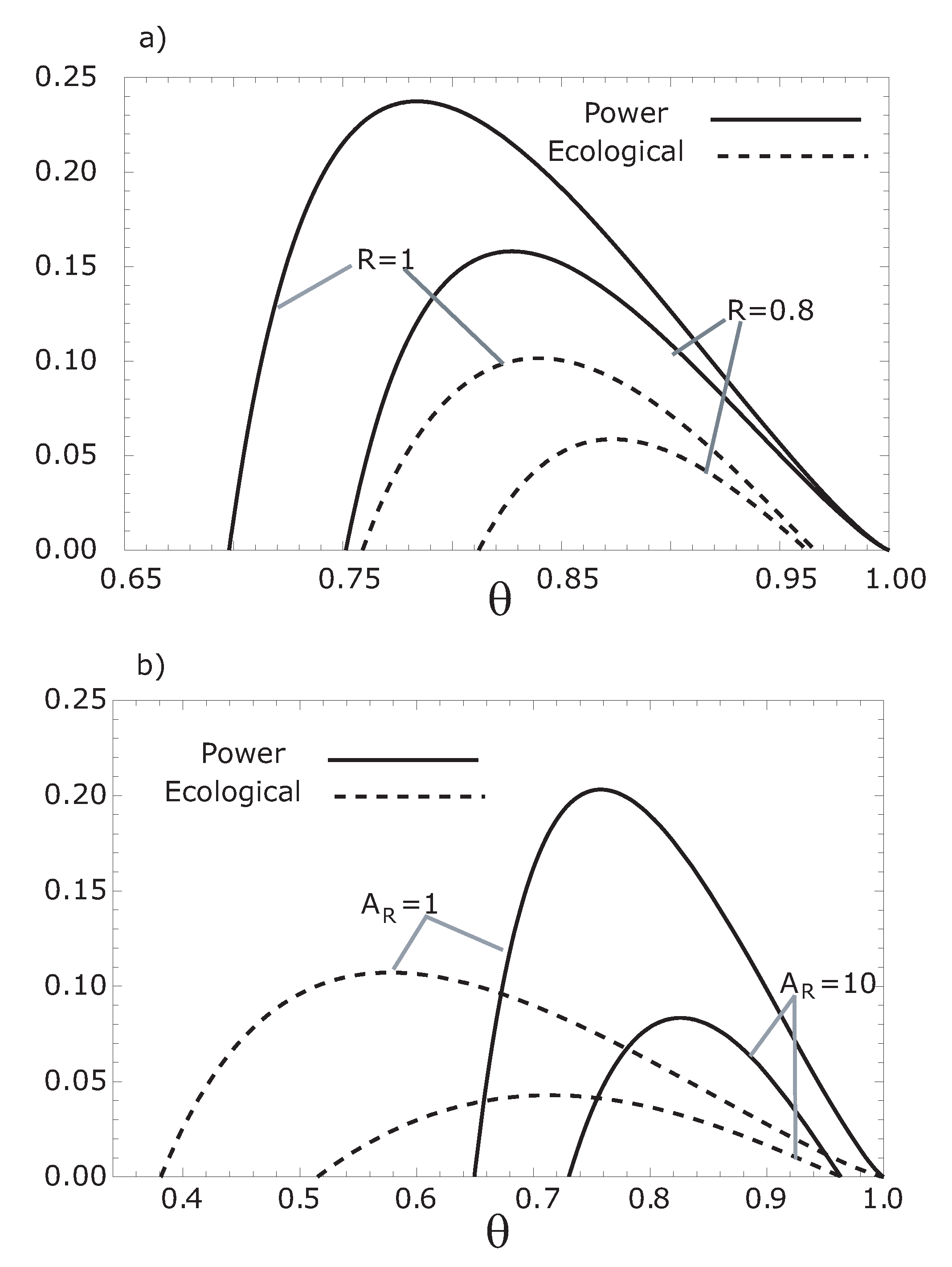
Figure 4.
Comparison of both the thermoeconomic objective functions, and , respect to , for (a) different values of the parameter R and for (b) several values of .
Figure 4.
Comparison of both the thermoeconomic objective functions, and , respect to , for (a) different values of the parameter R and for (b) several values of .
3. Numerical Results and Discussion
We can observe from
Figure 2 and
Figure 3 that the maximum thermoeconomic objective functions (
and
) diminish while the corresponding optimum hot working fluid temperatures shift towards
when the internal irreversibility parameter
R decreases. On the other hand, the thermoeconomic objective function at MER is less than the thermoeconomic objective function at MPR (see
Figure 4). In
Figure 5 and
Figure 6, for both MPR and MER cases, the variation of the dimensionless thermoeconomic objective functions with respect to thermal efficiency for several values of
R,
β,
, and
f are presented. From
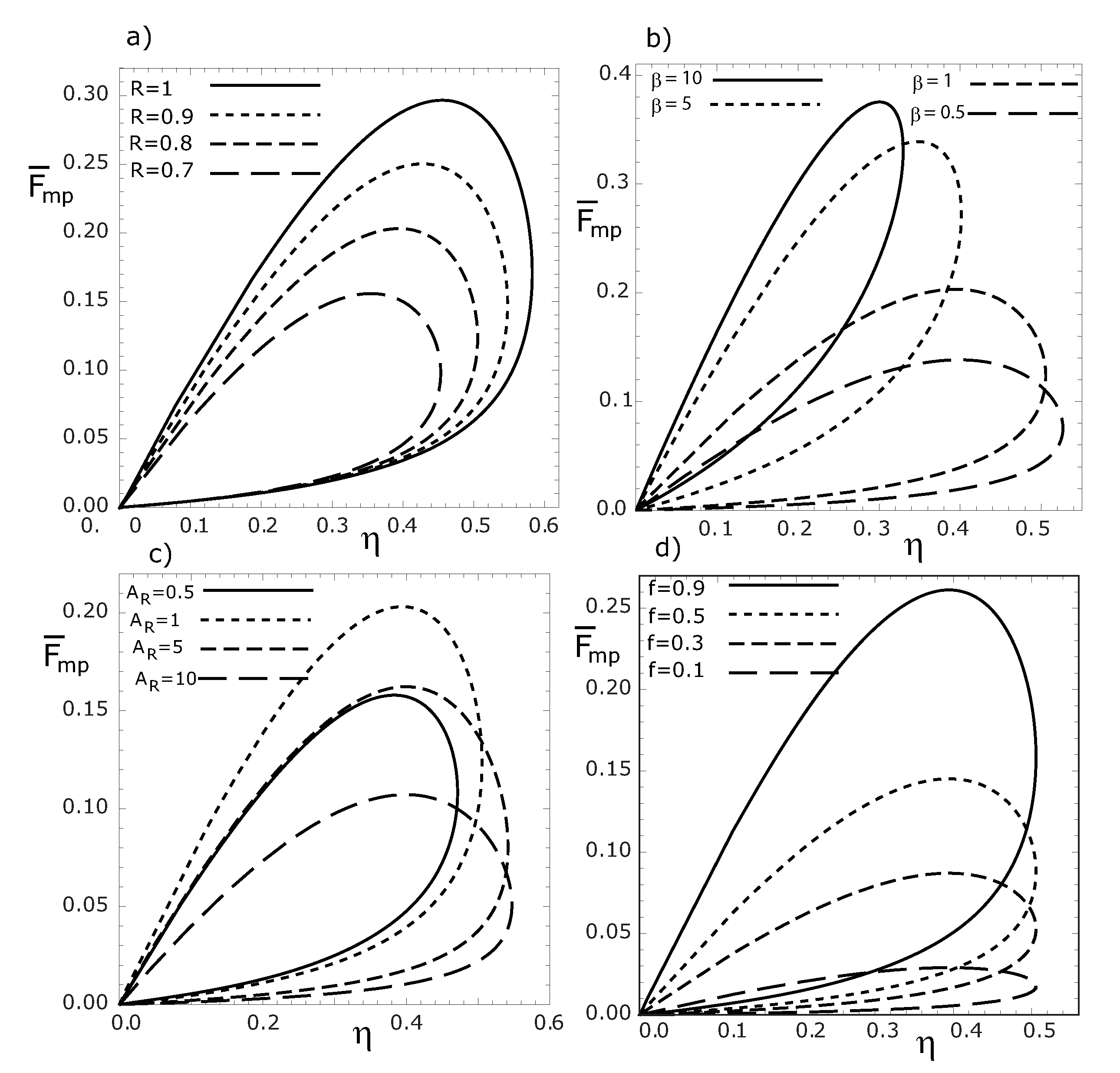
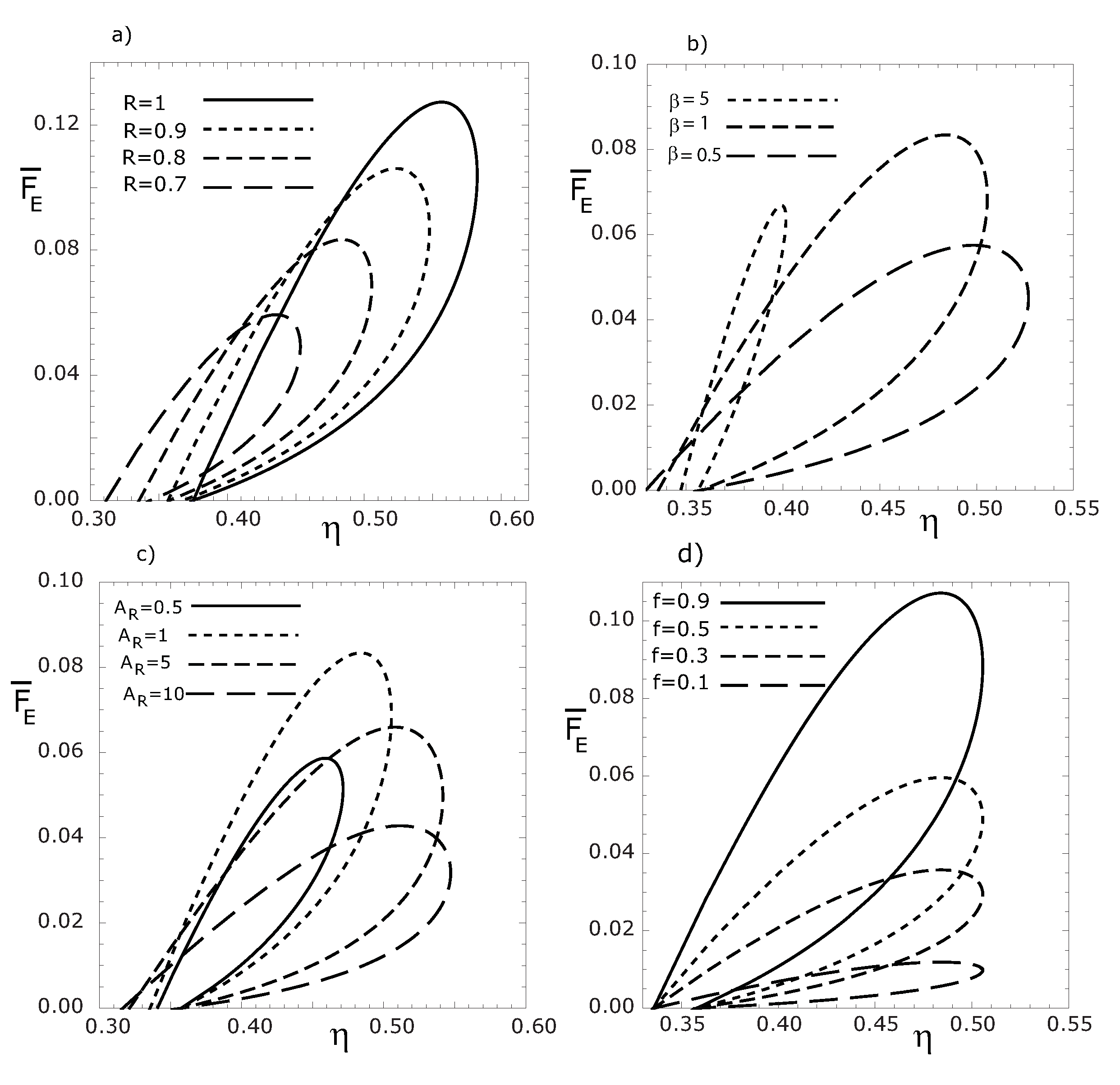
Figure 5a–
Figure 5d, we see that the loop shaped curves become smaller as
f,
β and
decrease. We can also see that the maximum thermal efficiency is independent of
f values, while the maximum
(or
), decreases for decreasing
f values (see
Figure 5d and
Figure 6d).
Figure 5.
Variations of the dimensionless thermoeconomic objective function , with respect to thermal efficiency for various R (a), β (b), (c) and f (d) values, respectively. ().
Figure 5.
Variations of the dimensionless thermoeconomic objective function , with respect to thermal efficiency for various R (a), β (b), (c) and f (d) values, respectively. ().
Figure 6.
Variations of the dimensionless thermoeconomic objective function , with respect to thermal efficiency for various R (a), β (b), (c) and f (d) values, respectively. ().
Figure 6.
Variations of the dimensionless thermoeconomic objective function , with respect to thermal efficiency for various R (a), β (b), (c) and f (d) values, respectively. ().
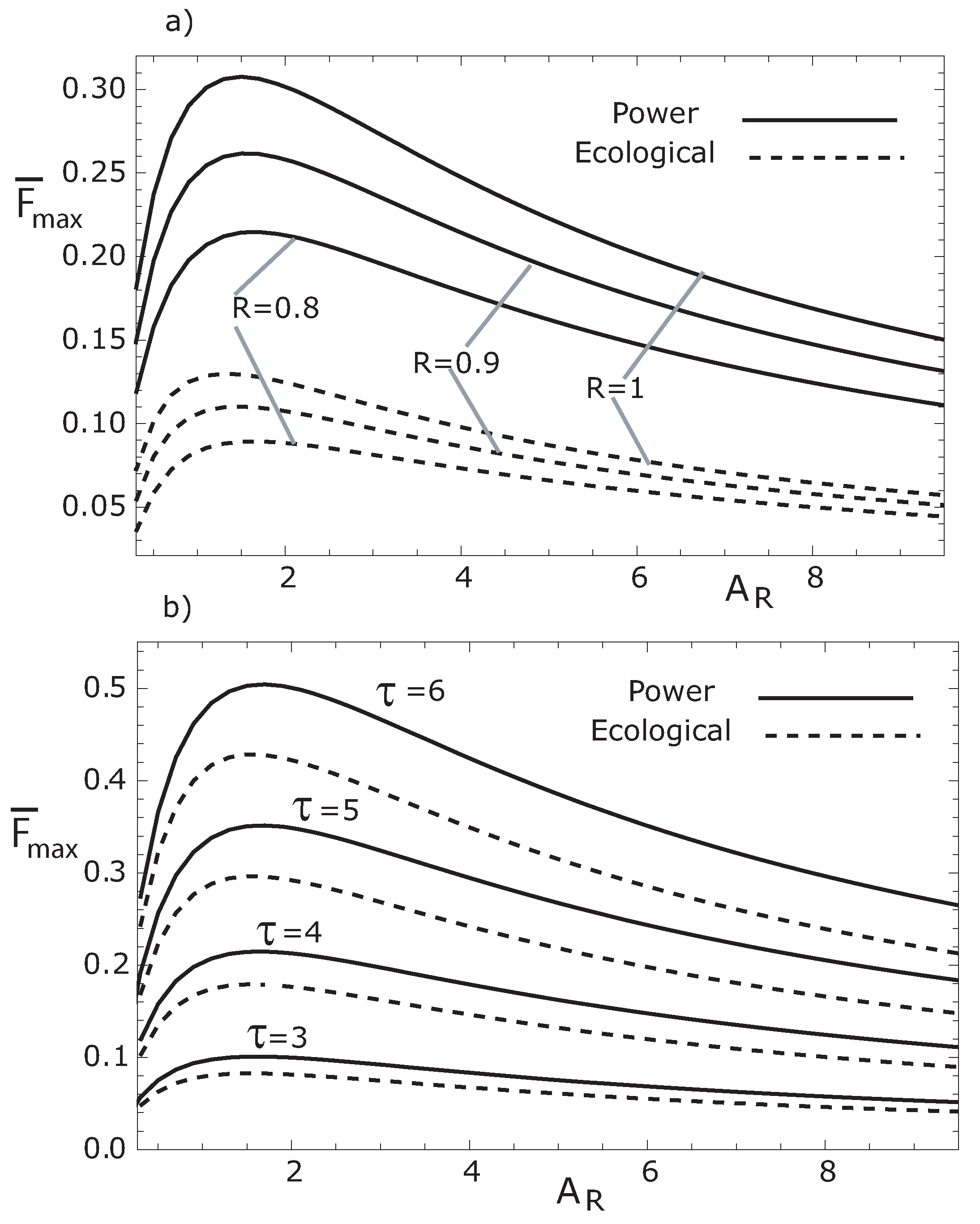
In
Figure 7 we show the variation of the maximum dimensionless thermoeconomic objective functions (
, for both MPR and MER cases) with respect to the ratio
for different values of the parameter
R (see
Figure 7a) and for several values of the temperature ratio
(see
Figure 7b). We can observe in
Figure 7, for both MPR and MER, that as
increases,
increases to its peak value, and then smoothly decreases. We can also observe that
increases and the optimal
value considerably decreases for increasing
R and
τ values.
Figure 7.
Variation of the two maximum thermoeconomic objective functions with respect to for: (a) two values of R with , and (b) for several values of τ with . In both cases, , and .
Figure 7.
Variation of the two maximum thermoeconomic objective functions with respect to for: (a) two values of R with , and (b) for several values of τ with . In both cases, , and .
In
Figure 8, we show the optimal thermal efficiencies for both MPR and MER cases. In this figure, we can observe that the optimal thermal efficiencies under MER (for all values of the parameter
R, see
Figure 8a) and for several values of the parameter
τ, see
Figure 8b) are bigger than the optimal thermal efficiencies at MPR. Besides, these optimal efficiencies satisfy the following inequality:
where the subscripts
C and
refer to Carnot and Curzon-Ahlborn respectively. The previous inequality was recently obtained by Barranco-Jiménez
et al. for the case of an endoreversible model of a solar driven-heat engine [
4].
Figure 8.
Optimal thermal efficiencies vs. for the two regimes. (a) For three values of R with and (b) For three values of τ with . In both cases, , and (these optimal thermal efficiencies were obtained by substituting in Equation (18)).
Figure 8.
Optimal thermal efficiencies vs. for the two regimes. (a) For three values of R with and (b) For three values of τ with . In both cases, , and (these optimal thermal efficiencies were obtained by substituting in Equation (18)).
On the other hand, regarding actual photothermal converters, their thermal efficiencies are not easily available in the technical literature. A reported case is that corresponding to the solar power plant Eurelios at Adrano (Italy) [
7]. The experimental efficiency for this plant is around 0.13, which is smaller than the theoretical value (around 0.68) given by the De Vos model [
7]. For the model of the present work, we can see in
Figure 8 that for
we obtain efficiencies around 0.3, which is bigger than the experimental one, but remarkably smaller than the De Vos value. If we locate the 0.13 point in
Figure 6 (a, b, c or d) it corresponds to a negative ecological function, that is, this plant seemingly has a dissipation larger than its power output. Thus, under the perspective here formulated, some solar power plants have yet a large range of efficiencies to be reached by means of design improvements. As it was asserted by Wu [
30], there are few solar engine data to compare with TTF corresponding models. However, Wu took two solar thermal power generation plants given by Hsieh with efficiency values of 0.36 and 0.37, respectively [
35]. Wu [
30] proposed a simple endoreversible model of the Curzon-Ahlborn type to compare with Hsieh’s data. His numerical comparison is very good and this author concludes that these solar power plants should be operated closer to the CA-efficiency. However, in
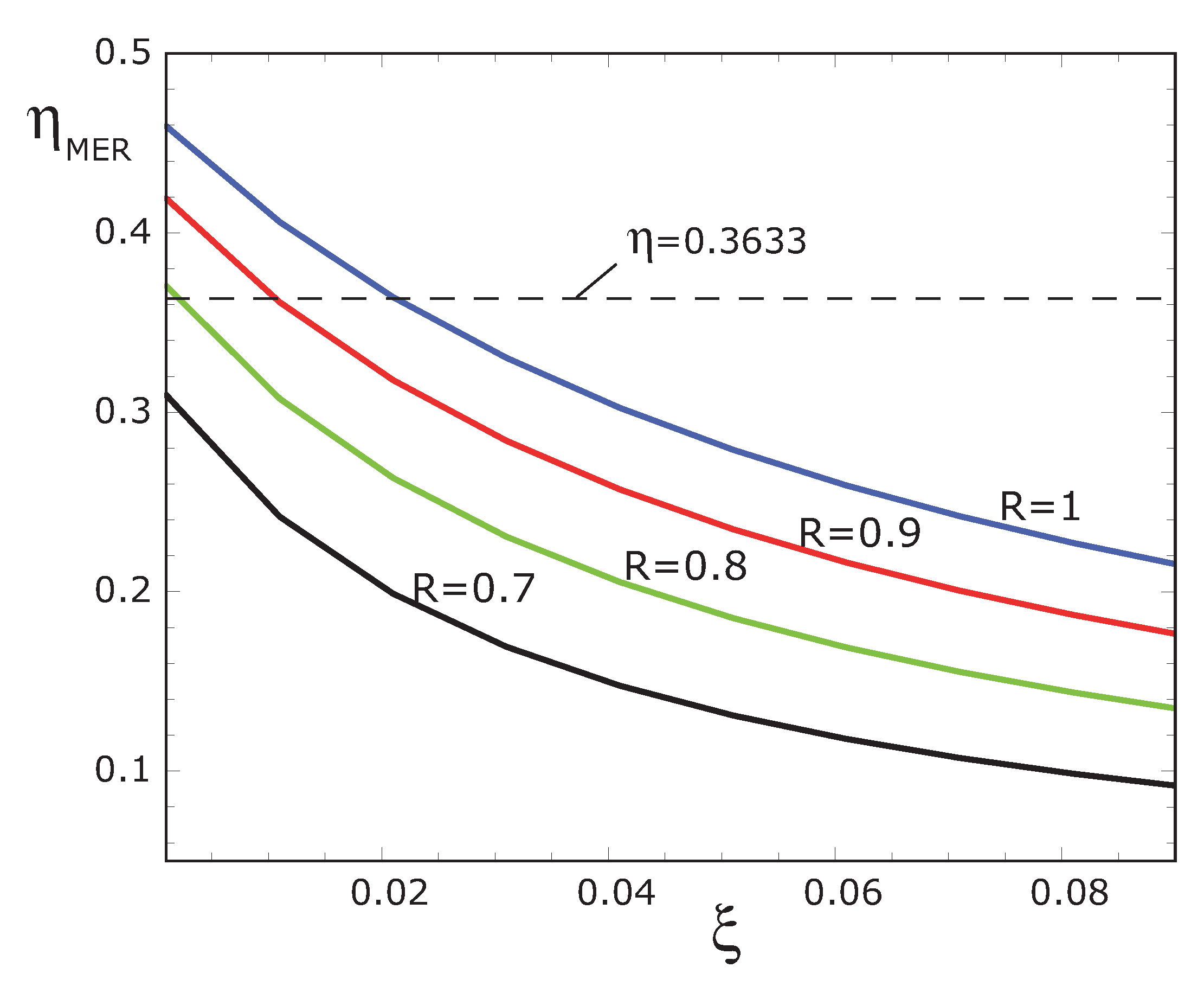
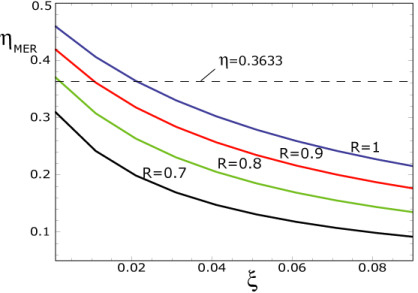
Figure 9 we show a numerical result corresponding to our model working at maximum ecological regime but with several irreversibilities, which in this case are:
,
and
,
, respectively. As it can be seen, we obtain efficiencies very close to the experimental ones but under some more realistic conditions. Thus, this scenario is also possible to describe the performance regime to the Hsieh’s plants.
Figure 9.
Optimal thermal efficiencies
vs. ξ at maximum ecological regime, for four values of
R with
,
and
for comparison with the values reported by Wu [
30].
Figure 9.
Optimal thermal efficiencies
vs. ξ at maximum ecological regime, for four values of
R with
,
and
for comparison with the values reported by Wu [
30].