On the Role of Entropy Generation in Processes Involving Fatigue
Abstract
:1. Introduction
2. Thermodynamics of Fatigue
2.1. Entropy Balance Equation
2.2. Entropy Generation Approach to Fatigue Failure
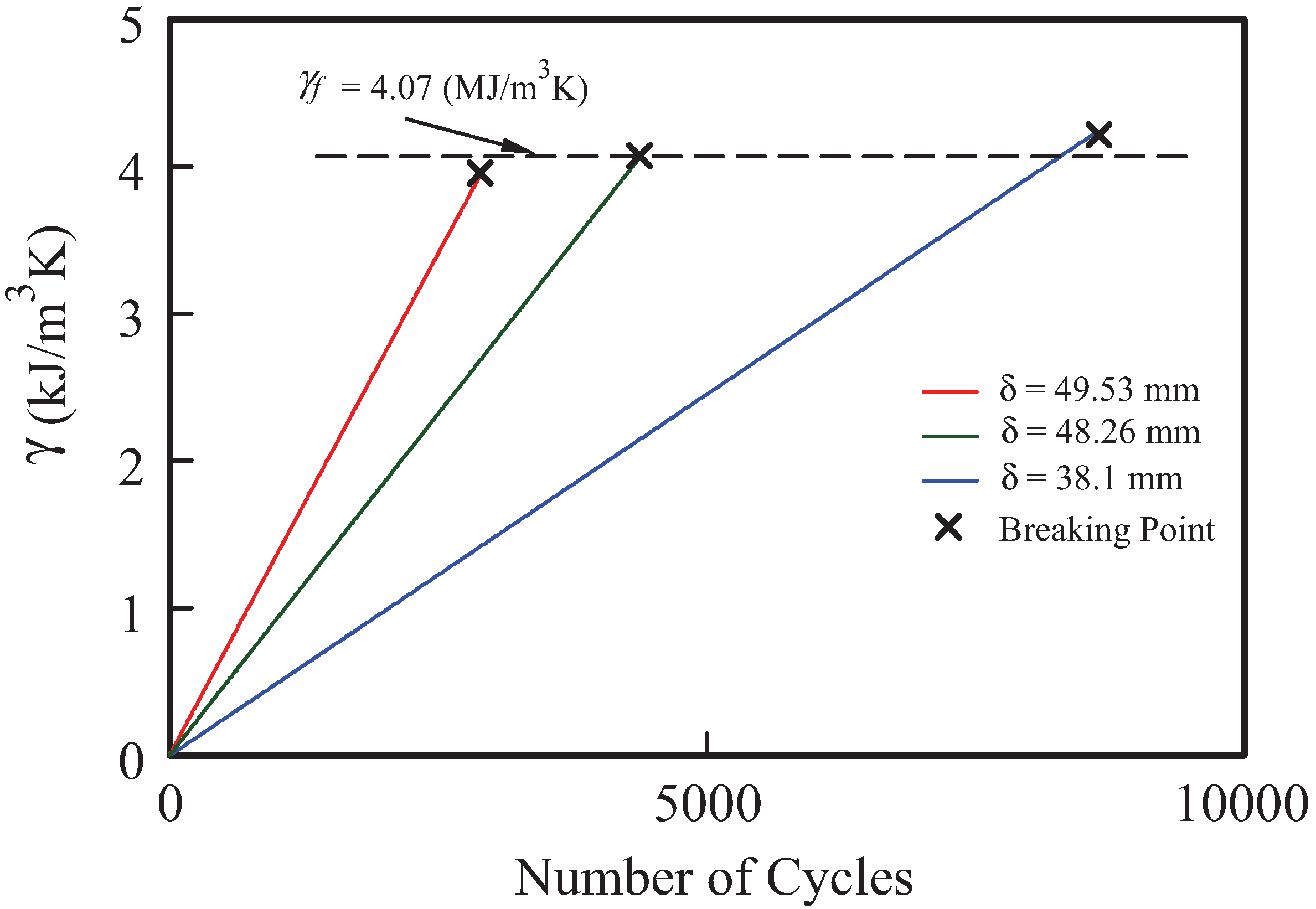
2.3. Application to Fatigue Life Prediction (Coffin-Manson Equation)
2.4. Application to Variable Load Amplitude (Miner’s Rule)
2.5. Degradation Coefficient (DEG Theorem)
2.6. Application to Paris-Erdogan Law
3. Conclusions
References
- Basaran, C.; Yan, C.Y. A thermodynamic framework for damage mechanics of solder joints. J. Elect. Pack. 1998, 120, 379–384. [Google Scholar] [CrossRef]
- Basaran, C.; Nie, S. An irreversible thermodynamics theory for damage mechanics of solids. Int. J. Damage Mech. 2004, 13, 205–223. [Google Scholar] [CrossRef]
- Lemaitre, J.; Chaboche, J.L. Mechanics of Solid Materials, 1st ed.; University Press: Cambridge, UK, 1990. [Google Scholar]
- Amiri, M.; Naderi, M.; Khonsari, M.M. An experimental approach to evaluate the critical damage. Int. J. Damage Mech. 2011, 20, 89–112. [Google Scholar] [CrossRef]
- Voyiadjis, G.Z.; Faghihi, D. Thermo-Mechanical Strain gradient plasticity with energetic and dissipative length scales. Int. J. Plasticity 2011. [Google Scholar] [CrossRef]
- Halford, G.R. The energy required for fatigue. J. Mater. 1966, 1, 3–18. [Google Scholar]
- Naderi, M.; Amiri, M.; Khonsari, M.M. On the thermodynamic entropy of fatigue fracture. Proc. R. Soc. A 2010, 466, 423–438. [Google Scholar] [CrossRef]
- Naderi, M.; Khonsari, M.M. An experimental approach to low-cycle fatigue damage based on thermodynamic entropy. Int. J. Solids Struct. 2010, 47, 875–880. [Google Scholar] [CrossRef]
- Naderi, M.; Khonsari, M.M. A thermodynamic approach to fatigue damage accumulation under variable loading. Mater. Sci. Eng. A 2010, 527, 6133–6139. [Google Scholar] [CrossRef]
- Ital’yantsev, Y.F. Thermodynamic state of deformed solids. Report 1. Determination of local function of state. Strength Mater. 1984, 16, 238–241. [Google Scholar] [CrossRef]
- Whaley, P.W. A thermodynamic approach to metal fatigue. In Proceedings of ASME International Conference on Advances in Life Prediction Methods, Albany, NY, USA, 18–20 April 1983; pp. 18–21.
- Morrow, J.D. Cyclic plastic strain energy and fatigue of metals, internal friction, damping, and cyclic plasticity. ASTM STP 1965, 378, 45–84. [Google Scholar]
- Amiri, M.; Khonsari, M.M. Rapid determination of fatigue failure based on the temperature: Fully reversed bending load. Int. J. Fatigue 2010, 32, 382–389. [Google Scholar] [CrossRef]
- Amiri, M.; Khonsari, M.M. Life prediction of metals undergoing fatigue load based on temperature evolution. Mater. Sci. Eng. A 2010, 527, 1555–1559. [Google Scholar] [CrossRef]
- Bryant, M.D.; Khonsari, M.M.; Ling, F.F. On the thermodynamics of degradation. Proc. R. Soc. A 2008, 464, 2001–2014. [Google Scholar] [CrossRef]
- Bodner, S.R.; Davidson, D.L.; Lankford, J. A description of fatigue crack growth in terms of plastic work. Eng. Frac. Mech. 1983, 17, 189–191. [Google Scholar] [CrossRef]
- Carpinteri, A.; Paggi, M. Self-similarity and crack growth instability in the correlation between the Paris’ constants. Eng. Frac. Mech. 2007, 74, 1041–1053. [Google Scholar] [CrossRef]
- Carpinteri, A.; Paggi, M. A Unified interpretation of power laws in fatigue and the analytical correlations between cyclic properties of engineering materials. Int. J. Fatigue 2009, 31, 1524–1531. [Google Scholar] [CrossRef]
- Paggi, M. Modeling fatigue in quasi-brittle materials with incomplete self-similarity concepts. RILEM Mater. Struct. 2011, 44, 659–670. [Google Scholar] [CrossRef]
- Ciavarella, M.; Paggi, M.; Carpinteri, A. One, no one, and one hundred thousand crack propagation laws: A generalized Barenballt and Botvia dimensional analysis approach to fatigue crack growth. J. Mech. Phys. Solids. 2008, 56, 3416–3432. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amiri, M.; Khonsari, M.M. On the Role of Entropy Generation in Processes Involving Fatigue. Entropy 2012, 14, 24-31. https://doi.org/10.3390/e14010024
Amiri M, Khonsari MM. On the Role of Entropy Generation in Processes Involving Fatigue. Entropy. 2012; 14(1):24-31. https://doi.org/10.3390/e14010024
Chicago/Turabian StyleAmiri, Mehdi, and M. M. Khonsari. 2012. "On the Role of Entropy Generation in Processes Involving Fatigue" Entropy 14, no. 1: 24-31. https://doi.org/10.3390/e14010024



