1. Introduction
The history and development of modern thermodynamics started during the industrialisation period and was closely related to the need to improve the efficiency of steam engines. Since the foundational work of Carnot [
1] in 1824, until 1960, thermodynamics has been a phenomenological theory mainly restricted to the description of either equilibrium states or transformations relating one equilibrium state to another [
2]. In essence, thermodynamics was essentially reduced to thermostatics or quasi-thermostatics.
In 1960, Stückelberg [
3] reformulated thermodynamics in an axiomatic way as a truly dynamic and phenomenological theory describing the evolution of a thermodynamic system by a set of first-order differential equations. By doing so, he actually extended the existing theory of equilibrium states, or thermostatic, in order to obtain a genuine thermodynamic theory,
i.e., a theory describing the evolution and the approach to equilibrium of thermodynamic systems. In his axiomatic approach, he introduced, two state functions, the “energy” and the “entropy” obeying the two fundamental laws of thermodynamics. He then derived from these two laws the equations for the time evolution of the system.
In his words, the state of a thermodynamic system is defined by a set of geometric variables and a set of thermal variables. The simplest system, which he called “system element”, is a system where one thermal variable and a set of geometric variables are sufficient to define entirely the state. The second law of thermodynamics requires the existence of a state function entropy for every system. Thus, the state of a system element is defined by the entropy and a set of geometric variables. We note that the notion of system element is analogous to the concept of point particle in mechanics. It is the building block required to develop the theory of general systems where several thermal variables are necessary to define the state.
In the present work, we essentially follow the original approach of Stückelberg [
3] and apply his formalism to a closed thermodynamic system consisting of
N point particles moving in a fluid. The system is thus defined by the particles and the fluid, but we assume no a priori knowledge of the fluid. It is a phenomenological approach where the theory is built upon the macroscopic description of the particles (which are the only observable objects). It is thus observed that the macroscopical mechanical variables (generalised coordinates and velocities) are not sufficient to obtain the time evolution of the system. Following the thermodynamic approach, we assume the simplest possible case where the system can be described by introducing only one more non-mechanical, or thermodynamical variable, the entropy. Applying the first and second law, we obtain a system of coupled differential equations describing the thermodynamic evolution of the system. In that system of differential equations, the coupling between the resulting Lagrange equations and the thermal equation is due to the viscous friction terms depending on the state variable.
In this phenomenological approach, the theory tells us what are the quantities that should be obtained from experiment, in particular in order to obtain typical thermodynamical properties such as the specific heat. Since we have introduced a model with only one thermal variable (the total entropy), it is not possible to derive transport properties (viscosity or thermal conductivity). To obtain such a transport theory, we would need a continuum description of the fluid (density field, entropy field, ...).
The structure of this publication is as follows. In
Section 2, we briefly recall Stückelberg’s axiomatic formulation of the first and second thermodynamic laws.
Section 3 is devoted to establishing the equations of evolution of a system of point particles based on the first law. In
Section 4, we extend the thermodynamics of a system of point particles to include the second law.
Section 5 shows how thermodynamics relates to mechanics and in particular to the Lagrange equations. Finally, in
Section 6, we apply our formalism to study the thermodynamics of an isolated system consisting of identical point particles interacting through a harmonic potential, which is the simplest phenomenological model of a solid.
3. System of Point Particles: First Law
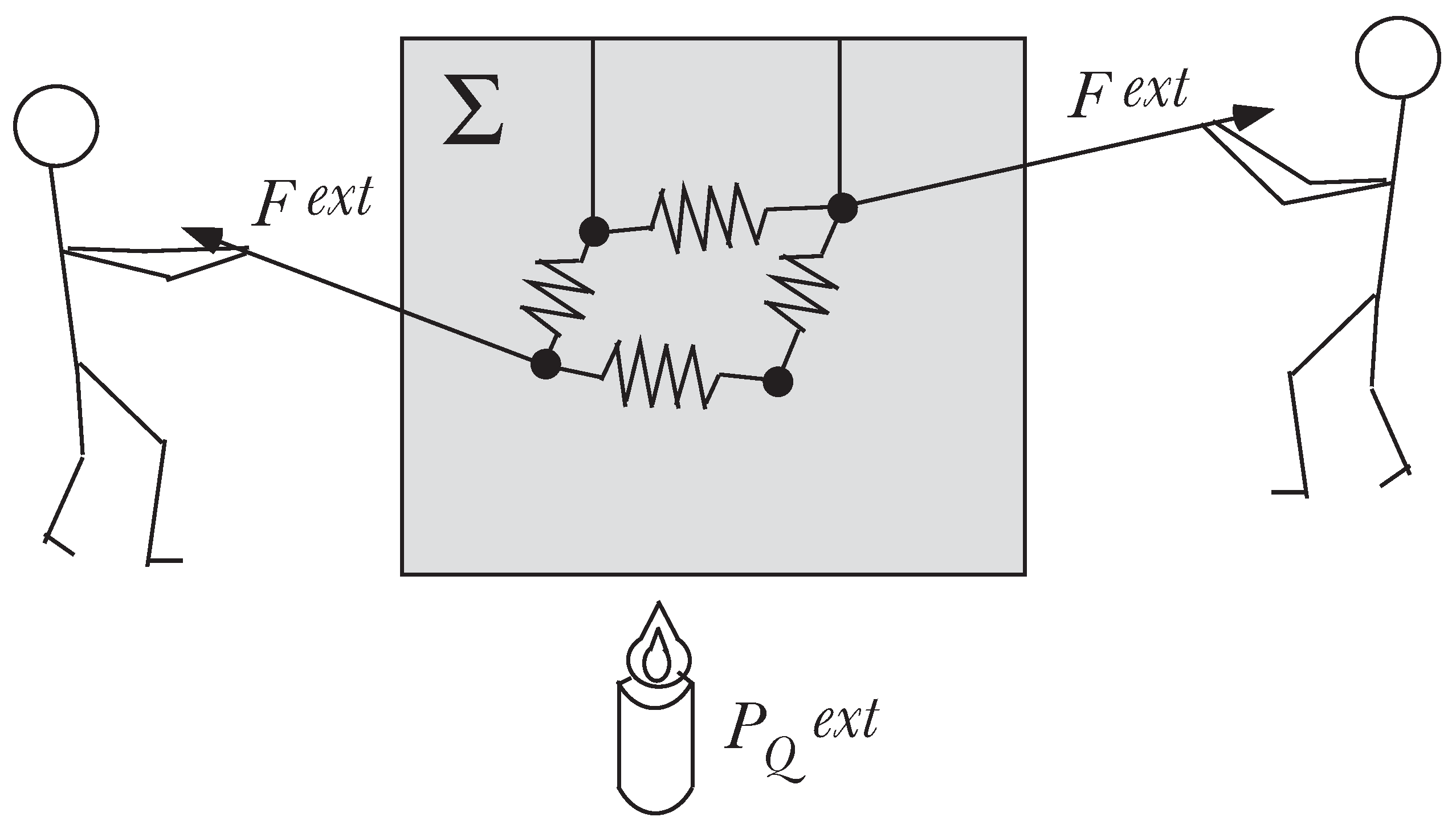
We consider a physical system Σ of
N point particles imbedded in a fluid (e.g., air, water, ...) as shown in
Figure 1. The point particles are submitted to holonomic, time-independent constraints, and we assume no a priori knowledge for the fluid.
The system is thus defined by the particles and the fluid. It is said to be isolated when there is no interaction between Σ and the outside (no external force, no heat exchange, no matter exchange). By definition, the mechanical state of Σ is defined by
independent variables
where
are the generalised coordinates and
, with
, are the generalised velocities. Moreover, under the above condition on the system, the kinetic energy
K for
N point particles of mass
, defined as,
is expressed in terms of the generalised coordinates
and velocities
, by the quadratic form (see [
6] Section 8.6)
Furthermore, the exterior of the system can act on the generalised coordinate by means of a generalised force , and the work done per unit time by this force on Σ is the work-power .
Figure 1.
The system Σ: N particles imbedded in a fluid.
Figure 1.
The system Σ: N particles imbedded in a fluid.
From observations, we conclude that the set of mechanical variables does not entirely define the state of the system and we are forced to introduce thermal variables. To simplify the following discussion, we assume that it is sufficient to add just one thermal variable to define entirely the state. Since in the axiomatic formulation of the second law there exists for any system a thermal observable, the entropy S, we shall define the state by the set of variables , which are all independent by definition of the holonomic constraints.
For our system, we assume that the energy
E, introduced in the first law, is the sum of the kinetic energy (
5) and a potential energy
U independent of the velocities,
i.e.,
The “potential energy”
describes the internal forces but may contain contributions from the outside of Σ, such as potentials of conservative external forces (e.g., the gravitational potential energy due to the earth). In this case, these forces are considered as internal forces of Σ, and not external.
Since the system is closed,
i.e., there is no exchange of matter with the outside, the first law of thermodynamics reduces to,
Using the fact that
, the LHS side of the first law (
7) gives,
At this point, we must insist on the fact that the choice of general coordinates was completely arbitrary. Therefore, we want to impose the covariance of the time evolution equations,
i.e., they must have the same structure for any coordinate transformation of the mechanical variables,
Under the covariance requirement,
must be a second order, symmetrical, covariant tensor so that
K is a scalar. In order to ensure that
K is positive definite,
must have a positive definite signature. Moreover, one can easily check that
is not covariant. However, the second term on the RHS of (8) is totally symmetric and invariant under cyclic permutation of indices, so that we have,
Introducing the symbols,
the LHS of the first law (
7) reduces to the covariant equation,
As for the RHS of (
7), we have [
6],
where
is the external generalised force associated with
.
It is useful to introduce two new state functions, defined respectively as,
where
is the internal force associated with the generalised coordinate
and
is called the “temperature” [
3]. Using these definitions, the first law (
7) reduces to the thermodynamic equation,
5. From Thermodynamics to Mechanics
The symmetric matrix
can be identified as a metric with a positive definite signature on the configuration space. Thus, the configuration space is a Riemannian manifold endowed with a torsion-free Levi-Civita connection [
11], where the components,
are commonly referred to as the Riemann-Christoffel symbols. Such a manifold preserves the generalised infinitesimal distances squared
defined in terms of the generalised coordinates as [
12],
and satisfies the metricity condition [
13], which requires the covariant derivative of the metric with respect to every coordinate
to vanish,
In the particular case of an isolated system where the friction coefficients matrix is strictly zero, then from the second relation in (
26), the entropy is necessarily constant. Furthermore, if the potential energy is zero,
i.e.,
, then the evolution of the system is a geodesic in configuration space given by,
thus satisfying the 1st law of Newton.
At this point, it is convenient to introduce the Lagrangian of the system defined as,
In order to recast the dynamical terms on the RHS of the thermodynamic equation (
17) in an analytical manner, we compute the partial derivatives of the Lagrangian and their time derivatives,
From the differential relations (
42) and (43), we derive the dynamic identity,
Using this identity, the thermodynamic equation (
35) reduces to,
In conclusion, if the state functions
U and
are independent of
S, or equivalently, if the time evolution happens at fixed temperature by contact with a thermal bath, the mechanical Lagrange equations decouple from the thermal equation. This is the usual case considered in mechanics.
6. Thermodynamics of an Isolated System of Point Particles Interacting through a Harmonic Potential
As an application of the formalism we developed, we now consider the thermodynamics of an isolated system where the state functions
U and
are independent of
S. The system consists of identical point particles interacting through a harmonic potential and is the simplest phenomenological model of a solid, where the harmonic oscillators represent the phonons. As generalised coordinates, we choose for simplicity cartesian coordinates of the point particles and denote them
. Thus, the metric reduces to the trivial Kronecker delta,
i.e.,
. The kinetic energy
of the point particles per unit mass and the harmonic interaction potential per unit mass
are respectively given by,
where the coefficient
is positive by the equilibrium condition of the second law and
ω represents the angular frequency of the identical harmonic oscillators. The Lagrangian of the system is given by,
The partial derivatives of the Lagrangian and their time derivatives are given by,
Finally, the system of coupled thermodynamical equations (26) is explicitly found to be,
The physical interpretation of these evolution equations is clear [
6]. The Lagrange equations are a system of coupled damped harmonic oscillators where the damping term is due to the action of a viscous friction force. These equations are in turn coupled to the thermal equation through the friction force. Since
and
do not depend on
S, the Lagrange equations can be solved independently to find
, which in turn will give
using the thermal equation.
If the friction matrix
is positive, the condition that the system evolves to a state of maximal entropy implies that the system evolves towards the equilibrium state (
,
). For a strictly mechanical system,
i.e., if
, then
S is a constant; in this case the system will oscillate around the equilibrium state according to,