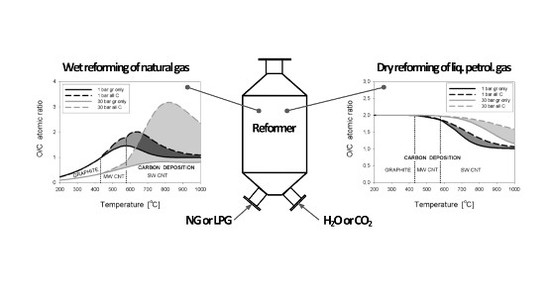
On the Deposition Equilibrium of Carbon Nanotubes or Graphite in the Reforming Processes of Lower Hydrocarbon Fuels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Reactions
2.2. Thermodynamic Relationships
2.3. Modeling Procedure
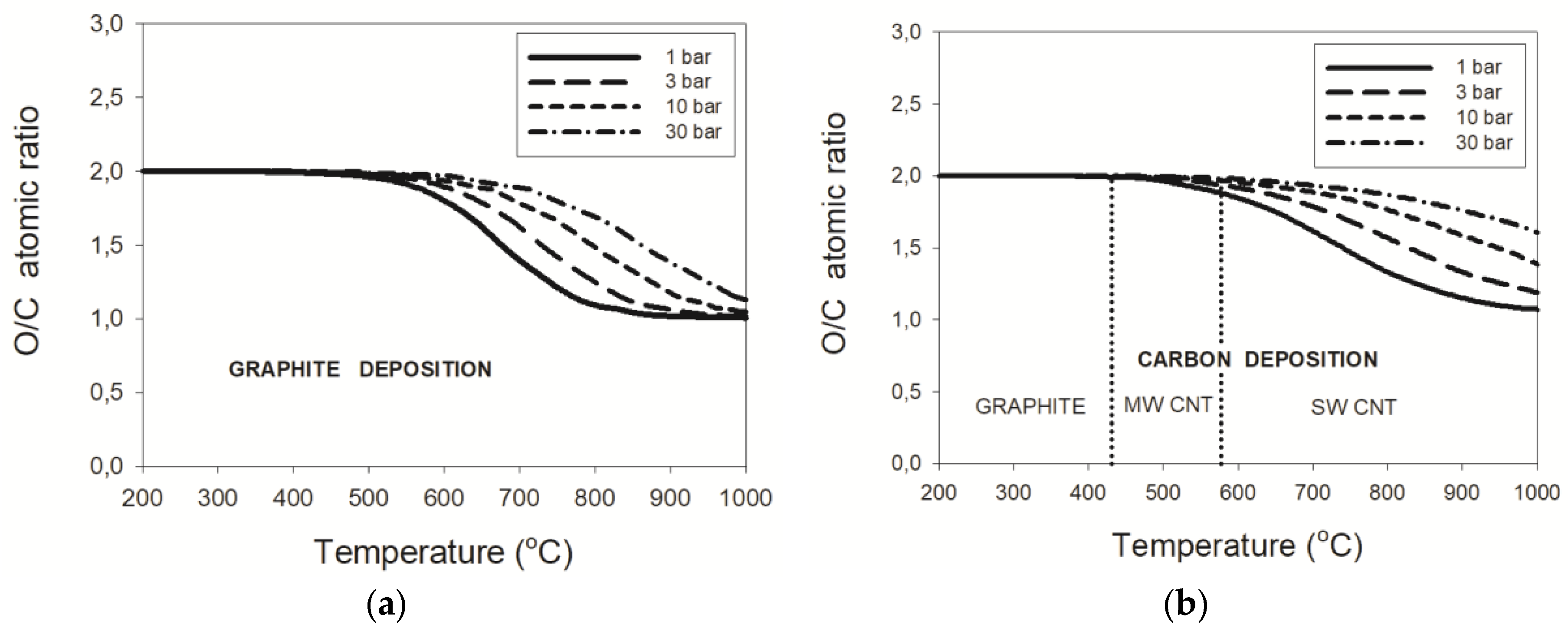
3. Results
3.1. Wet Reforming
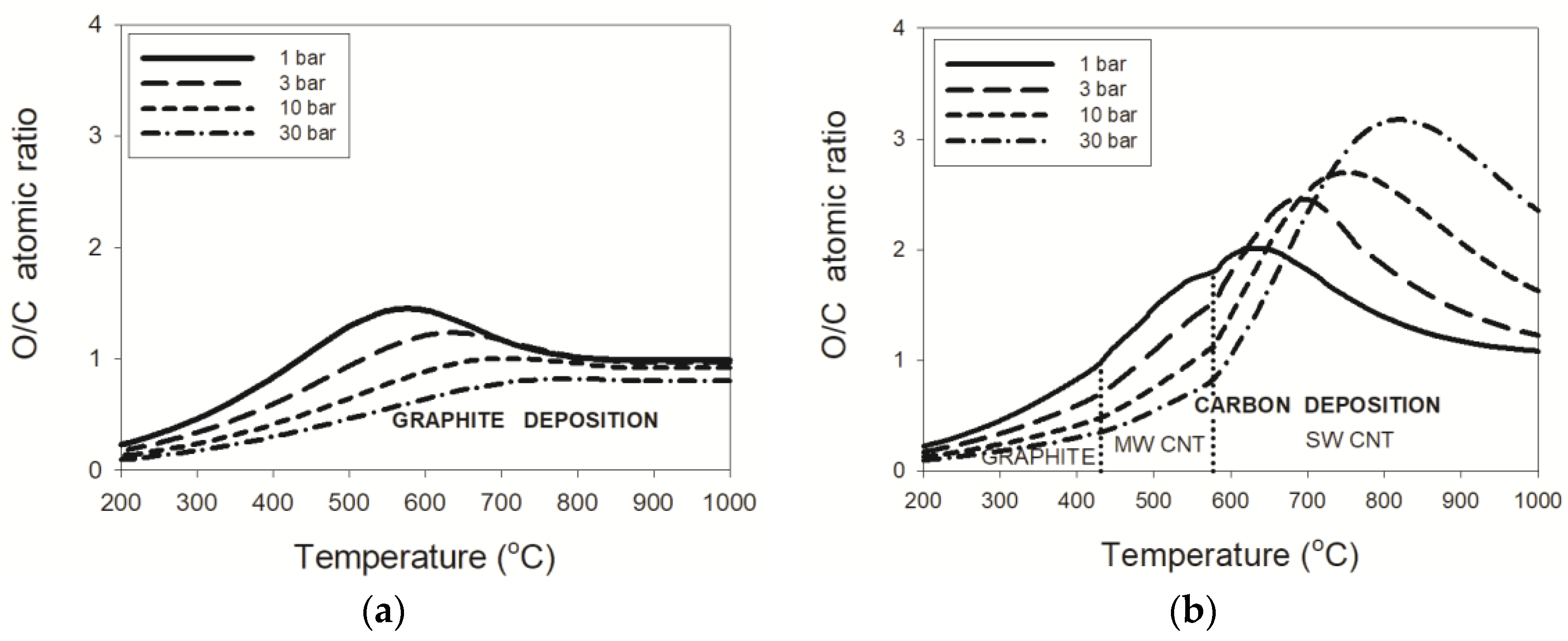
3.1.1. Natural Gas (NG)–Water Mixtures
3.1.2. LPG–Water Mixtures
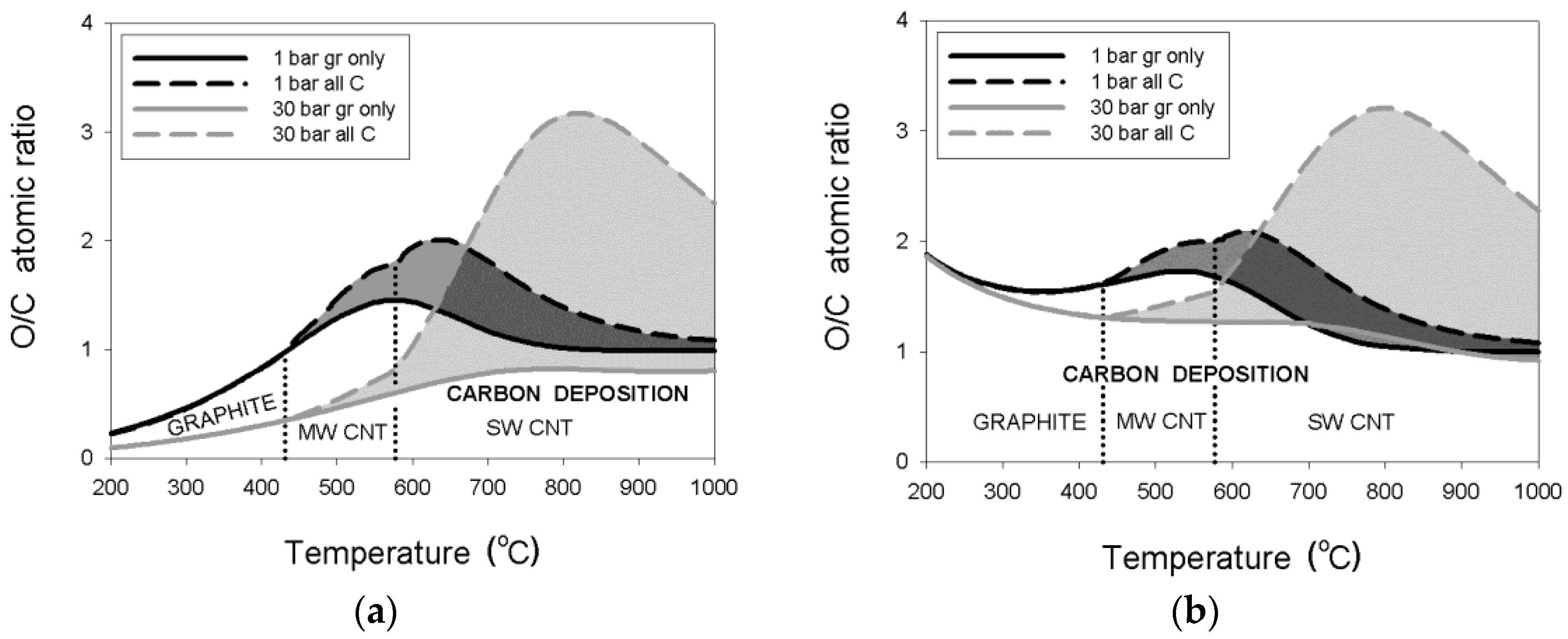
3.1.3. Pressure Effects
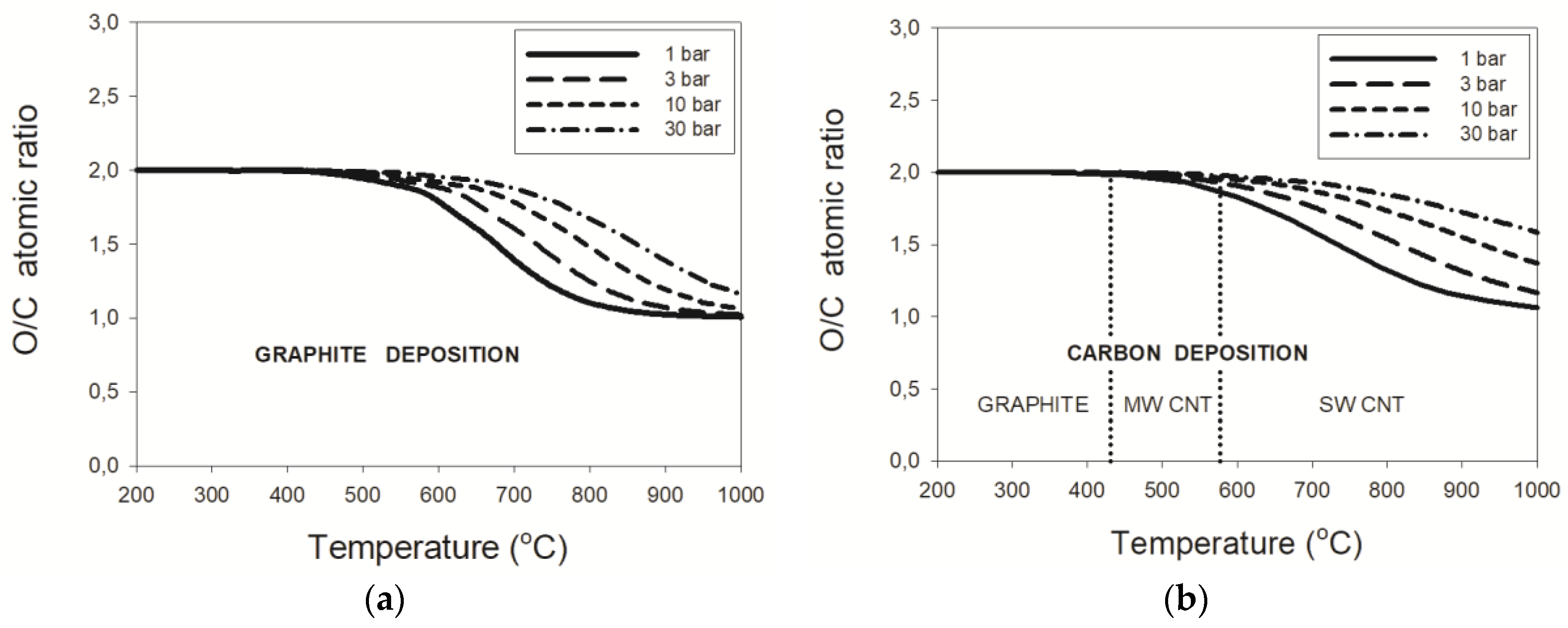
3.2. Dry Reforming
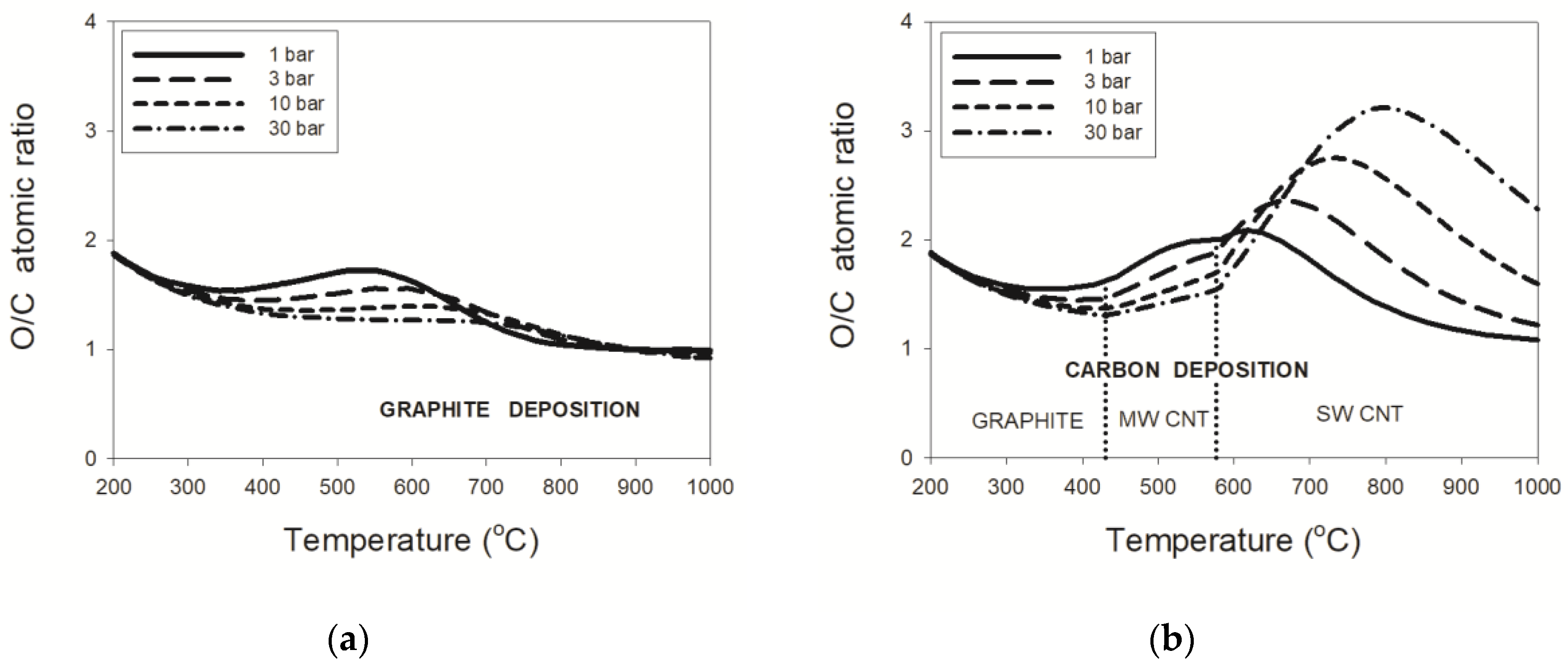
3.2.1. Natural Gas–CO2 Mixtures
3.2.2. LPG–CO2 Mixtures
3.2.3. Pressure Effects
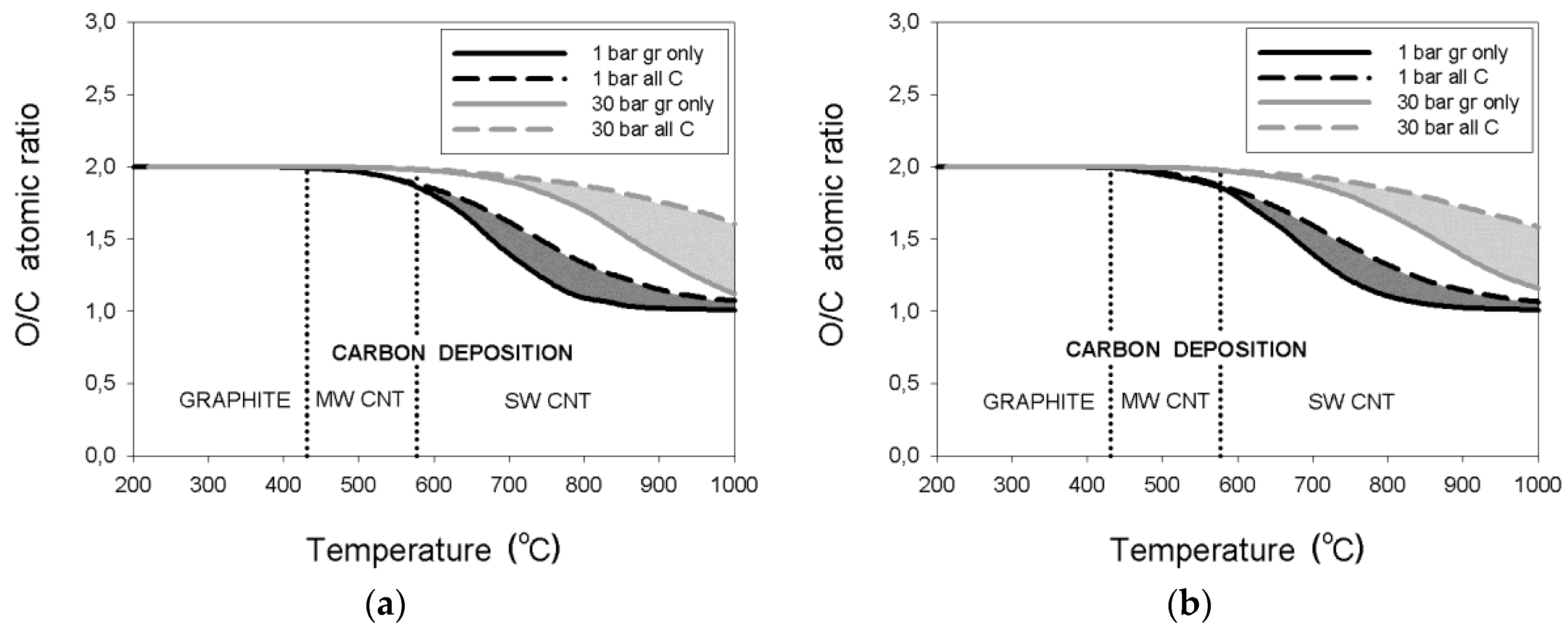
4. Discussion
5. Conclusions
- (i)
- thermodynamic data for purified carbon nanotubes indicate a substantially different dependence of the critical O/C ratio on temperature, in comparison to that for graphite deposition;
- (ii)
- the threshold temperature of 431 °C or 577 °C for the deposition boundaries between, respectively, graphite and MWCNT or MWCNT and SWCNT were confirmed regardless of pressure;
- (iii)
- an enlargement of the critical O/C ratio for SWCNT relative to that for graphite was computed at 30 bar to a maximum of about 290% or 37%, respectively, for the wet or dry reformate; and
- (iv)
- maximum critical O/C ratios were found for the wet reforming of NG and LPG at 2.0, 2.4, 2.7, and 3.2 for the process pressures of 1, 3, 10, and 30 bar, respectively.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mundhwa, M.; Thurgood, C.P. Numerical study of methane steam reforming and methane combustion over the segmented and continuously coated layers of catalysts in a plate reactor. Fuel Process. Technol. 2017, 158, 57–72. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Christiansen, L.J.; Hansen, J.H. Activity of steam reforming catalysts: Role and assessment. Appl. Catal. A 1988, 32, 287–303. [Google Scholar] [CrossRef]
- Maier, L.; Schadel, B.; Delgado, K.H.; Tischer, S.; Deutschmann, O. Steam reforming of methane over nickel: Development of a multi-step surface reaction mechanism. Top. Catal. 2011, 54, 845–858. [Google Scholar] [CrossRef]
- Dixit, M.; Baruah, R.; Parikh, D.; Sharma, S.; Bhargav, A. Authothermal reforming of methane on rhodium catalysts: Microkinetic analysis for model reduction. Comput. Chem. Eng. 2016, 89, 149–157. [Google Scholar] [CrossRef]
- Michael, B.C.; Donazzi, A.; Schmidt, L.D. Effects of H2O and CO2 addition in catalytic partial oxidation of methane on Rh. J. Catal. 2009, 265, 117–129. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wang, X.; Zhang, Y.; Lu, X.; Ding, W. Steam reforming of coke oven gas for hydrogen production over a NiO/MgO solid solution catalyst. Energy Fuels 2010, 24, 785–788. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Norskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam reforming and graphite formation on Ni catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Tang, Y.H.; Li, X.C.; Li, J.L.; Lin, L.W.; Xu, H.F.; Huang, B.Y. Experimental evidence for the formation mechanism of metallic catalyst-free carbon nanotubes. Nano-Micro Lett. 2010, 2, 18–21. [Google Scholar] [CrossRef]
- Avetisov, A.K.; Rostrup-Nielsen, J.R.; Kuchaev, V.L.; Bak Hansen, J.H.; Zyskin, A.G.; Shapatina, E.N. Steady-state kinetics and mechanism of methane reforming with steam and carbon dioxide over Ni catalyst. J. Mol. Catal. A Chem. 2010, 315, 155–162. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.S.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Chen, D.; Lodeng, R.; Svendsen, H.; Holmen, A. Hierarchical multiscale odelling of methane steam reforming reactions. Ind. Eng. Chem. Res. 2011, 50, 2600–2612. [Google Scholar] [CrossRef]
- Snoeck, J.W.; Froment, G.F.; Fowles, M. Filamentous carbon formation and gasification: Thermodynamics, driving force, nucleation and steady-state growth. J. Catal. 1997, 169, 240–249. [Google Scholar] [CrossRef]
- Snoeck, J.W.; Froment, G.F.; Fowles, M. Kinetic study of the carbon filament formation by methane cracking on a nickel catalyst. J. Catal. 1997, 169, 250–262. [Google Scholar] [CrossRef]
- Silva, P.P.; Ferreira, R.A.R.; Noronha, F.B.; Hori, C.E. Hydrogen production from steam and oxidative steam reforming of liquefied petroleum gas over cerium and strontium doped LaNiO3 catalysts. Catal. Today 2017, 289, 211–221. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Hydrogen production from steam and autothermal reforming of LPG over high surface area ceria. J. Power Sources 2006, 158, 1348–1357. [Google Scholar] [CrossRef]
- Jaworski, Z.; Zakrzewska, B.; Pianko-Oprych, P. On thermodynamic equilibrium of carbon deposition from gaseous C-H-O mixtures: Updating for nanotubes. Rev. Chem. Eng. 2017, 33, 217–235. [Google Scholar] [CrossRef]
- Wagg, L.M.; Hornyak, G.L.; Grigorian, L.; Dillon, A.C.; Jones, K.M.; Blackburn, J.; Parilla, P.A.; Heben, M.J. Experimental Gibbs free energy considerations in the nucleation and growth of single-walled carbon nanotubes. J. Phys. Chem. B 2005, 109, 10435–10440. [Google Scholar] [CrossRef] [PubMed]
- Yaws, C.L. Yaws’ Critical Property Data for Chemical Engineers and Chemists; Knovel: New York, NY, USA, 2012; ISBN 978-1-61344-932-5. Available online: http://app.knovel.com/hotlink/toc/id:kpYCPDCECD/yaws-critical-property (accessed on 12 September 2016).
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw Hill Higher Education: New York, NY, USA, 2004; ISBN 0-07-310445-0. [Google Scholar]
- Miller, S.A.; Jolivet, C.S.; Stoner, J.O., Jr. New methods for testing cyclotron carbon stripper foils. Nucl. Instrum. Methods Phys. Res. Sect. A 2008, 590, 57–65. [Google Scholar] [CrossRef]
- Castillo, J.; Grossmann, I.E. Computation of phase and chemical equilibria. Comput. Chem. Eng. 1981, 5, 99–108. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Outotec (Finland) Oy, Research Center Pori. Outotec’s HSC Chemistry v.8.0 Software; Outotec (Finland) Oy, Research Center Pori: Pori, Finland, 2014. [Google Scholar]
- Armor, J.N. The multiple roles for catalysis in the production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Jaworski, Z.; Pianko-Oprych, P. On nanotube carbon deposition at equilibrium in catalytic partial oxidation of selected hydrocarbon fuels. Int. J. Hydrog. Energy 2017, 42, 6920–6931. [Google Scholar] [CrossRef]
- Gozzi, D.; Iervolino, M.; Latini, A. The thermodynamics of the transformation of graphite to multiwalled carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 10269–10275. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, D.; Latini, A.; Lazzarini, L. Experimental thermodynamics of high temperature transformations in single-walled carbon nanotube bundles. J. Am. Chem. Soc. 2009, 131, 12474–12482. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.R.; Malone, M.A.; Nanney, W.A.; Maddux, C.J.A.; Coe, J.V.; Martinez, H.L. Generalizing thermodynamic properties of bulk single-walled carbon nanotubes. AIP Adv. 2014, 4, 127149. [Google Scholar] [CrossRef] [PubMed]
| Reaction “i” | Equation | kJ mol−1 | kJ mol−1 |
|---|---|---|---|
| 2 | CO + H2O = CO2 + H2 | −28.7 | −41.2 |
| 4 | CnH2n+2 + nH2O(g) = nCO + (2n + 1)H2 | ≅61.6 + 79.6 n | ≅56.4 + 148.4 n |
| 5 | CnH2n+2 + nCO2 = 2nCO + (n + 1) H2 | ≅61.6 + 108.2 n | ≅56.4 + 189.6 n |
| 6 | CH4 = C(g) + 2H2 | 721.8 | 791.2 |
| 7 | 2CO = C(g) + CO2 | 551.2 | 544.2 |
| 8 | CO + H2 = C(g) + H2O | 579.8 | 585.4 |
| 9 | 2H2 + CO2 = C(g) + 2H2O | 608.5 | 626.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworski, Z.; Pianko-Oprych, P. On the Deposition Equilibrium of Carbon Nanotubes or Graphite in the Reforming Processes of Lower Hydrocarbon Fuels. Entropy 2017, 19, 650. https://doi.org/10.3390/e19120650
Jaworski Z, Pianko-Oprych P. On the Deposition Equilibrium of Carbon Nanotubes or Graphite in the Reforming Processes of Lower Hydrocarbon Fuels. Entropy. 2017; 19(12):650. https://doi.org/10.3390/e19120650
Chicago/Turabian StyleJaworski, Zdzisław, and Paulina Pianko-Oprych. 2017. "On the Deposition Equilibrium of Carbon Nanotubes or Graphite in the Reforming Processes of Lower Hydrocarbon Fuels" Entropy 19, no. 12: 650. https://doi.org/10.3390/e19120650