Physical Properties of High Entropy Alloys
Abstract
:1. Introduction
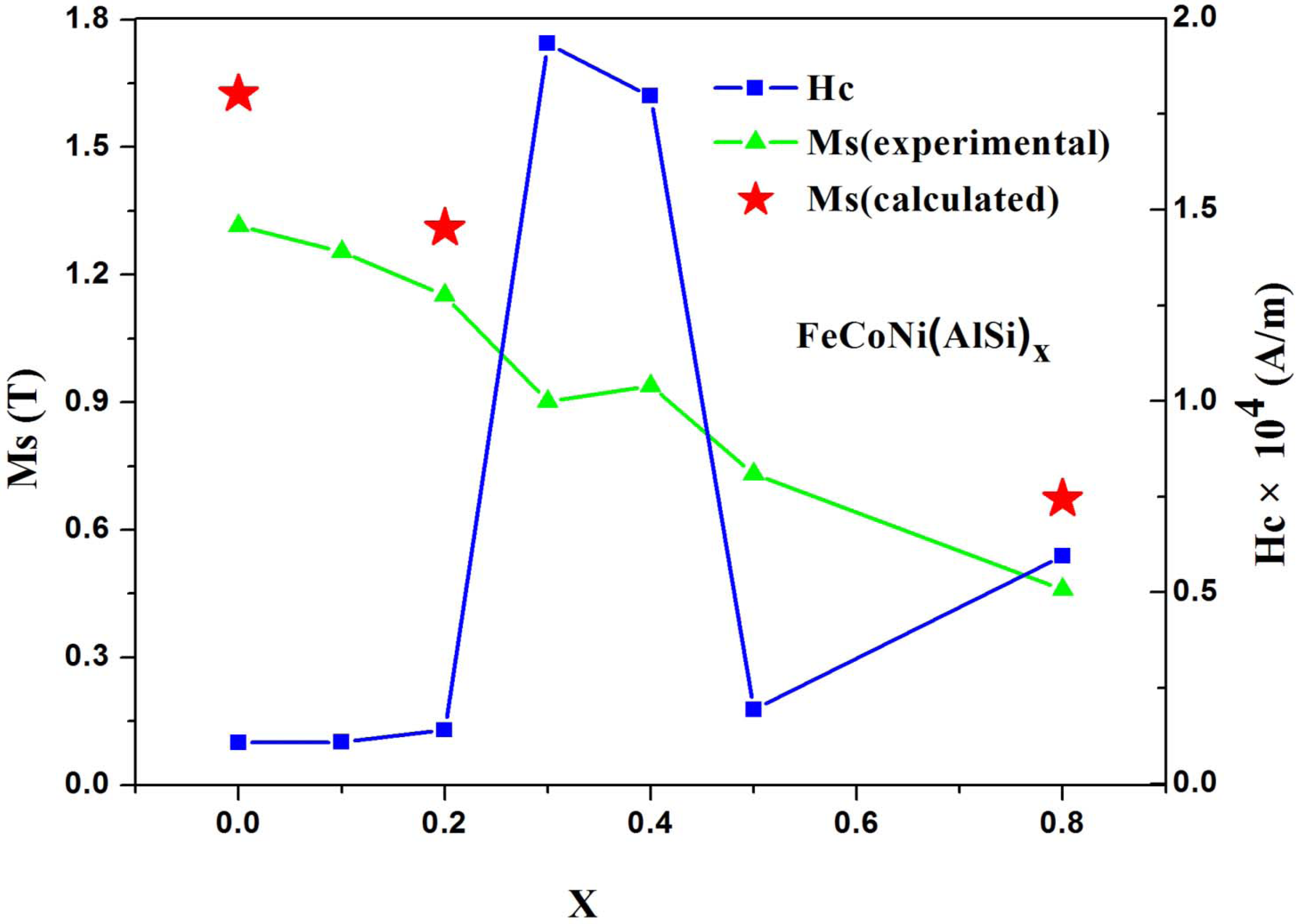
2. Magnetic Properties
| Alloy | Phase | Ms (emu/g) | Ms (emu/cc) | Hc (Oe) |
|---|---|---|---|---|
| FeCoNi [24] | FCC | 1047 | 13 | |
| FeCoNiCr [18,19] | FCC | Paramag. | Paramag. | |
| FeCoNiCrCu [16] | FCC1+FCC(Cu-rich) | Paramag. | Paramag. | |
| FeCoNiCrAl [18,22] | BCC+B2 | 65 | 462 | 52 |
| FeCoNiCrAlCu [17,19,20] | BCC+B2+FCC(Cu-rich) | 38–46 | 281–340 | 45 |
| FeCoNiCrAlTi [23] | BCC+FCC+FeTi+Co2Ti | 15 | 100 | 226 |
| FeCoNiCrPd [19] | FCC | 33 | 296 | N/A |
| FeCoNiCrTi [23] | FCC+BCC+Co2Ti | 5 | 37 | 20 |
| FeCoNiAl0.2Si0.2 [24] | BCC+unknown phase | 915 | 18 |
3. Electrical Properties
| Category | Composition/Alloy | Electrical Resistivity (μΩ-cm) | Reference | Thermal conductivity (W/m K) | Reference |
|---|---|---|---|---|---|
| High Entropy Alloy | CoCrFeNi | 142 | [18] | 12 | [28] |
| AlCoCrFeNi | 221 | 11 | |||
| Al2CoCrFeNi | 211 | 16 | |||
| Pure Element | Al | 3 | [29,30] | 237 | [31] |
| Fe | 10 | 80 | |||
| Ni | 7 | 91 | |||
| Ti | 42 | 22 | |||
| Cu | 2 | 398 | |||
| Conventional Alloy | 7075 Al alloy | 6 | [30] | 121 | [30,31] |
| Low Carbon Steel | 17 | 52 | |||
| 304 Stainless Steel | 69 | 15 | |||
| Inconel 718 | 125 | 11 | |||
| Ti-6Al-4V | 168 | 6 | |||
| Bulk Metallic Glass | Zr41Ti14Cu12.5Ni10Be22.5 | 171 | [32] | N/A | N/A |
| Fe78Si9B13 | 137 | [33] | N/A | N/A | |
| Co63Fe9Zr8B20 | 188 | [33] | N/A | N/A |
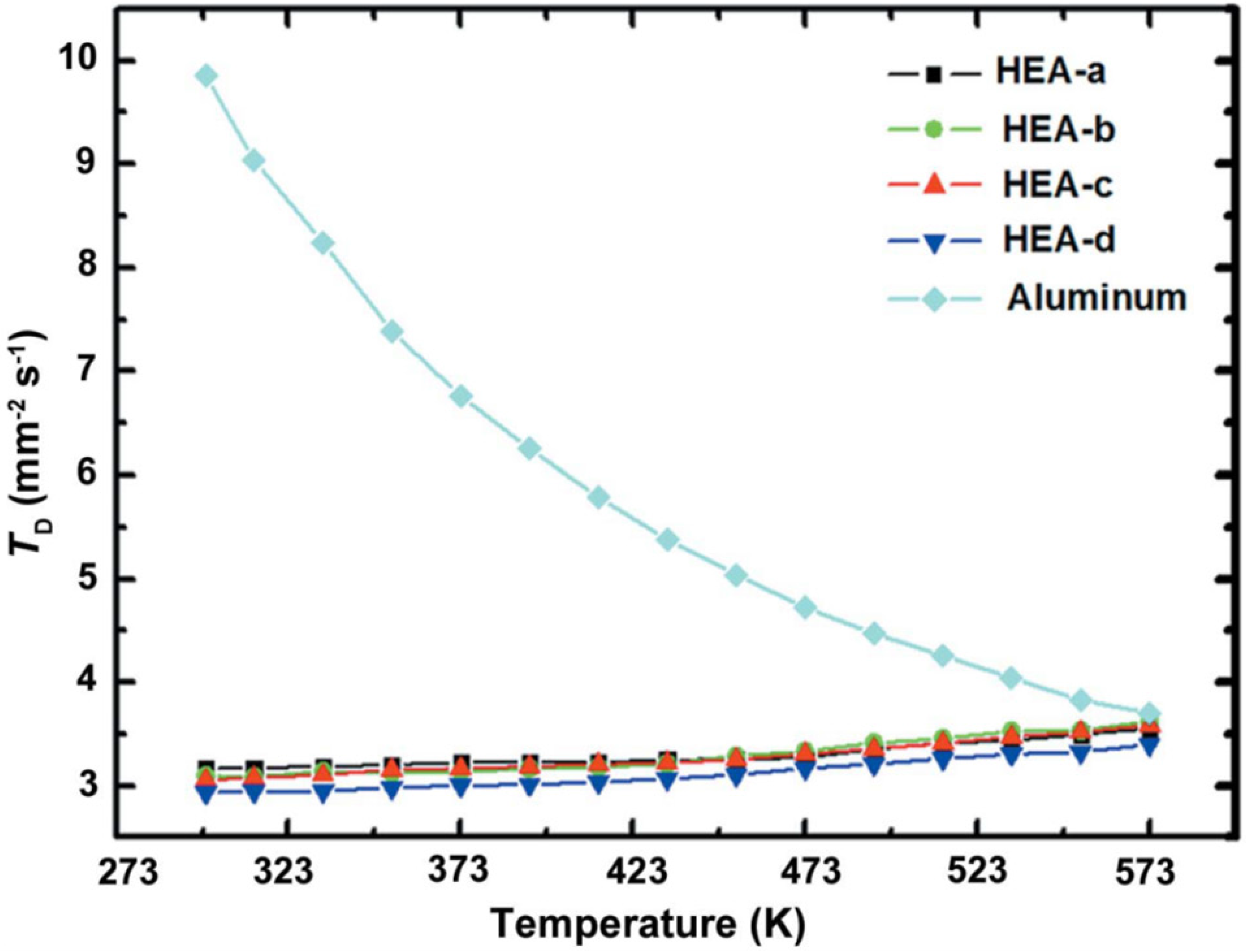
4. Thermal Properties
5. Conclusions and Remarks
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Annales de Chimie Science des Matériaux. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Lin, S.J.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Wen, L.H.; Kou, H.C.; Li, J.S.; Chang, H.; Xue, X.Y.; Zhou, L. Effect of aging temperature on microstructure and properties of AlCoCrCuFeNi high-entropy alloy. Intermetallics 2009, 17, 266–269. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Synthesis and properties of multiprincipal component AlCoCrFeNiSix alloys. Mater. Sci. Eng. A 2010, 527, 7210–7214. [Google Scholar] [CrossRef]
- Chen, S.T.; Tang, W.Y.; Kuo, Y.F.; Chen, S.Y.; Tsau, C.H.; Shun, T.T.; Yeh, J.W. Microstructure and properties of age-hardenable AlxCrFe1.5MnNi0.5 alloys. Mater. Sci. Eng. A 2010, 527, 5818–5825. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Tsai, C.W.; Yang, N.H.; Chang, S.Y.; Yeh, J.W.; Chen, S.K.; Lin, S.J. Intrinsic surface hardening and precipitation kinetics of Al0.3CrFe1.5MnNi0.5 multi-component alloy. J. Alloys Compd. 2013, 551, 12–18. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Wang, W.R.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. On the superior hot hardness and softening resistance of AlCoCr(x)FeMo(0.5)Ni high-entropy alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Tsai, M.H.; Wang, C.W.; Tsai, C.W.; Shen, W.J.; Yeh, J.W.; Gan, J.Y.; Wu, W.W. Thermal Stability and Performance of NbSiTaTiZr High-Entropy Alloy Barrier for Copper Metallization. J. Electrochem. Soc. 2011, 158, H1161–H1165. [Google Scholar] [CrossRef]
- Chou, Y.L.; Wang, Y.C.; Yeh, J.W.; Shih, H.C. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the structure and properties evolution of the CoCrFeNiCuAl high-entropy alloy. J. Alloys Compd. 2010, 502, 295–299. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, S.K.; Chen, T.J.; Chu, P.C.; Yeh, J.W.; Lin, S.J. Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mauger, L.; Munoz, J.A.; Xiao, Y.M.; Sheets, A.O.; Semiatin, S.L.; Horwath, J.; Turgut, Z. Magnetic and vibrational properties of high-entropy alloys. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Kiefer, K.; Siemensmeyer, K.; Banhart, J. Effect of decomposition of the Cr-Fe-Co rich phase of AlCoCrCuFeNi high entropy alloy on magnetic properties. Ultramicroscopy 2011, 111, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, J.B.; Li, J.C.; Jiang, Q. Microstructure and Magnetic Properties of FeNiCuMnTiSnx High Entropy Alloys. Adv. Eng. Mater. 2012, 14, 919–922. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y. Effects of annealing treatment on properties of CoCrFeNiTiAlx multi-component alloys. Intermetallics 2012, 28, 34–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-entropy Alloys with High Saturation Magnetization, Electrical Resistivity, and Malleability. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.Y.; Varga, L.K.; Chen, N.X.; Delczeg, L.; Vitos, L. Ab initio investigation of high-entropy alloys of 3d elements. Phys. Rev. B 2013, 87. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Chen, S.K.; Kao, Y.F. Near-constant resistivity in 4.2–360 K in a B2 Al2.08CoCrFeNi. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 <= x <= 2) high-entropy alloys. Mater. Sci. Eng. B 2009, 163, 184–189. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003.
- Gale, W.F.; Totemeier, T.C. (Eds.) Smithells Metals Reference Book, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2004.
- Shackelford, J.F.; Alexander, W. (Eds.) CRC Materials Science and Engineering Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000.
- Li, Y.; Bai, H.Y. Superconductivity in a representative Zr-based bulk metallic glass. J. Non-Cryst. Solids 2005, 351, 2378–2382. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Lu, C.L.; Lu, S.Y.; Yeh, J.W.; Hsu, W.K. Thermal expansion and enhanced heat transfer in high-entropy alloys. J. Appl. Crystallogr. 2013, 46, 736–739. [Google Scholar] [CrossRef]
- Lucas, M.S.; Belyea, D.; Bauer, C.; Bryant, N.; Michel, E.; Turgut, Z.; Leontsev, S.O.; Horwath, J.; Semiatin, S.L.; McHenry, M.E.; Miller, C.W. Thermomagnetic analysis of FeCoCrxNi alloys: Magnetic entropy of high-entropy alloys. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, M.-H. Physical Properties of High Entropy Alloys. Entropy 2013, 15, 5338-5345. https://doi.org/10.3390/e15125338
Tsai M-H. Physical Properties of High Entropy Alloys. Entropy. 2013; 15(12):5338-5345. https://doi.org/10.3390/e15125338
Chicago/Turabian StyleTsai, Ming-Hung. 2013. "Physical Properties of High Entropy Alloys" Entropy 15, no. 12: 5338-5345. https://doi.org/10.3390/e15125338




