Sleep Stage Classification Using EEG Signal Analysis: A Comprehensive Survey and New Investigation
Abstract
:1. Introduction
1.1. Background and Motivation
1.2. Contribution and Paper Organization
2. Structure and Materials
2.1. General Structure of ASSC
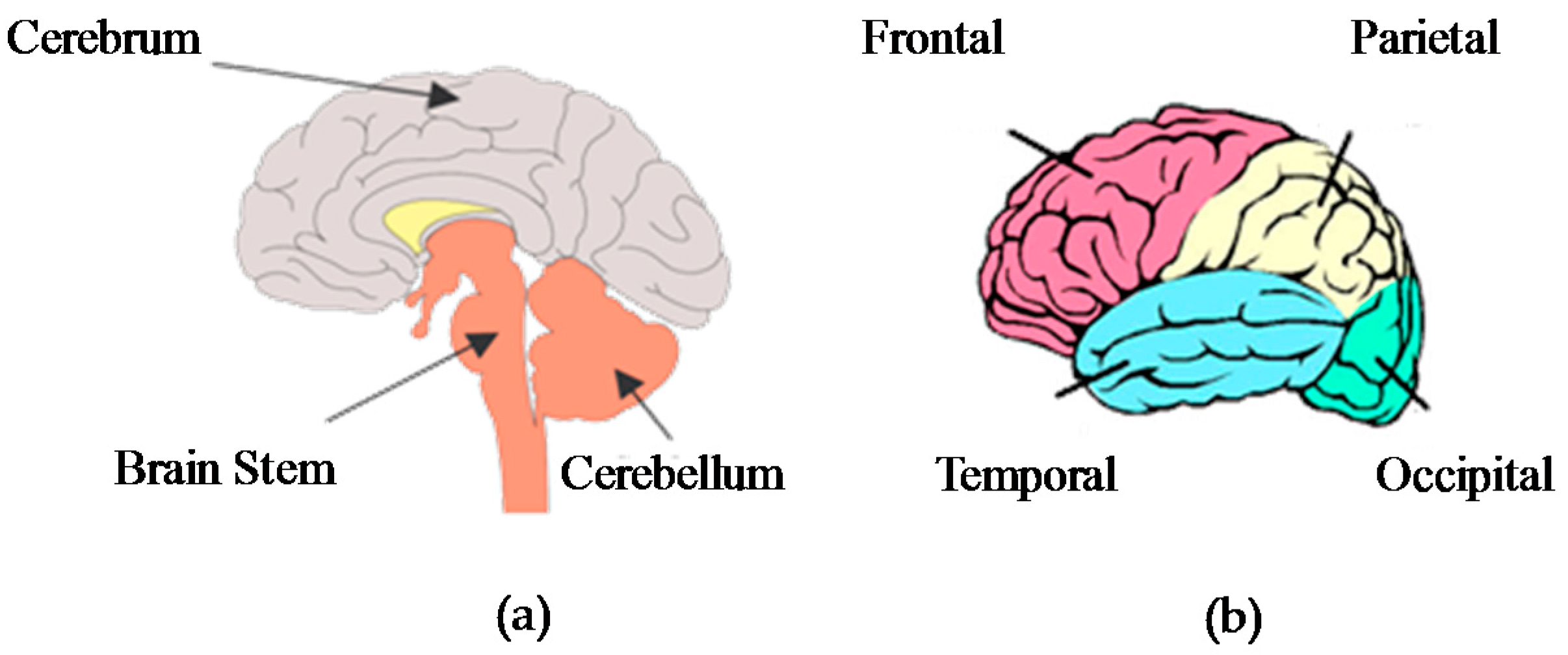
2.2. Electroencephalogram
3. State of the Art
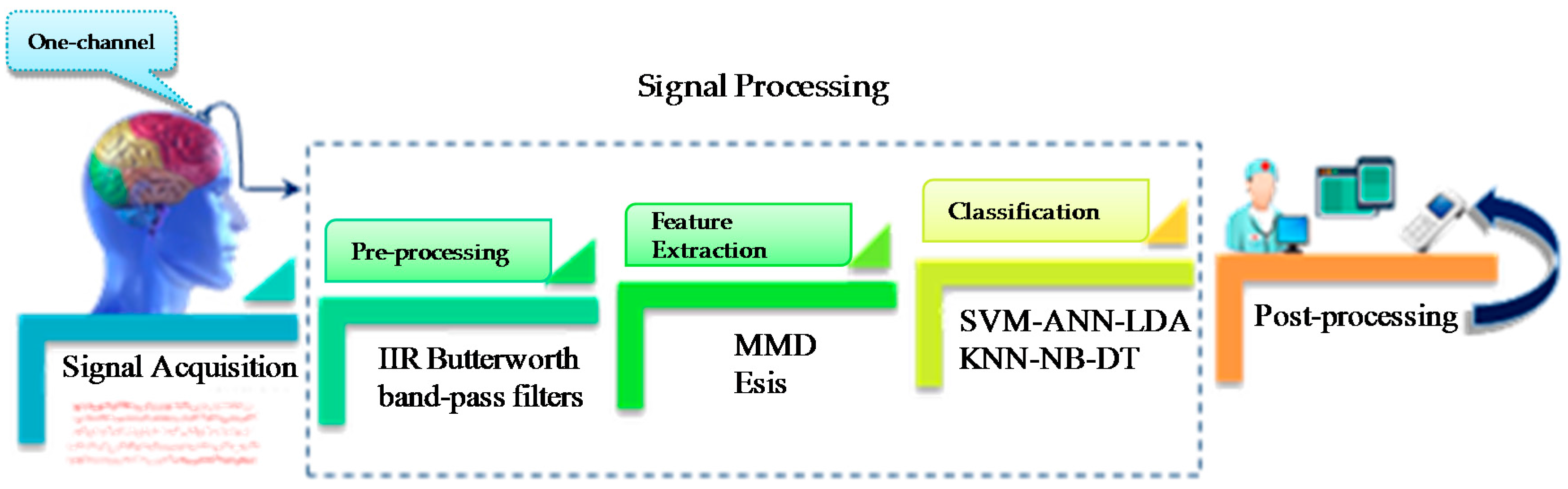
4. Proposed Method and Procedures
4.1. Input EEG Signal
4.2. Pre-Processing
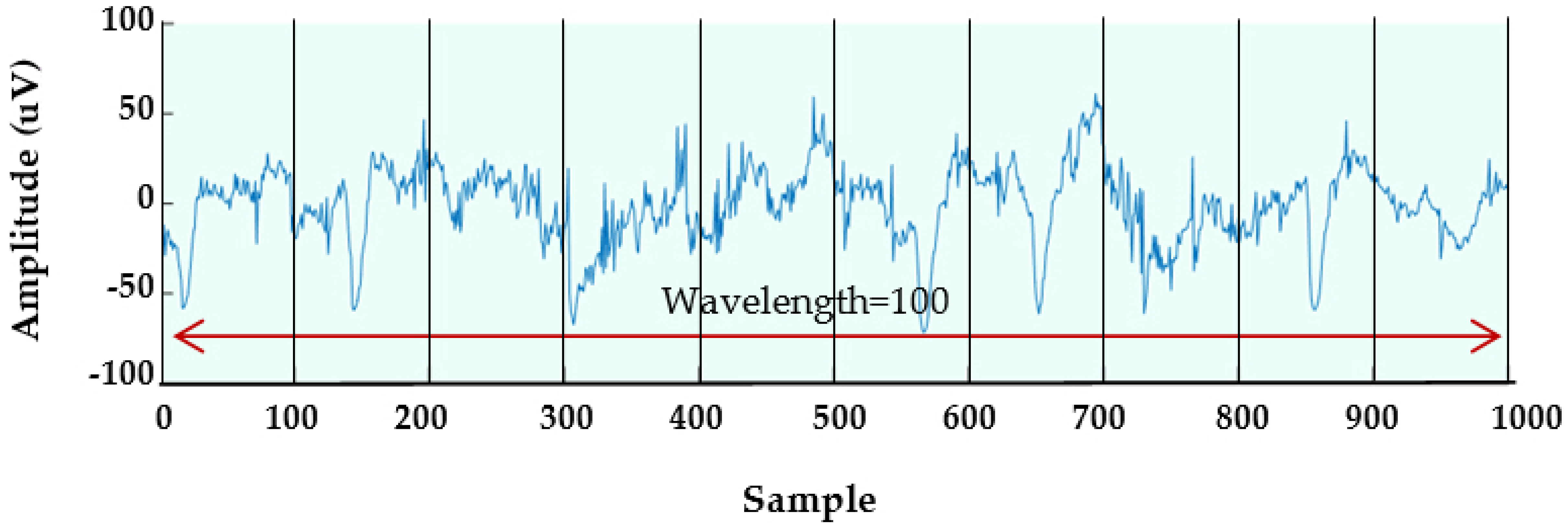
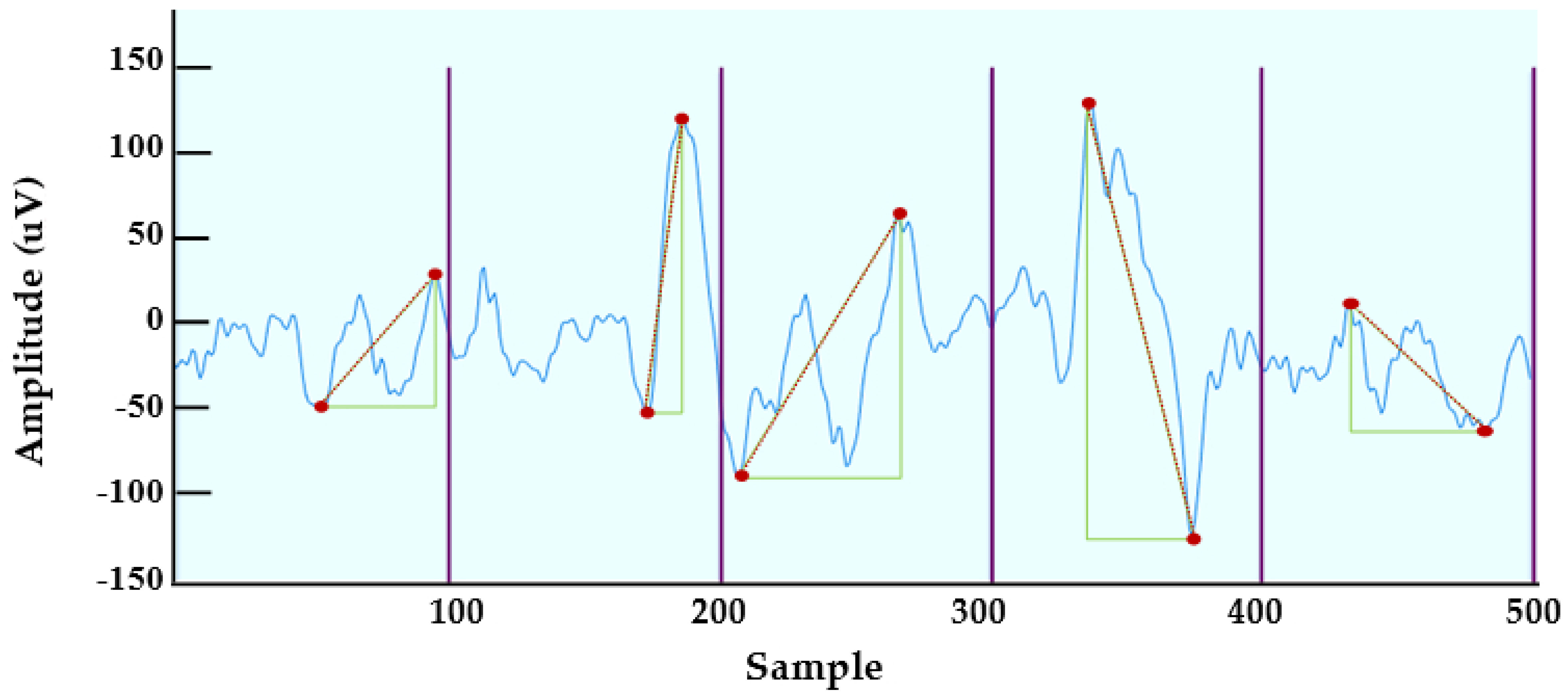
4.3. New Feature Extraction
4.4. Machine Learning and Classification
5. Discussion
5.1. Signal Pre-Processing
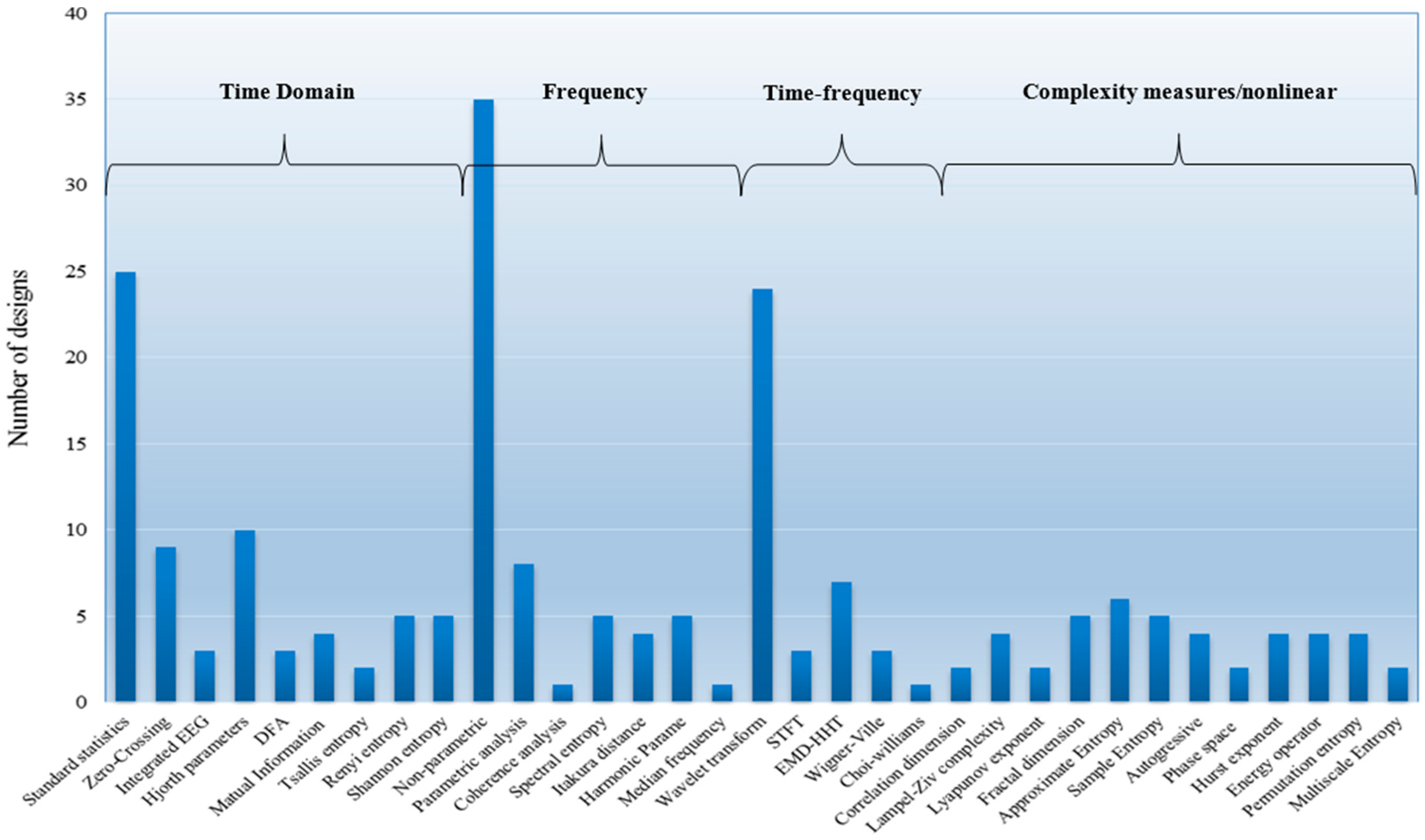
5.2. Feature Extraction
5.2.1. Standard Statistics
5.2.2. Non-Parametric Statistics of the Spectral Domain
5.2.3. Wavelet Transforms
5.3. Feature Selection/Dimensionality Reduction
5.3.1. Principal Component Analysis
5.3.2. Sequential Selection Methods
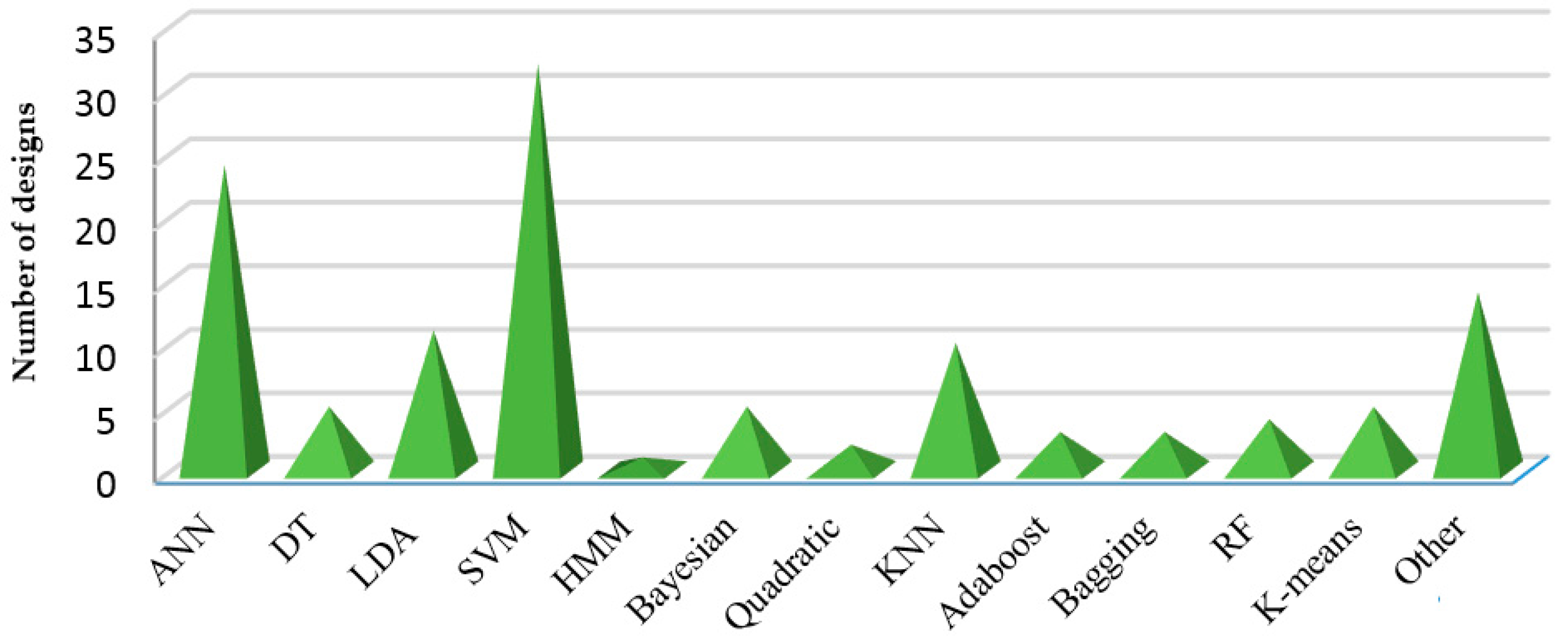
5.4. Classification
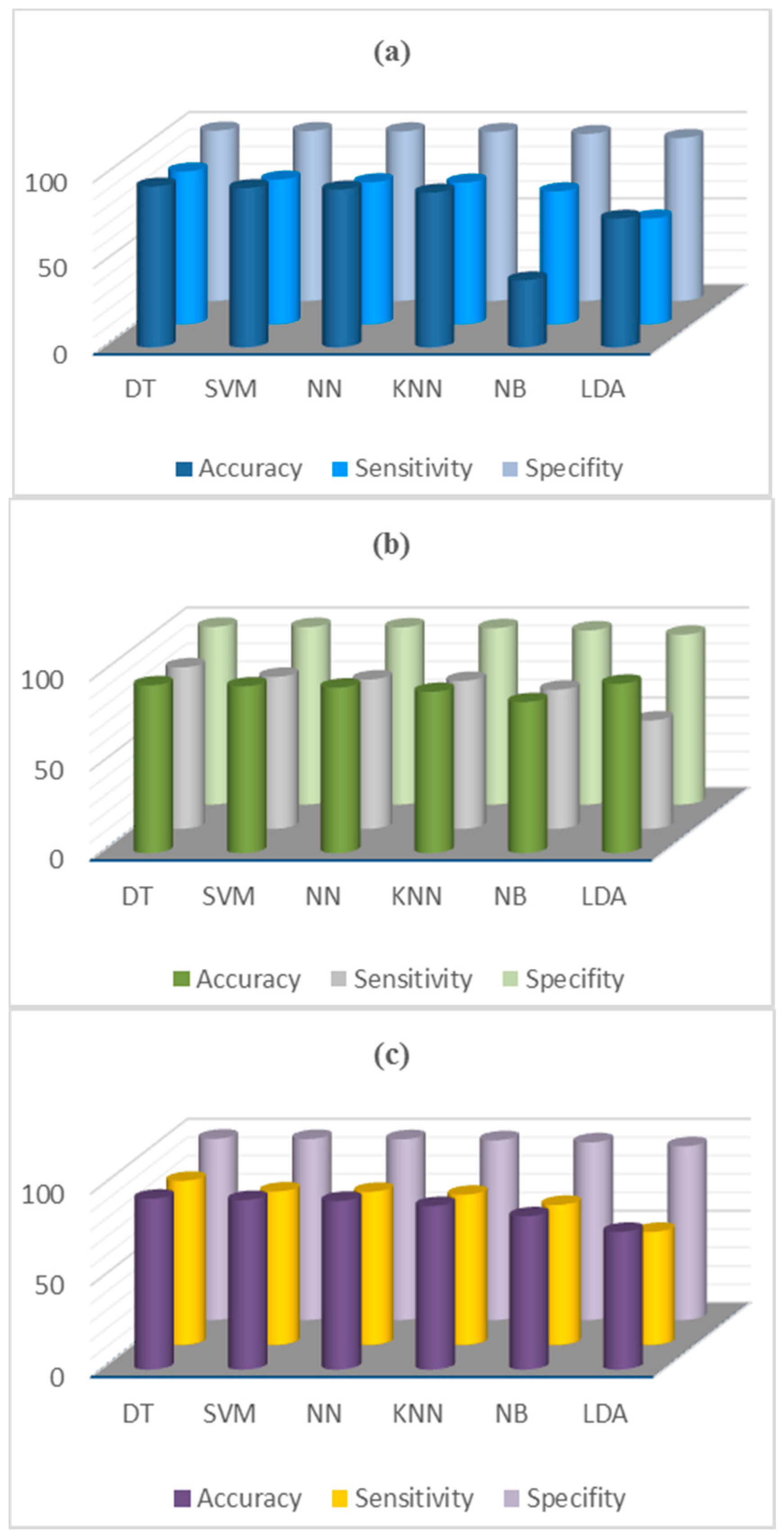
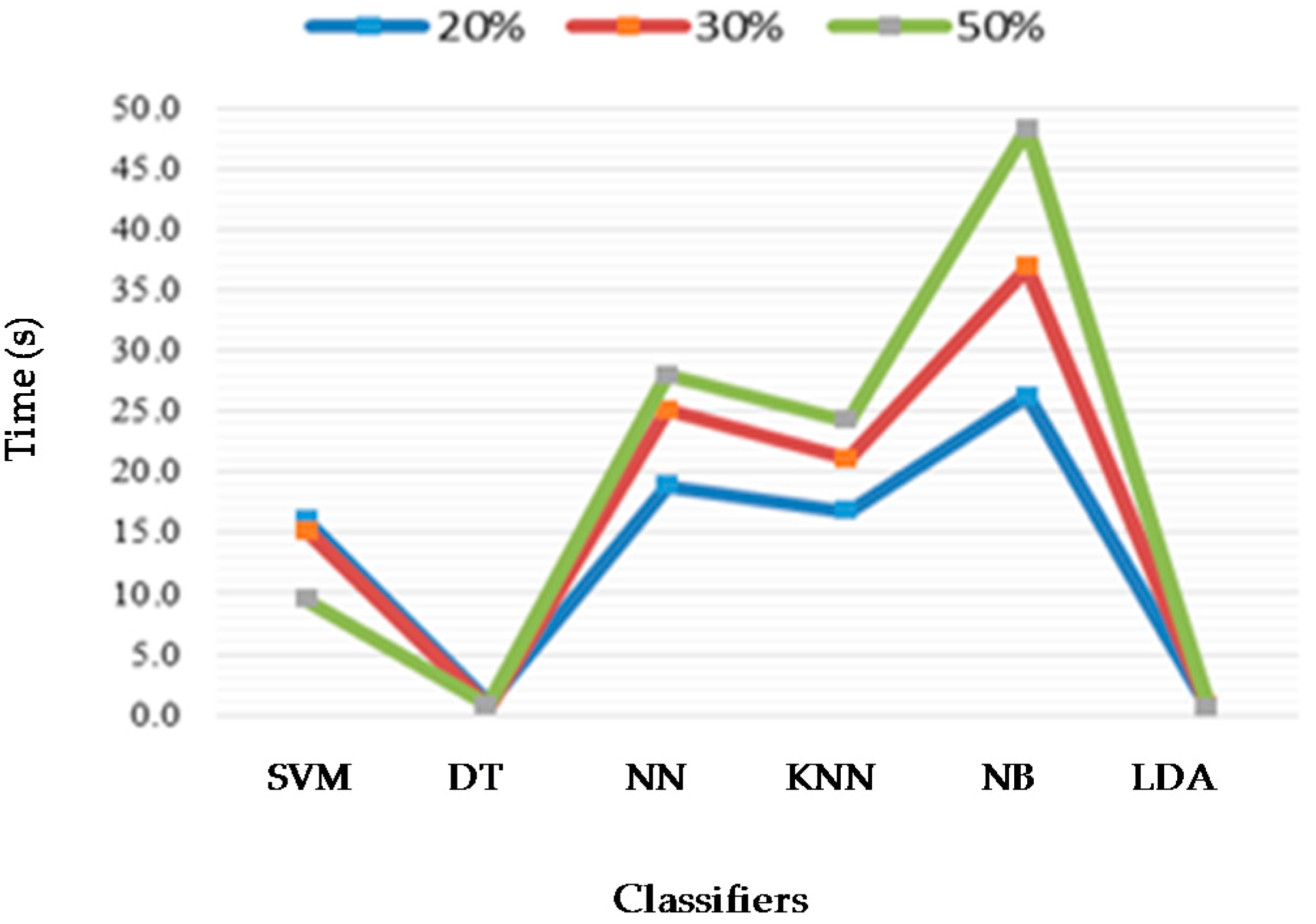
6. Results
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Estrada, E.; Nazeran, H.; Nava, P.; Behbehani, K.; Burk, J.; Lucas, E. Itakura distance: A useful similarity measure between EEG and EOG signals in computer-aided classification of sleep stages. In Proceedings of the 27th IEEE Annual International Conference of Engineering in Medicine and Biology Society, Shanghai, China, 1–4 September 2005; pp. 1189–1192.
- Li, Y.; Yingle, F.; Gu, L.; Qinye, T. Sleep stage classification based on EEG Hilbert–Huang transform. In Proceedings of the 4th IEEE Conference on Industrial Electronics and Applications (ICIEA), Xi’an, China, 25–27 May 2009; pp. 3676–3681.
- Aboalayon, K.A.; Faezipour, M. Multi-class SVM based on sleep stage identification using EEG signal. In Proceedings of the IEEE Healthcare Innovation Conference (HIC), Seattle, WA, USA, 8–10 October 2014; pp. 181–184.
- Huang, C.-S.; Lin, C.-L.; Ko, L.-W.; Liu, S.-Y.; Sua, T.-P.; Lin, C.-T. A hierarchical classification system for sleep stage scoring via forehead EEG signals. In Proceedings of the IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 1–5.
- Huang, C.-S.; Lin, C.-L.; Yang, W.-Y.; Ko, L.-W.; Liu, S.-Y.; Lin, C.-T. Applying the fuzzy c-means based dimension reduction to improve the sleep classification system. In Proceedings of the IEEE International Conference on Fuzzy Systems (FUZZ), Hyderabad, India, 7–10 July 2013; pp. 1–5.
- Lee, Y.-H.; Chen, Y.-S.; Chen, L.-F. Automated sleep staging using single EEG channel for REM sleep deprivation. In Proceedings of the Ninth IEEE International Conference on Bioinformatics and BioEngineering, Taichung, Taiwan, 22–24 June 2009; pp. 439–442.
- Hassan, A.R.; Bhuiyan, M.I.H. Automatic sleep scoring using statistical features in the EMD domain and ensemble methods. Biocybern. Biomed. Eng. 2016, 36, 248–255. [Google Scholar] [CrossRef]
- Khalighi, S.; Sousa, T.; Pires, G.; Nunes, U. Automatic sleep staging: A computer assisted approach for optimal combination of features and polysomnographic channels. Expert Syst. Appl. 2013, 40, 7046–7059. [Google Scholar] [CrossRef] [Green Version]
- Şen, B.; Peker, M.; Çavuşoğlu, A.; Çelebi, F.V. A comparative study on classification of sleep stage based on EEG signals using feature selection and classification algorithms. J. Med. Syst. 2014, 38, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.; Garcia-Molina, G.; Poel, M.; Tononi, G. Comparison of feature and classifier algorithms for online automatic sleep staging based on a single EEG signal. In Proceedings of the 36th IEEE Annual International Conference of Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1876–1880.
- Hassan, A.R.; Bashar, S.K.; Bhuiyan, M.I.H. On the classification of sleep states by means of statistical and spectral features from single channel electroencephalogram. In Proceedings of the IEEE International Conference on Advances in Computing, Communications and Informatics (ICACCI), Kochi, India, 10–13 August 2015; pp. 2238–2243.
- Hassan, A.R.; Bhuiyan, M.I.H. Computer-aided sleep staging using complete ensemble empirical mode decomposition with adaptive noise and bootstrap aggregating. Biomed. Signal Process. Control 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Ge, J.; Zhou, P.; Zhao, X.; Wang, M. Sample entropy analysis of sleep EEG under different stages. In Proceedings of the IEEE/ICME International Conference on Complex Medical Engineering, Beijing, China, 23–27 May 2007.
- Kuo, C.-E.; Liang, S.-F. Automatic stage scoring of single-channel sleep EEG based on multiscale permutation entropy. In Proceedings of the IEEE Conference on Biomedical Circuits and Systems (BioCAS), San Diego, CA, USA, 10–12 November 2011; pp. 448–451.
- Liang, S.-F.; Kuo, C.-E.; Hu, Y.-H.; Cheng, Y.-S. A rule-based automatic sleep staging method. In Proceedings of the 33rd IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6067–6070.
- Rodríguez-Sotelo, J.L.; Osorio-Forero, A.; Jiménez-Rodríguez, A.; Cuesta-Frau, D.; Cirugeda-Roldán, E.; Peluffo, D. Automatic sleep stages classification using EEG entropy features and unsupervised pattern analysis techniques. Entropy 2014, 16, 6573–6589. [Google Scholar] [CrossRef]
- Lan, K.-C.; Chang, D.-W.; Kuo, C.-E.; Wei, M.-Z.; Li, Y.-H.; Shaw, F.-Z.; Liang, S.-F. Using off-the-shelf lossy compression for wireless home sleep staging. J. Neurosci. Methods 2015, 246, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Estrada, E.; Nazeran, H.; Ebrahimi, F.; Mikaeili, M. EEG signal features for computer-aided sleep stage detection. In Proceedings of the 4th International IEEE/EMBS Conference on Neural Engineering, Antalya, Turkey, 29 April–2 May 2009; pp. 669–672.
- Estrada, E.; Nazeran, H. EEG and HRV signal features for automatic sleep staging and apnea detection. In Proceedings of the 20th IEEE International Conference on Electronics, Communications and Computer (CONIELECOMP), Cholula, Mexico, 22–24 February 2010; pp. 142–147.
- Leistedt, S.; Dumont, M.; Lanquart, J.-P.; Jurysta, F.; Linkowski, P. Characterization of the sleep EEG in acutely depressed men using detrended fluctuation analysis. Neurophysiol. Clin. 2007, 118, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Redmond, S.J.; Heneghan, C. Cardiorespiratory-based sleep staging in subjects with obstructive sleep apnea. IEEE Trans. Biomed. Eng. 2006, 53, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.; Hornero, R.; Marcos, J.V.; del Campo, F.; Lopez, M. Spectral analysis of electroencephalogram and oximetric signals in obstructive sleep apnea diagnosis. In Proceedings of the 31st IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 400–403.
- Correa, A.G.; Leber, E.L. An automatic detector of drowsiness based on spectral analysis and wavelet decomposition of EEG records. In Proceedings of the 32nd IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1405–1408.
- Gurudath, N.; Riley, H.B. Drowsy driving detection by EEG analysis using wavelet transform and k-means clustering. Procedia Comput. Sci. 2014, 34, 400–409. [Google Scholar] [CrossRef]
- Correa, A.G.; Orosco, L.; Laciar, E. Automatic detection of drowsiness in EEG records based on multimodal analysis. Med. Eng. Phys. 2014, 36, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K. Review on drowsy driving: Becoming dangerous problem. Int. J. Sci. Res. 2014, 3, 49–51. [Google Scholar]
- Tsai, P.-Y.; Hu, W.; Kuo, T.B.; Shyu, L.-Y. A portable device for real time drowsiness detection using novel active dry electrode system. In Proceedings of the 31st IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 3775–3778.
- Yu, S.; Li, P.; Lin, H.; Rohani, E.; Choi, G.; Shao, B.; Wang, Q. Support vector machine based detection of drowsiness using minimum EEG features. In Proceedings of the IEEE International Conference on Social Computing (SocialCom), Alexandria, VA, USA, 8–14 September 2013; pp. 827–835.
- Fraiwan, L.; Lweesy, K.; Khasawneh, N.; Fraiwan, M.; Wenz, H.; Dickhaus, H. Time frequency analysis for automated sleep stage identification in fullterm and preterm neonates. J. Med. Syst. 2011, 35, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, L.; Lweesy, K.; Khasawneh, N.; Wenz, H.; Dickhaus, H. Automated sleep stage identification system based on time–frequency analysis of a single EEG channel and random forest classifier. Comput. Methods Progr. Biomed. 2012, 108, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Mikaili, M.; Estrada, E.; Nazeran, H. Assessment of itakura distance as a valuable feature for computer-aided classification of sleep stages. In Proceedings of the 29th IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 3300–3303.
- Mora, A.M.; Fernandes, C.M.; Herrera, L.J.; Castillo, P.A.; Merelo, J.; Rojas, F.; Rosa, A.C. Sleeping with ants, SVMs, multilayer perceptrons and SOMs. In Proceedings of the 10th International Conference on Intelligent Systems Design and Applications, Cairo, Egypt, 29 November–1 December 2010; pp. 126–131.
- Vatankhah, M.; Akbarzadeh-T, M.R.; Moghimi, A. An intelligent system for diagnosing sleep stages using wavelet coefficients. In Proceedings of the IEEE International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain, 18–23 July 2010; pp. 1–5.
- Brignol, A.; Al-Ani, T.; Drouot, X. EEG-based automatic sleep-wake classification in humans using short and standard epoch lengths. In Proceedings of the 12th IEEE International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 276–281.
- Jo, H.G.; Park, J.Y.; Lee, C.K.; An, S.K.; Yoo, S.K. Genetic fuzzy classifier for sleep stage identification. Comput. Biol. Med. 2010, 40, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, Y.; Wen, P.P. Analysis and classification of sleep stages based on difference visibility graphs from a single-channel EEG signal. IEEE J. Biomed. Health Inform. 2014, 18, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Runarsson, T.P.; Sigurdsson, S. Automatic sleep staging using support vector machines with posterior probability estimates. In Proceedings of the International Conference on Computational Intelligence for Modelling, Control and Automation and International Conference on Intelligent Agents, Web Technologies and Internet Commerce (CIMCA-IAWTIC), Vienna, Austria, 28–30 November 2005; pp. 366–372.
- Hsu, Y.-L.; Yang, Y.-T.; Wang, J.-S.; Hsu, C.-Y. Automatic sleep stage recurrent neural classifier using energy features of EEG signals. Neurocomputing 2013, 104, 105–114. [Google Scholar] [CrossRef]
- Le, Q.K.; Truong, Q.D.K.; Vo, V.T. A tool for analysis and classification of sleep stages. In Proceedings of the IEEE International Conference on Advanced Technologies for Communications (ATC), Da Nang, Vietnam, 2–4 August 2011; pp. 307–310.
- Shuyuan, X.; Bei, W.; Jian, Z.; Qunfeng, Z.; Junzhong, Z.; Nakamura, M. An improved k-means clustering algorithm for sleep stages classification. In Proceedings of the 54th IEEE Annual Conference on Society of Instrument and Control Engineers of Japan (SICE), Hangzhou, China, 28–30 July 2015; pp. 1222–1227.
- Lajnef, T.; Chaibi, S.; Ruby, P.; Aguera, P.-E.; Eichenlaub, J.-B.; Samet, M.; Kachouri, A.; Jerbi, K. Learning machines and sleeping brains: Automatic sleep stage classification using decision-tree multi-class support vector machines. J. Neurosci. Methods 2015, 250, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Krakovská, A.; Mezeiová, K. Automatic sleep scoring: A search for an optimal combination of measures. Artif. Intell. Med. 2011, 53, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Güneş, S.; Polat, K.; Yosunkaya, Ş. Efficient sleep stage recognition system based on EEG signal using k-means clustering based feature weighting. Expert Syst. Appl. 2010, 37, 7922–7928. [Google Scholar] [CrossRef]
- Charbonnier, S.; Zoubek, L.; Lesecq, S.; Chapotot, F. Self-evaluated automatic classifier as a decision-support tool for sleep/wake staging. Comput. Biol. Med. 2011, 41, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Koley, B.; Dey, D. An ensemble system for automatic sleep stage classification using single channel EEG signal. Comput. Biol. Med. 2012, 42, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Ronzhina, M.; Janoušek, O.; Kolářová, J.; Nováková, M.; Honzík, P.; Provazník, I. Sleep scoring using artificial neural networks. Sleep Med. Rev. 2012, 16, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Zoubek, L.; Charbonnier, S.; Lesecq, S.; Buguet, A.; Chapotot, F. Feature selection for sleep/wake stages classification using data driven methods. Biomed. Signal Process. Control 2007, 2, 171–179. [Google Scholar] [CrossRef]
- Phan, H.; Do, Q.; Do, T.-L.; Vu, D.-L. Metric learning for automatic sleep stage classification. In Proceedings of the 35th IEEE Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5025–5028.
- Kayikcioglu, T.; Maleki, M.; Eroglu, K. Fast and accurate PLS-based classification of EEG sleep using single channel data. Expert. Syst. Appl. 2015, 42, 7825–7830. [Google Scholar] [CrossRef]
- Sousa, T.; Cruz, A.; Khalighi, S.; Pires, G.; Nunes, U. A two-step automatic sleep stage classification method with dubious range detection. Comput. Biol. Med. 2015, 59, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, S.; Sousa, T.; Oliveira, D.; Pires, G.; Nunes, U. Efficient feature selection for sleep staging based on maximal overlap discrete wavelet transform and SVM. In Proceedings of the 33rd IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3306–3309.
- Zhovna, I.; Shallom, I.D. Automatic detection and classification of sleep stages by multichannel EEG signal modeling. In Proceedings of the 30th IEEE EMBS 2008 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 2665–2668.
- Yu, S.; Chen, X.; Wang, B.; Wang, X. Automatic sleep stage classification based on ECG and EEG features for day time short nap evaluation. In Proceedings of the 10th World Congress on Intelligent Control and Automation (WCICA), Beijing, China, 6–8 July 2012; pp. 4974–4977.
- Ebrahimi, F.; Mikaeili, M.; Estrada, E.; Nazeran, H. Automatic sleep stage classification based on EEG signals by using neural networks and wavelet packet coefficients. In Proceedings of the 30th IEEE EMBS Annual International Conference of the Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1151–1154.
- Fell, J.; Röschke, J.; Mann, K.; Schäffner, C. Discrimination of sleep stages: A comparison between spectral and nonlinear EEG measures. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 401–410. [Google Scholar] [CrossRef]
- Weiss, B.; Clemens, Z.; Bódizs, R.; Halász, P. Comparison of fractal and power spectral EEG features: Effects of topography and sleep stages. Brain Res. Bull. 2011, 84, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, L.; Zeng, B.; Wang, W. Automatic sleep stage scoring using Hilbert–Huang transform with BP neural network. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Dursun, M.; Gunes, S.; Ozsen, S.; Yosunkaya, S. Comparison of artificial immune clustering with fuzzy c-means clustering in the sleep stage classification problem. In Proceedings of the International Symposium on Innovations in Intelligent Systems and Applications (INISTA), Trabzon, Turkey, 2–4 July 2012; pp. 1–4.
- Estrada, E.; Nazeran, H.; Nava, P.; Behbehani, K.; Burk, J.; Lucas, E. EEG feature extraction for classification of sleep stages. In Proceedings of the 26th IEEE EMBS Annual International Conference of the engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 196–199.
- Obayya, M.; Abou-Chadi, F. Automatic classification of sleep stages using EEG records based on fuzzy c-means (fcm) algorithm. In Proceedings of the 31st National Radio Science Conference (NRSC), Cairo, Egypt, 28–30 April 2014; pp. 265–272.
- Ahmad, R.F.; Malik, A.S.; Kamel, N.; Amin, H.; Zafar, R.; Qayyum, A.; Reza, F. Discriminating the different human brain states with EEG signals using fractal dimension: A nonlinear approach. In Proceedings of the IEEE International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), Kuala Lumpur, Malaysia, 25–25 November 2014; pp. 1–5.
- Electroencephalography I Laboratory. Available online: https://glneurotech.com/docrepo/teaching-labs/EEG_I_Teacher.pdf (accessed on 22 August 2016).
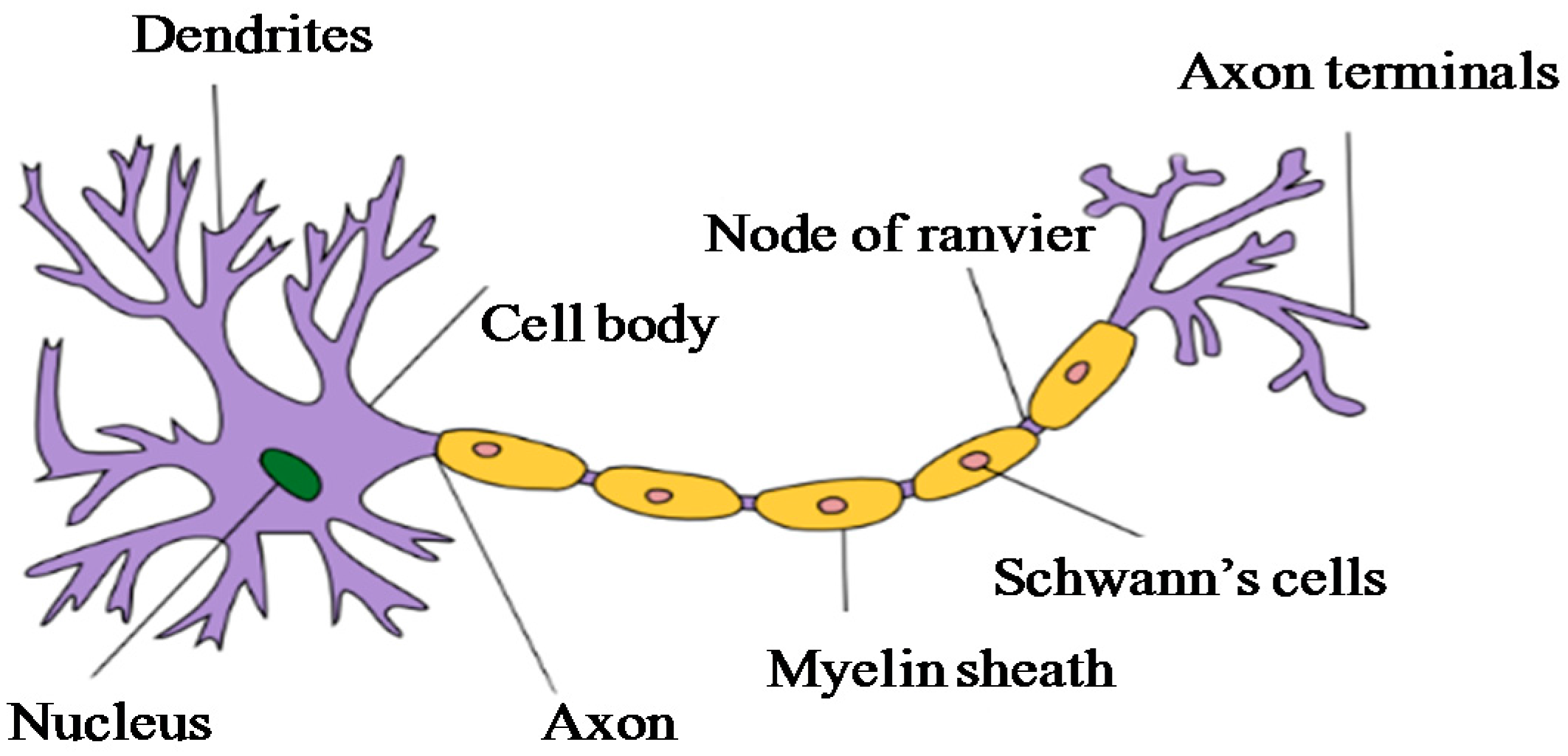
- Borzenko, A. Neuron mechanism of human languages. In Proceedings of the International Joint Conference on Neural Networks, Atlanta, GA, USA, 14–19 June 2009; pp. 375–382.
- Myelin. Available online: https://en.wikipedia.org/wiki/Myelin (accessed on 22 August 2016).
- Khalifa, W.; Salem, A.; Roushdy, M.; Revett, K. A survey of EEG based user authentication schemes. In Proceedings of the 8th International Conference on Informatics and Systems (INFOS), Cairo, Egypt, 14–16 May 2012.
- Hu, X.-S.; Hong, K.-S.; Ge, S.S. Fnirs-based online deception decoding. J. Neural Eng. 2012, 9, 26012–26019. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Hong, K.-S.; Jeong, M.-Y.; Ge, S.S. Detection of event-related hemodynamic response to neuroactivation by dynamic modeling of brain activity. Neuroimage 2012, 63, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Hong, K.-S. Passive BCI based on drowsiness detection: An fNIRS study. Biomed. Opt. Express 2015, 6, 4063–4078. [Google Scholar] [CrossRef] [PubMed]
- Naseer, N.; Hong, K.-S. FNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.R.; Hong, M.J.; Kim, Y.-H.; Hong, K.-S. Single-trial lie detection using a combined fNIRS-polygraph system. Front. Psychol. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-S.; Naseer, N. Reduction of delay in detecting initial dips from functional near-infrared spectroscopy signals using vector-based phase analysis. Int. J. Neural. Syst. 2016, 26, 1650012. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-S.; Santosa, H. Decoding four different sound-categories in the auditory cortex using functional near-infrared spectroscopy. Hear. Res. 2016, 333, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Derakhshani, R. A comparison of EEG preprocessing methods using time delay neural networks. In Porceedings of the 2nd IEEE EMBS International Conference on Neural Engineering, Arlington, VA, USA, 16–19 March 2005; pp. 262–264.
- Mantri, S.; Patil, V.; Mitkar, R. EEG based emotional distress analysis–a survey. Int. J. Eng. Dev. 2012, 4, 24–28. [Google Scholar]
- Roy, V.; Shukla, S. A survey on artifacts detection techniques for electro-encephalography (EEG) signals. Int. J. Multimed. Ubiquitous Eng. 2015, 10, 425–442. [Google Scholar] [CrossRef]
- Padmanabh, M.L.; Shastri, R.K.; Biradar, D. EEG signal processing techniques for mental task classification. Int. J. Adv. Comput. Electron. Technol. 2015, 2, 66–73. [Google Scholar]
- Patil, S.S.; Pawar, M.K. Quality advancement of EEG by wavelet denoising for biomedical analysis. In Proceedings of the International Conference on Communication, Information & Computing Technology (ICCICT), Mumbai, India, 19–20 October 2012; pp. 1–6.
- Gorur, D.; Halici, U.; Aydin, H.; Ongun, G.; Ozgen, F.; Leblebicioglu, K. Sleep spindles detection using short time fourier transform and neural networks. In Proceedings of the International Joint Conference on Neural Networks, Honolulu, HI, USA, 12–17 May 2002; pp. 1631–1636.
- Huang, L.; Sun, Q.; Cheng, J. Novel method of fast automated discrimination of sleep stages. In Proceedings of the 25th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Cancun, Mexico, 17–21 September 2003; pp. 2273–2276.
- Abeyratne, U.R.; Swarnkar, V.; Rathnayake, S.I.; Hukins, C. Sleep-stage and event dependency of brain asynchrony as manifested through surface EEG. In Proceedings of the 29th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 709–712.
- See, A.R.; Liang, C.-K. A study on sleep EEG using sample entropy and power spectrum analysis. In Porceedings of the Defense Science Research Conference and Expo (DSR), Singapore, 3–5 August 2011; pp. 1–4.
- Čić, M.; Šoda, J.; Bonković, M. Automatic classification of infant sleep based on instantaneous frequencies in a single-channel EEG signal. Comput. Biol. Med. 2013, 43, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
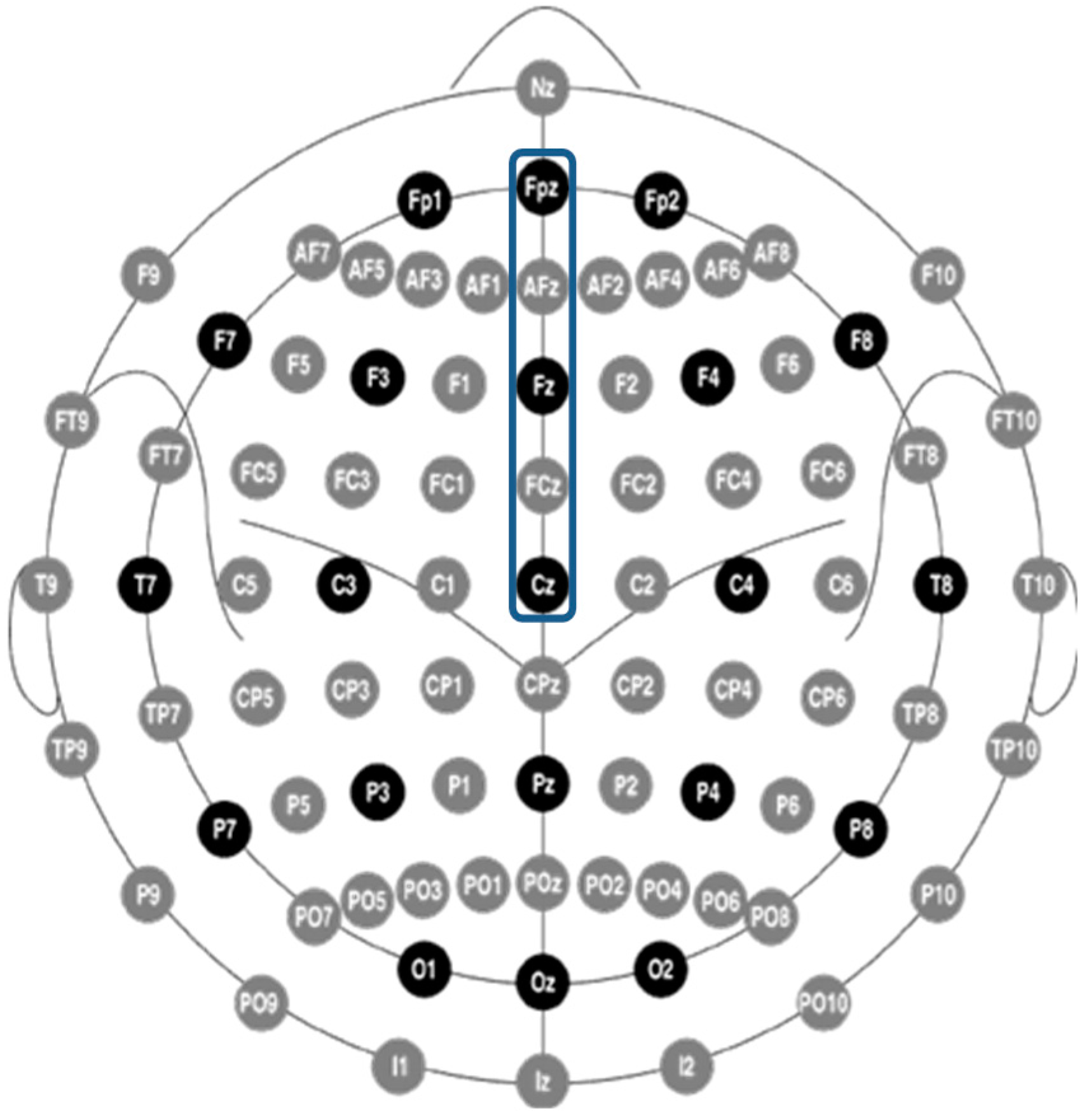
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. Neuroimage 2007, 34, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Neurophysiol. Clin. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Chen, X.; Wang, B.; Wang, X. Automatic sleep stage classification for daytime nap based on hopfield neural network. In Proceedings of the Chinese Control and Decision Conference (CCDC), Guiyang, China, 25–27 May 2013; pp. 2671–2674.
- Kempfner, J.; Jennum, P.; Sorensen, H.B.; Christensen, J.A.; Nikolic, M. Automatic sleep staging: From young aduslts to elderly patients using multi-class support vector machine. In Proceedings of the IEEE Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5777–5780.
- Sanders, T.H.; McCurry, M.; Clements, M.A. Sleep stage classification with cross frequency coupling. In Proceedings of the 36th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4579–4582.
- Koch, H.; Christensen, J.A.; Frandsen, R.; Zoetmulder, M.; Arvastson, L.; Christensen, S.R.; Jennum, P.; Sorensen, H.B. Automatic sleep classification using a data-driven topic model reveals latent sleep states. J. Neurosci. Methods 2014, 235, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Gialelis, J.; Panagiotou, C.; Karadimas, D.; Samaras, I.; Chondros, P.; Serpanos, D.; Koubias, S. Sleep monitoring classification strategy for an unobtrusive EEG system. In Proceedings of the 4th Mediterranean Conference on Embedded Computing (MECO), Budva, Montenegro, 14–18 June 2015; pp. 402–406.
- Agarwal, R.; Gotman, J. Computer-assisted sleep staging. IEEE Trans. Biomed. Eng. 2001, 48, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Janjarasjitt, S. Examination of temporal characteristic of sleep EEG subbands based on the local min-max. In Proceedings of the Biomedical Engineering International Conference (BMEiCON), Ubon Ratchathani, Thailand, 5–7 December 2012; pp. 1–4.
- Djordjevic, V.; Reljin, N.; Gerla, V.; Lhotska, L.; Krajca, V. Feature extraction and classification of EEG sleep recordings in newborns. In Proceedings of the 9th International Conference on Information Technology and Applications in Biomedicine, Larnaca, Cyprus, 4–7 November 2009; pp. 1–4.
- Herrera, L.J.; Mora, A.M.; Fernandes, C.; Migotina, D.; Guillén, A.; Rosa, A.C. Symbolic representation of the EEG for sleep stage classification. In Proceedings of the 11th International Conference on Intelligent Systems Design and Applications (ISDA), Córdoba, Spain, 22–24 November 2011; pp. 253–258.
- Khushaba, R.N.; Kodagoda, S.; Lal, S.; Dissanayake, G. Driver drowsiness classification using fuzzy wavelet-packet-based feature-extraction algorithm. IEEE Trans. Biomed. Eng. 2011, 58, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Grall-Maes, E.; Beauseroy, P. Features extraction for signal classification based on Wigner–Ville distribution and mutual information criterion. In Proceedings of the the IEEE-SP International Symposium on Time-Frequency and Time-Scale Analysis, Pittsburgh, PA, USA, 6–9 October 1998; pp. 589–592.
- Chen, L.-L.; Zhao, Y.; Zhang, J.; Zou, J.-Z. Automatic detection of alertness/drowsiness from physiological signals using wavelet-based nonlinear features and machine learning. Expert Syst. App. 2015, 42, 7344–7355. [Google Scholar] [CrossRef]
- Brignol, A.; Al-Ani, T.; Drouot, X. Phase space and power spectral approaches for EEG-based automatic sleep–wake classification in humans: A comparative study using short and standard epoch lengths. Comput. Methods Progr. Biomed. 2013, 109, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, K.; Jeong, D.-U. Hybrid neural-network and rule-based expert system for automatic sleep stage scoring. In Proceedings of the 22nd IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Chicago, IL, USA, 23–28 July 2000; pp. 1316–1319.
- Schlüter, T.; Conrad, S. An approach for automatic sleep stage scoring and apnea-hypopnea detection. IEEE Int. Conf. Data Min. 2010, 6, 230–241. [Google Scholar]
- Madan, T.; Agarwal, R.; Swamy, M. Compression of long-term EEG using power spectral density. In Proceedings of the 26th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 180–183.
- Koch, H.; Christensen, J.A.; Frandsen, R.; Arvastson, L.; Christensen, S.R.; Sorensen, H.B.; Jennum, P. Classification of iRBD and parkinson’s patients using a general data-driven sleep staging model built on EEG. In Proceedings of the 35th IEEE Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4275–4278.
- Liu, D.; Pang, Z.; Lloyd, S.R. A neural network method for detection of obstructive sleep apnea and narcolepsy based on pupil size and EEG. IEEE Trans. Neural Netw. 2008, 19, 308–318. [Google Scholar] [PubMed]
- Rossow, A.B.; Salles, E.O.T.; Côco, K.F. Automatic sleep staging using a single-channel EEG modeling by kalman filter and hmm. In Proceedings of the ISSNIP Biosignals and Biorobotics Conference, Vitoria, Spain, 6–8 January 2011; pp. 1–6.
- He, W.-X.; Yan, X.-G.; Chen, X.-P.; Liu, H. Nonlinear feature extraction of sleeping EEG signals. In Proceedings of the 27th IEEE of the Engineering in Medicine and Biology, Shanghai, China, 17–18 January 2006; pp. 4614–4617.
- Jain, V.P.; Mytri, V.; Shete, V.; Shiragapur, B. Sleep stages classification using wavelettransform & neural network. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 71–74.
- Tsinalis, O.; Matthews, P.M.; Guo, Y. Automatic sleep stage scoring using time-frequency analysis and stacked sparse autoencoders. Ann. Biomed. Eng. 2016, 44, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, L.; Lweesy, K.; Khasawneh, N.; Fraiwan, M.; Wenz, H.; Dickhaus, H. Classification of sleep stages using multi-wavelet time frequency entropy and lda. Methods Inf. Med. 2010, 49, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Estrada, E.; Nazeran, H.; Sierra, G.; Ebrahimi, F.; Setarehdan, S.K. Wavelet-based EEG denoising for automatic sleep stage classification. In Proceedings of the 21st International Conference on Electrical Communications and Computers (CONIELECOMP), San Andres Cholula, Mexico, 28 February–2 March 2011; pp. 295–298.
- Ahmed, B.; Redissi, A.; Tafreshi, R. An automatic sleep spindle detector based on wavelets and the teager energy operator. In Proceedings of the IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2596–2599.
- Maeda, M.; Takajyo, A.; Inoue, K.; Kumamaru, K.; Matsuoka, S. Time-frequency analysis of human sleep EEG and its application to feature extraction about biological rhythm. In Proceedings of the SICE Annual Conference, Takamatsu, Japan, 17–20 September 2007; pp. 1939–1944.
- Gabran, S.; Zhang, S.; Salama, M.; Mansour, R.; George, C. Real-time automated neural-network sleep classifier using single channel EEG recording for detection of narcolepsy episodes. In Proceedings of the 30th IEEE Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1136–1139.
- Vivaldi, E.A.; Bassi, A. Frequency domain analysis of sleep EEG for visualization and automated state detection. In Proceedings of the 28th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 3740–3743.
- Cho, S.-P.; Lee, J.; Park, H.; Lee, K. Detection of arousals in patients with respiratory sleep disorders using a single channel EEG. In Proceedings of the 27th IEEE Annual Conference of the Engineering in Medicine and Biology, Shanghai, China, 1–4 September 2006; pp. 2733–2735.
- Song, I.; Ji, Y.; Cho, B.; Ku, J.; Chee, Y.; Lee, J.; Lee, S.; Kim, I.; Kim, S.I. Multifractal analysis of sleep EEG dynamics in humans. In Proceedings of the 3rd IEEE/EMBS International Conference on Neural Engineering, Kohala Coast, HI, USA, 2–5 May 2007; pp. 546–549.
- Ma, Q.; Ning, X.; Wang, J.; Li, J. Sleep-stage characterization by nonlinear EEG analysis using wavelet-based multifractal formalism. In Proceedings of the 27th IEEE Annual Conference of the Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 4526–4529.
- Fraiwan, L.; Lweesy, K. Newborn sleep stage identification using multiscale entropy. In Proceedings of the 2nd Middle East Conference on Biomedical Engineering, Doha, Qatar, 17–20 February 2014; pp. 361–364.
- Ventouras, E.M.; Panagi, M.; Tsekou, H.; Paparrigopoulos, T.J.; Ktonas, P.Y. Amplitude normalization applied to an artificial neural network-based automatic sleep spindle detection system. In Proceedings of the 36th IEEE Annual International Conference of the Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3240–3243.
- Naseer, N.; Hong, K.-S. Decoding answers to four-choice questions using functional near infrared spectroscopy. J. Near Infrared Spectrosc. 2015, 23, 23–31. [Google Scholar] [CrossRef]
- Hong, K.-S.; Naseer, N.; Kim, Y.-H. Classification of prefrontal and motor cortex signals for three-class fNIRS–BCI. Neurosci. Lett. 2015, 587, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Hong, M.J.; Hong, K.-S. Decoding of four movement directions using hybrid NIRS-EEG brain-computer interface. Front. Hum. Neurosci. 2014, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Naseer, N.; Hong, M.J.; Hong, K.-S. Online binary decision decoding using functional near-infrared spectroscopy for the development of brain–computer interface. Exp. Brain Res. 2014, 232, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Naseer, N.; Hong, K.-S. Classification of functional near-infrared spectroscopy signals corresponding to the right-and left-wrist motor imagery for development of a brain–computer interface. Neurosci. Lett. 2013, 553, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Talmon, R.; Lo, Y.-L. Assess sleep stage by modern signal processing techniques. IEEE Trans. Biomed. Eng. 2015, 62, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Krajca, V.; Petranek, S.; Paul, K.; Matousek, M.; Mohylova, J.; Lhotska, L. Automatic detection of sleep stages in neonatal EEG using the structural time profiles. In Proceedings of the 27th IEEE-EMBS Annual International Conference, Shanghai, China, 17–18 January 2006; pp. 1–4.
- Lai, P.L.; Yang, J.L.; Inselberg, A. Classification and visualization for EEG data. In Proceedings of the Third International Conference on Innovative Computing Technology (INTECH), London, UK, 29–31 August 2013; pp. 452–455.
- Ilyas, M.Z.; Saad, P.; Ahmad, M.I. A survey of analysis and classification of EEG signals for brain-computer interfaces. In Proceedings of the 2nd International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 30–31 March 2015; pp. 1–6.
- Zhang, D.-X.; Wu, X.-P.; Guo, X.-J. The EEG signal preprocessing based on empirical mode decomposition. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 2131–2134.
- Widmann, A.; Schröger, E.; Maess, B. Digital filter design for electrophysiological data—A practical approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Motamedi-Fakhr, S.; Moshrefi-Torbati, M.; Hill, M.; Hill, C.M.; White, P.R. Signal processing techniques applied to human sleep EEG signals—A review. Biomed. Signal Process. Control 2014, 10, 21–33. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Lin, C.-J. A comparison of methods for multiclass support vector machines. IEEE Trans. Neural Netw. 2002, 13, 415–425. [Google Scholar] [PubMed]
- Theodoridis, S.; Pikrakis, A.; Koutroumbas, K.; Cavouras, D. Introduction to Pattern Recognition: A Matlab Approach; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Theodoridis, S.; Koutroumbas, K. Pattern Recognition; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Han, J.; Pei, J.; Kamber, M. Data Mining: Concepts and Techniques; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Principe, J.C.; de Vries, B.; De Oliveira, P.G. The gamma-filter-a new class of adaptive IIR filters with restricted feedback. IEEE Trans. Signal Process. 1993, 41, 649–656. [Google Scholar] [CrossRef]
- Lakshmi, M.R.; Prasad, D.T.; Prakash, D.V.C. Survey on EEG signal processing methods. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2014, 4, 84–91. [Google Scholar]
- Lan, T. Feature Extraction Feature Selection and Dimensionality Reduction Techniques for Brain Computer Interface. Ph.D. Thesis, Oregon Health & Science University, Portland, OR, USA, July 2011. [Google Scholar]
- Bashashati, A.; Fatourechi, M.; Ward, R.K.; Birch, G.E. A survey of signal processing algorithms in brain—Computer interfaces based on electrical brain signals. J. Neural Eng. 2007, 4, R32. [Google Scholar] [CrossRef] [PubMed]
- Khatwani, P.; Tiwari, A. A survey on different noise removal techniques of EEG signals. Int. J. Adv. Res. Comput. Commun. Eng. 2013, 2, 1091–1095. [Google Scholar]

| Bands | Frequencies (Hz) | Amplitude (μV) |
|---|---|---|
| Delta (δ) | 0–4 | 20–100 |
| Theta (θ) | 4–8 | 10 |
| Alpha (α) | 8–13 | 2–100 |
| Beta (β) | 13–22 | 5–10 |
| Gamma (γ) | > 30 | - |
| Technique | Features | Sleep Stage Classification | Sleep Stage Characteristics | Sleep Disorders Detection: OSA, Arousal & Others |
|---|---|---|---|---|
| Time Domain | Standard statistics | [3,7,8,9,10,11,12,17,25,38,41,42,44,45,47,48,51,53,58,86,89,90] | [18,91,92] | [19] |
| Zero crossing | [9,10,23,25,27,40,42,45,89] | - | - | |
| Integrated EEG | [23,25,27] | - | - | |
| Hjorth parameters | [8,9,10,37,44,45,51,89] | [18,92] | [19,21] | |
| Detrended fluctuation analysis | [16,45] | [18] | [19] | |
| Mutual information | [79,93,94] | [95] | - | |
| Tsallis entropy | [8,51] | - | - | |
| Renyi entropy | [8,9,30,51,96] | - | - | |
| Shannon entropy | [8,16,29,44,51] | - | - | |
| Frequency Domain | Non-parametric analysis | [4,5,6,8,10,11,15,17,28,34,35,37,40,41,42,43,44,45,47,50,51,53,56,60,82,85,87,96,97,98,99] | [39,55,100] | [21,22,80,101,102] |
| Parametric analysis | [23,25,49,52,90,103] | [1,59] | [19] | |
| Coherence analysis | [42] | - | - | |
| Spectral entropy | [9,10,37] | [55,104] | [22] | |
| Itakura distance | - | [1,18,31,59] | [19] | |
| Harmonic Parameter | [8,50] | [18,59] | [19] | |
| Median frequency | [37] | - | [22] | |
| Time-frequency Domain | WT | [8,9,24,29,30,32,33,47,50,51,54,60,81,87,94,99,105,106,107] | [92,108,109,110] | [102,111] |
| STFT | [87] | [78,112] | [113] | |
| EMD | [2,7,12,29,30,57,82] | - | - | |
| WVD | [9,29] | [95] | - | |
| Choi-williams | [30] | - | - | |
| Complexity measures & non-linear parameters | Correlation dimension | [45] | [55] | - |
| Lempel-Ziv | [10,45,79] | [104] | - | |
| Lyapunov exponent | [45] | [55] | - | |
| Fractal dimension | [9,10,16,42,45] | - | - | |
| Approximate Entropy | [9,16,45,96] | [104] | [19] | |
| Sample Entropy | [16,81,96] | [13,104] | - | |
| Autoregressive | [8,14,51,89] | - | - | |
| Phase space | [34,97] | - | - | |
| Hurst exponent | [9,56] | [114,115] | - | |
| Energy operator | [9,41,90] | [109] | - | |
| Permutation entropy | [9,14,41,48] | - | - | |
| Multiscale Entropy | [16] | [116] | - |
| Technique | Technique Variations | Sleep Stage Classification | Sleep Stage Characteristics | Sleep Disorders Detection: OSA, Arousal & Others |
|---|---|---|---|---|
| ANN | - | [7,9,12,16,23,25,27,29,38,44,47,54,57,58,79,85,98,105] | [73,78,116,117] | [102,111] |
| Statistical | LDA | [7,8,12,14,41,49,50,56,87,94,107] | - | - |
| SVM | [3,4,5,6,7,8,9,10,28,32,33,34,36,37,41,45,50,51,53,81,82,86,88,93,94,96,97,118,119,120,121,122] | [78,92,123] | [113] | |
| Hidden Markov Model | [103] | - | - | |
| Bayesian | [7,8,12,49] | [92] | - | |
| Quadratic | [47] | - | [21] | |
| Instance base | KNN | [2,7,12,32,37,43,47,48,49,94] | - | - |
| Decision tree | DT | [9,15,17,43,99] | - | - |
| Ensemble | Adaboost | [7,8,12] | - | - |
| Bagging | [7,11,12] | - | - | |
| RF | [9,10,30] | [116] | - | |
| Clustering | K-means classifier | [24,40,90] | [124] | [101] |
| Other classifiers | - | [16,32,34,35,49,52,60,96,97,106] | [112,116,125] | - |
| Technique Variations | Sleep Stage Classification | Sleep Stage Characteristics |
|---|---|---|
| Minimum Redundancy Maximum-Relevance (mRMR) | [8,51,93] | - |
| Sequential methods | [4,8,41,42,44,47,50] | [92] |
| Best Subsets Procedure | [42] | - |
| t-test | [9,41] | - |
| SVM-Recursive Feature Elimination (RFE) | [45] | - |
| Differential Evolution Feature | [8] | - |
| Fisher score | [9] | - |
| ReliefF method | [9,10] | - |
| Fast correlation based filter | [9] | - |
| Principal component analysis | [5,58,60] | [73,112] |
| Linear Discriminant Analysis | [5,25,126] | - |
| Large Margin NN | [48] | - |
| Fuzzy C-means clustering | [5,58] | - |
| Artificial Immune Clustering | [58] | - |
| Stage | W | S1 | S2 | S3 | S4 | REM |
|---|---|---|---|---|---|---|
| Total | 5961 | 3552 | 7175 | 4321 | 1900 | 897 |
| FIR Filter | IIR Filter | |
|---|---|---|
| Phase | Liner or Non-linear | Non-linear |
| Cutoff frequency | Usually as −6 dB | Usually as −3 dB |
| Stability | Stable at all time | Can be unstable Stability need to be checked |
| Feedback coefficient | No non-zero | One or more non-zero |
| Order | High | Low |
| Analog Equivalent | Yes | No |
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 91.19 | 96.43 | 91.25 | 86.88 | 98.64 | 66.32 | 93.29 |
| Sp | 98.59 | 98.09 | 98.10 | 98.70 | 99.44 | 98.94 | ||
| 30 | Se | 91.06 | 95.84 | 90.95 | 86.15 | 97.41 | 76.26 | 93.18 |
| Sp | 98.89 | 97.91 | 98.13 | 98.87 | 99.55 | 98.40 | ||
| 50 | Se | 91.46 | 95.05 | 90.83 | 87.60 | 97.63 | 72.22 | 92.92 |
| Sp | 98.58 | 98.16 | 98.11 | 98.72 | 99.32 | 98.58 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 92.49 | 96.78 | 91.71 | 86.88 | 98.13 | 36.22 | 92.31 |
| Sp | 97.34 | 97.48 | 97.89 | 99.02 | 99.63 | 99.18 | ||
| 30 | Se | 94.13 | 95.75 | 92.65 | 86.88 | 97.13 | 39.68 | 92.59 |
| Sp | 97.46 | 97.93 | 97.77 | 99.15 | 99.64 | 99.01 | ||
| 50 | Se | 94.66 | 96.93 | 91.39 | 82.88 | 96.80 | 37.47 | 92.22 |
| Sp | 97.36 | 97.64 | 97.41 | 99.04 | 99.68 | 99.31 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 90.72 | 96.44 | 90.69 | 84.67 | 97.27 | 33.14 | 91.45 |
| Sp | 97.56 | 96.62 | 97.41 | 98.78 | 99.54 | 99.47 | ||
| 30 | Se | 89.32 | 96.68 | 92.72 | 84.73 | 97.31 | 36.75 | 91.91 |
| Sp | 97.87 | 96.87 | 97.36 | 99.26 | 99.45 | 99.11 | ||
| 50 | Se | 87.92 | 96.83 | 94.29 | 82.70 | 97.20 | 40.00 | 91.75 |
| Sp | 98.05 | 96.65 | 97.27 | 99.33 | 99.41 | 98.98 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 90.48 | 95.22 | 85.16 | 85.99 | 94.21 | 40.88 | 89.72 |
| Sp | 96.54 | 96.29 | 97.76 | 98.29 | 99.46 | 98.99 | ||
| 30 | Se | 86.27 | 92.46 | 90.95 | 82.98 | 94.57 | 45.56 | 89.48 |
| Sp | 96.96 | 97.34 | 96.81 | 98.59 | 99.54 | 98.10 | ||
| 50 | Se | 87.55 | 95.22 | 83.41 | 87.84 | 93.78 | 41.57 | 88.94 |
| Sp | 96.57 | 95.34 | 98.20 | 97.99 | 99.60 | 98.66 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 76.04 | 85.89 | 84.46 | 69.39 | 96.17 | 48.97 | 83.95 |
| Sp | 97.88 | 95.30 | 94.73 | 98.33 | 97.01 | 96.97 | ||
| 30 | Se | 77.67 | 84.33 | 85.30 | 69.76 | 94.93 | 51.36 | 83.84 |
| Sp | 97.64 | 95.57 | 94.68 | 98.45 | 96.68 | 97.08 | ||
| 50 | Se | 74.05 | 85.23 | 85.16 | 66.95 | 96.30 | 48.69 | 83.34 |
| Sp | 97.74 | 95.42 | 94.46 | 98.29 | 96.66 | 96.93 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | Wake | REM | |||
| 20 | Se | 89.39 | 95.69 | 43.55 | 56.87 | 81.15 | 0 | 74.60 |
| Sp | 93.82 | 80.18 | 93.78 | 98.75 | 99.80 | 99.97 | ||
| 30 | Se | 88.24 | 94.30 | 42.44 | 54.67 | 81.25 | 0 | 74 |
| Sp | 93.89 | 79.89 | 93.44 | 98.52 | 99.86 | 100 | ||
| 50 | Se | 88.25 | 96.25 | 40.86 | 60.02 | 82.61 | 0 | 74.87 |
| Sp | 93.67 | 79.38 | 95.13 | 98.65 | 99.88 | 100 | ||
| No. | ASSC Approaches | Sleep Stages | Extracted Features | Classifier | Subject, Dataset & Channels | Performance |
|---|---|---|---|---|---|---|
| 1 | Li et al. 2009 [2] | Wake, S1 + REM, S2, S3/4 | 560 30 s epochs; EMD and Hilbert spectrum based on Hilbert-Huang Transform (HHT) | KNN | 8 recordings, Sleep-EDF dataset EEG (Fpz-Cz, Pz-Oz) | Acc 81.7% |
| 2 | Huanga, et al. 2013 [4] | Wake, S1/S2, S1/S4, REM | 8251 30 s epochs, PSD based on STFT & Sequential method | SVM | 10 recording, Sleep laboratory of NCTU dataset EEG (FP1,FP2) | Acc 77.1% |
| 3 | Huang et al. 2013 [5] | Wake, S1, S2, SWS, REM | 8251 30 s epochs; PSD based on STFT, FCM, PCA & Discriminant analysis feature extraction | SVM | 10 recordings, dataset EEG (FP1,FP2) | Acc 70.92% Kappa 0.6130 |
| 4 | Hassan et al. 2015 [7] in press | (Wake, S1, S2, S3, S4,REM) (Wake, S1, S2, S3–S4, REM) (Wake, S1–S2, SWS, REM) | 15188 30 s epochs; EMD &Statistical features | NB, LDA, QDA, MDA, NN, LS-SVM, SVM, KNN, Adaboost, Bagging | 8 recordings, Sleep-EDF database EEG (Fz-Oz) | Acc 88.62% Acc 90.11% Acc 91.2% |
| 5 | Şen et al. 2014 [9] | Wake, S1, S2, S3, S4, REM | 5160 30 s epochs; Zero crossings , Hjorth parameters, Petrosian fractal dimension , Mean teager energy, Hurst exponent, WVD, WT, Spectral entropy, Rényi entropy, ApEn, Permutation entropy & Feature selection: FCBF, t-test, ReliefF, Fisher score, mRMR algorithms and efficient feature selection algorithms | DT FFNN SVM RBF RF | 25 recordings, St. Vincent’s University Hospital and University College Dublin EEG (C3-A2) | Acc: RF 97.03%, DT 92.35%, RBF 89.45%, SVM 93.21%, FFNN 71.88% |
| 6 | Hassan et al. 2015 [11] | (Wake, S1, S2, S3, S4, REM) (Wake, S1, S2, S3–S4, REM) (Wake, S1–S2, SWS, REM) | 15188 30 s epochs; 4 Standard statistics features & 5 Spectral features | Bootstrap Aggregating (Bagging) | 8 recording, Sleep-EDF database EEG (Fz-Oz) | Acc 85.57% Acc 86.53% Acc 87.49% |
| 7 | Hassan et al. 2016 [12] | (Wake, S1, S2, S3, S4, REM) (Wake, S1, S2, S3–S4, REM) (Wake, S1–S2, SWS, REM) | 15188 30 s epochs; EMD & Standard statistics | NB, LDA, QDA, MDA, NN, KNN, Adaboost, Bagging | 8 recordings, Sleep-EDF database EEG (Fz-Oz) | 86.89%, 90.69% 92.14% using Bagging |
| 8 | Rodríguez-Sotelo et al. 2014 [16] | W, S1, S2, S3, REM | 40826 30 s epochs; Fractal dimension, Detrended fluctuation analysis , Shannon entropy, (ApEn), Sample entropy, Multiscale entropy & PCA | ANN | 20 recordings, Sleep-EDF dataset EEG (Fpz-Cz, Pz-Oz) | Acc 80% |
| 9 | Fraiwan et al. 2012 [30] | Wake, S1, S2, S3, REM | 20269 30 s epochs; Renyi’s entropy based on Choi–williams distribution, CWT & HHT | RF | 16 recordings, thoracic clinic at the University of Heidelberg, Germany EEG (C3-A1) | CWT Acc 0.83% & Kappa 0.76. CWD Acc 0.78% & Kappa 0.70 HHT Acc 0.75% & Kappa 0.65 |
| 10 | Vatankhah et al. 2010 [33] | Wake, S1, S2, S3, S4, REM | 2400 30 s epochs; WT coefficients | SVM | 2 recordings, Sleep-EDF dataset EEG (Fpz-Cz, Pz-Oz) | Acc 98% Wake from REM |
| 12 | Zhu et al. 2014 [36] | Wake, S1, S2, S3, S4, REM | 14963 30 s epochs; DVG & HVG graph domain features | SVM | 8 recordings, Sleep-EDF EEG (Pz-Oz) | Acc 87.5% |
| 13 | Gudmundsson et al. 2005 [37] | Wake, S1 + S2, SWS, REM | 4122 30 s epochs; Hjorth Parameter, Power spectrum on Welch’s, Histogram waveform | SVM, KNN | 4 recordings, EEG (C3-A2) | Acc 81% |
| 14 | Hsu et al. 2013 [38] | Wake, S1, S2, SWS, REM | 4800 30 s epochs; Energy statistic features | Elman network | 8 recordings, Sleep-EDF database EEG (Fpz-cz) | Acc 87.2% |
| 15 | Gunes et al. 2010 [43] | Wake, REM, S1, S2, S3 | 4196 30 s epochs; Welch based on FFT spectral analysis, Feature weighting process using K-meansclustering | KNN DT | 5 recordings, EEG (C4-A1) | Acc 82.21% |
| 16 | Koley & Dey 2012 [45] | Wake, S1, S2, SWS, REM | 15541 30 s epochs; Standard statistics, Zero crossing, Hjorth parameters, PSD features based on Welch method, Dimension (D2), Lyapunov exponent & ApEn, Detrended fluctuation analysis, Lempel-ziv, Higuchi fractal dimension , SVM-Recursive feature elimination | 1vA SVM | 28 recordings, recordings were performed at the Center for Sleep Disorder Diagnosis (CSDD) located in West Bengal, India EEG (C4-A1) | Se 88.32%, Sp 97.42%, Acc 95.88% Error 10.61 |
| 17 | Phan et al. 2013 [48] | Wake, S1 + REM, S2, SWS | 11314 30 s epochs, Standard statistics, Spindle score, Permutation entropy, Power band, total power, PSD & Power fraction | KNN | 4 recordings, Sleep-EDF EEG (Fpz-Cz) | Acc 94.49% |
| 18 | Ebrahimi et al. 2008 [54] | Wake, S1 + REM, S2, SWS | 5779 30 s epochs; 5 features: WT coefficients | ANN | 8 recordings, Sleep-EDF dataset, EEG (Pz-Oz) | 93.0% Acc; 48.2% Se; 94.4% Sp |
| 19 | Liu et al. 2010 [57] | Wake, S1 + REM, S2, SWS | 6645 30 s epochs; Marginal energy based on HHT | NN | 7 recordings, Sleep-EDF dataset, EEG (Pz-Oz) | Acc of 95% W, 87.1% (S1 + REM), 82.0% S2, 92.9% SWS |
| 20 | Obayya1 & Abou-Chadi 2014 [60] | Wake, S1, S2, S3, S4, REM | 30 s epoch; Power spectral based on FFT, WT coefficients, PCA | FCM | 12 recordings, Cairo Center for Sleep dataset EEG (SAHC) | Acc 92.27% |
| 21 | Sanders et al. 2014 [87] | Wake, S1, S2, S3/S4, REM | 9830 30 s epochs; Average spectral power, Preferential frequency band & CFC method | LDA | 10 recording, Sleep-EDF database EEG (Fpz-Cz) | Acc 75% |
| 22 | Fraiwan et al. 2010 [128] | Wake, S1, S2, S3, S4, REM | 41778 30 s epochs; WT coefficients, Entropy | LDA | 32 recordings, MIT-BIT dataset EEG (C3-A2) | Acc 84% |
| 23 | Jain et al. 2012 [129] | Wake, S1, S2, S3, REM | 3000 30 s epochs; WT coefficients | ANN | Sleep Centre, MCH-Westeinde Hospital, Den Haag, The Netherlands | Acc 93% for S2 |
| 24 | Tsinalis et al. 2015 [131] | W, S1, S2, S3, REM | 37022 30 s epochs; WT coefficients | Stacked sparse autoencoders NN | 20 recordings, Sleep-EDF database EEG (Fpz-Cz) | Acc 78%, mean 84% |
| 25 | Herrera et al. 2011 [93] | Wake, S1/S2, S3/S4, REM | 9000 30 s epochs; Self-Organizing Maps % Mutual information based mRMR algorithm | SVM | 10 recordings, EEG (C3-M2) | Acc 70% |
| 26 | Rossow et al. [103] | W, S1, S2, S3, S4, REM | 1257 30 s epochs; The ARMA coefficients | Hidden Markov Model | 5 recordings, MIT-BIT dataset EEG (C4/A1) | Acc 59.51% |
| 27 | Gabran1 et al. 2008 [111] | S1, S2, S3, S4, REM | 200 30 s epochs; WT coefficients | LVQ, PNN, NN | EEG | Acc 85% |
| 28 | Proposed method | (Wake, S1, S2, S3, S4, REM) (Wake, S1, S2, S3 + S4, REM) (Wake, S1 + S2, S3 + S4, REM) (Wake, S1 + REM, S2, S3 + S4) | 23806 10 s epochs; MMD and Esis | DT, SVM, NN, KNN, LDA, NB | 39 PSG recordings, Sleep-EDF database EEG (Fpz-Cz) | Acc 93.61%, Se 89.06%, Sp 98.61% Acc 95.17%, Se 95.75 %, Sp 98.83% Acc 96.06%, Se 96.06%, Sp 96.11% Acc 97.05%, Se 97 %, Sp 99% |
| Test Percentage | Sleep EEG Classes | Acc | |||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 + S4 | Wake | REM | |||
| 20 | Se | 92.53 | 96.34 | 97.82 | 97.88 | 67.23 | 95.46 |
| Sp | 98.71 | 98.33 | 99.00 | 99.58 | 98.71 | ||
| 30 | Se | 91.18 | 96.38 | 97.42 | 97.28 | 72.14 | 95.19 |
| Sp | 98.87 | 98.04 | 98.96 | 99.47 | 98.62 | ||
| 50 | Se | 91.40 | 95.54 | 97.49 | 97.73 | 66.88 | 94.87 |
| Sp | 98.40 | 98.10 | 99.01 | 99.44 | 98.64 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||
| S1 + S2 | S3 + S4 | Wake | REM | |||
| 20 | Se | 96.33 | 98.19 | 98.13 | 70.05 | 96.30 |
| Sp | 97.11 | 99.05 | 99.49 | 98.93 | ||
| 30 | Se | 95.50 | 98.52 | 98.24 | 68.63 | 95.98 |
| Sp | 97.21 | 98.81 | 99.30 | 98.87 | ||
| 50 | Se | 96.41 | 98.00 | 97.82 | 64.85 | 95.90 |
| Sp | 96.19 | 99.32 | 99.49 | 98.81 | ||
| Test Percentage | Sleep EEG Classes | Acc | ||||
| S1 + REM | S2 | S3 + S4 | Wake | |||
| 20 | Se | 96.22 | 95.76 | 98.14 | 97.92 | 96.99 |
| Sp | 99.00 | 98.52 | 98.90 | 99.52 | ||
| 30 | Se | 95.53 | 96.36 | 98.32 | 98.69 | 97.29 |
| Sp | 99.12 | 98.55 | 99.32 | 99.34 | ||
| 50 | Se | 94.48 | 96.64 | 97.76 | 98.15 | 96.89 |
| Sp | 99.27 | 97.94 | 99.13 | 99.42 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboalayon, K.A.I.; Faezipour, M.; Almuhammadi, W.S.; Moslehpour, S. Sleep Stage Classification Using EEG Signal Analysis: A Comprehensive Survey and New Investigation. Entropy 2016, 18, 272. https://doi.org/10.3390/e18090272
Aboalayon KAI, Faezipour M, Almuhammadi WS, Moslehpour S. Sleep Stage Classification Using EEG Signal Analysis: A Comprehensive Survey and New Investigation. Entropy. 2016; 18(9):272. https://doi.org/10.3390/e18090272
Chicago/Turabian StyleAboalayon, Khald Ali I., Miad Faezipour, Wafaa S. Almuhammadi, and Saeid Moslehpour. 2016. "Sleep Stage Classification Using EEG Signal Analysis: A Comprehensive Survey and New Investigation" Entropy 18, no. 9: 272. https://doi.org/10.3390/e18090272






