Symbolic Analysis of Brain Dynamics Detects Negative Stress
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Preprocessing Applied to the EEG Recording
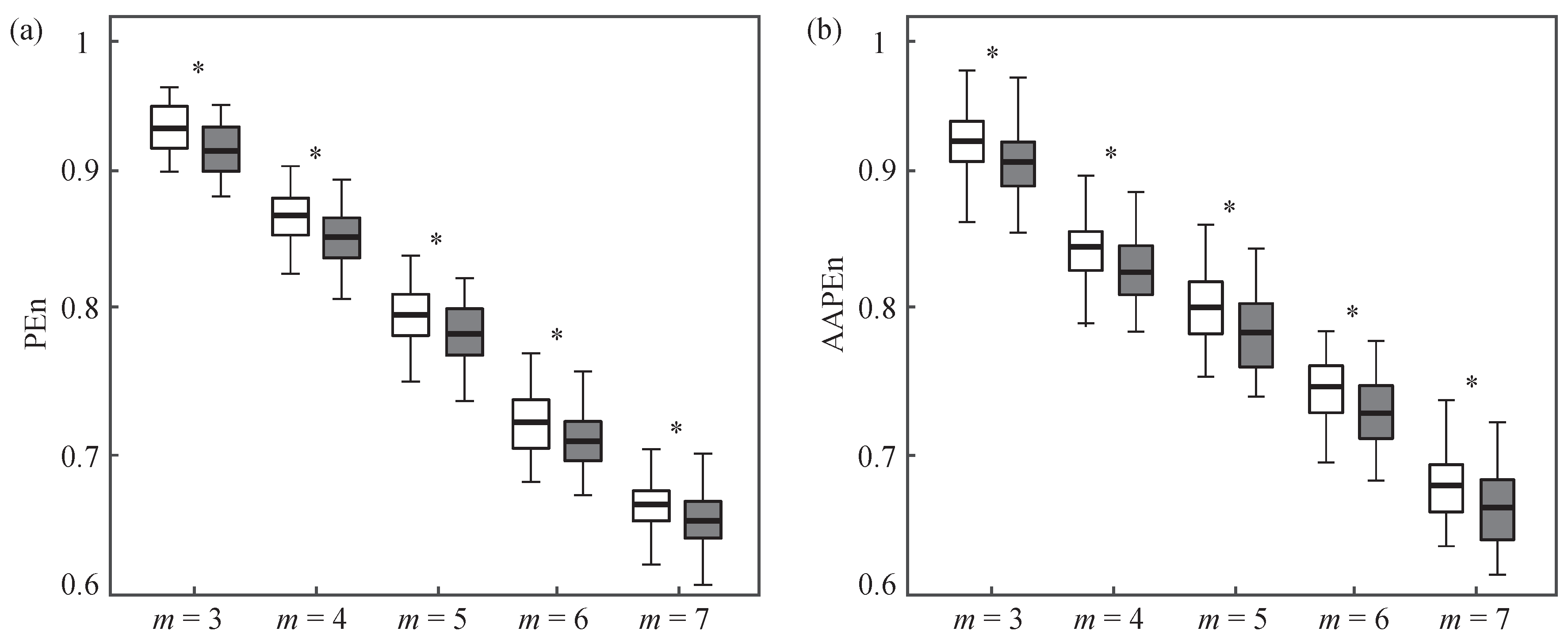
2.3. Permutation Entropy
2.4. Performance Assessment
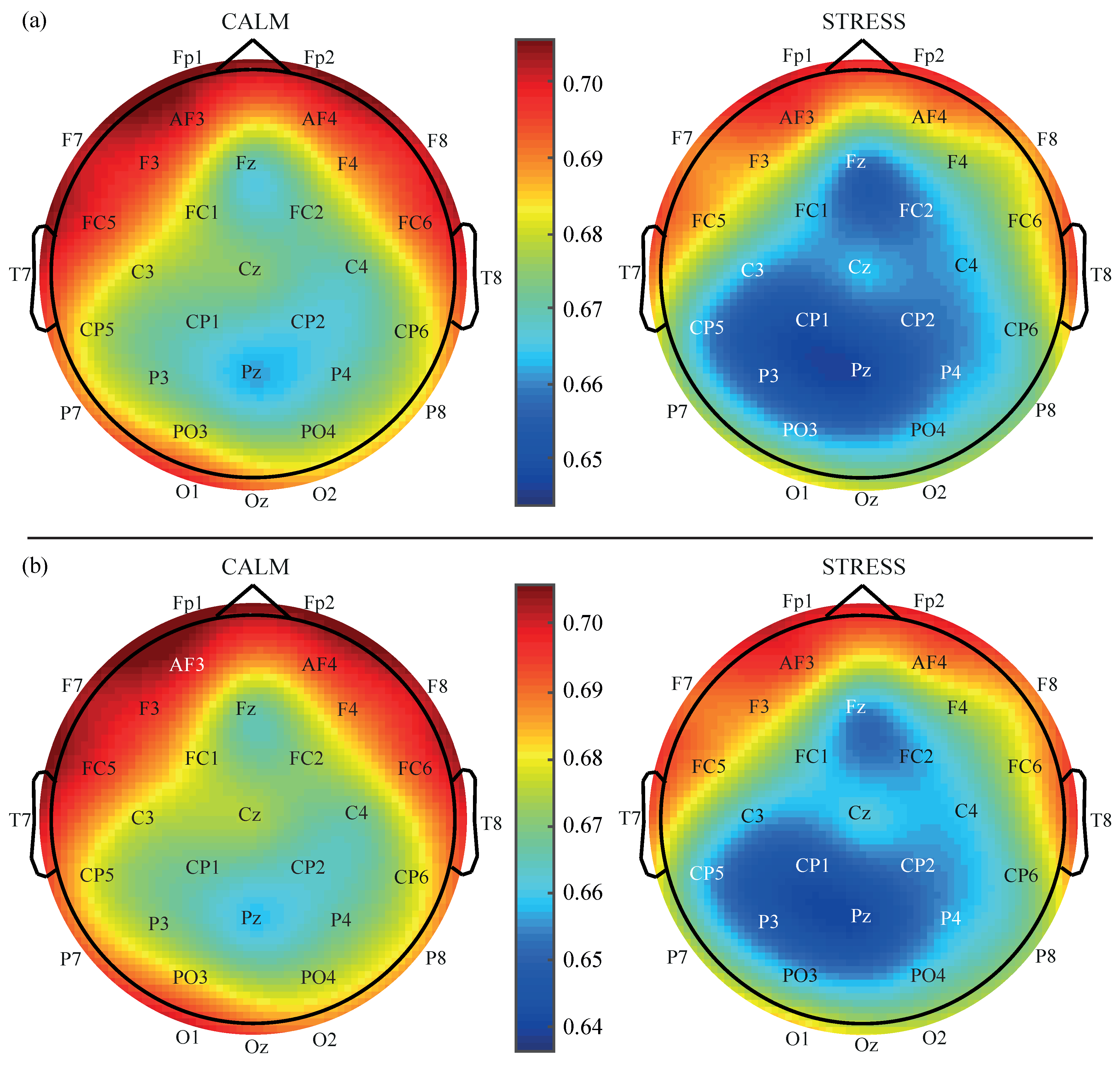
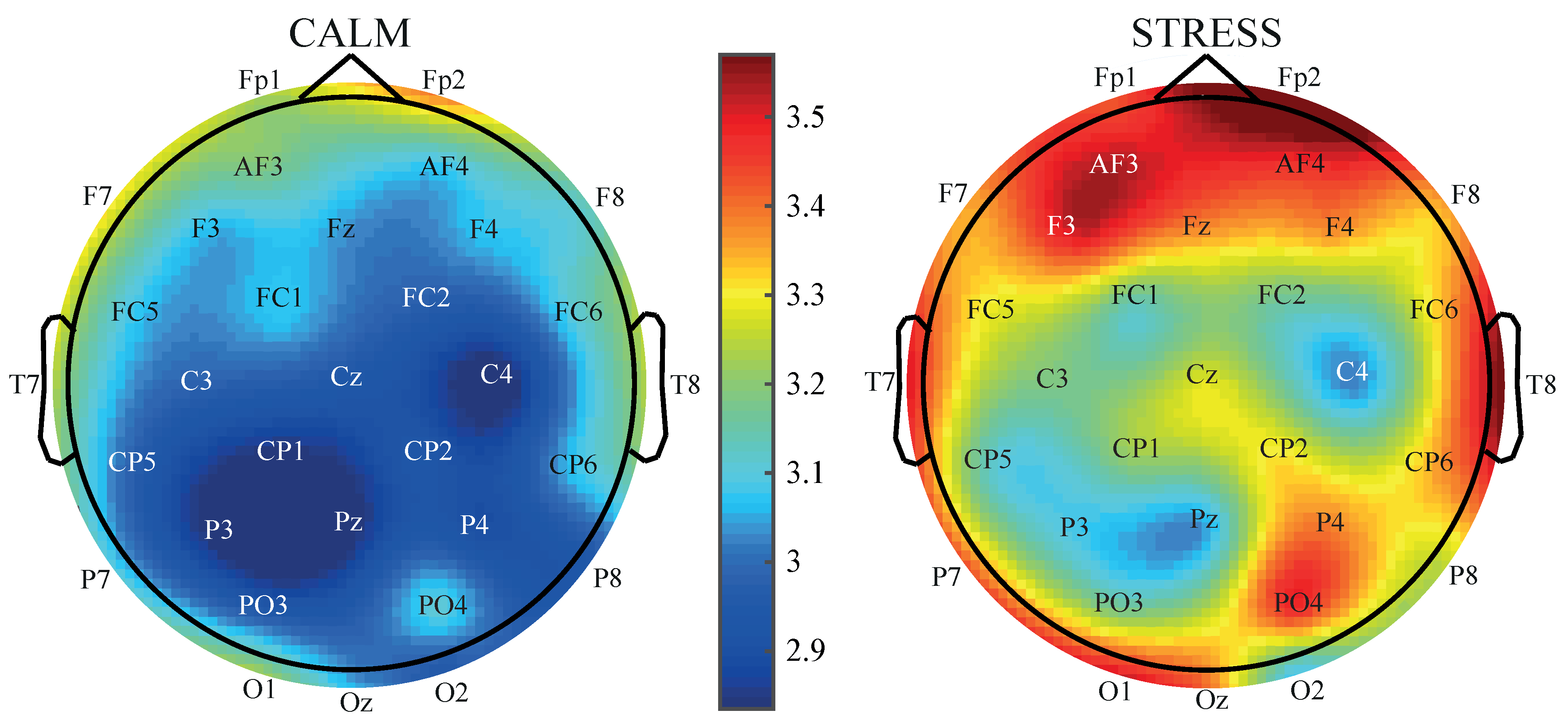
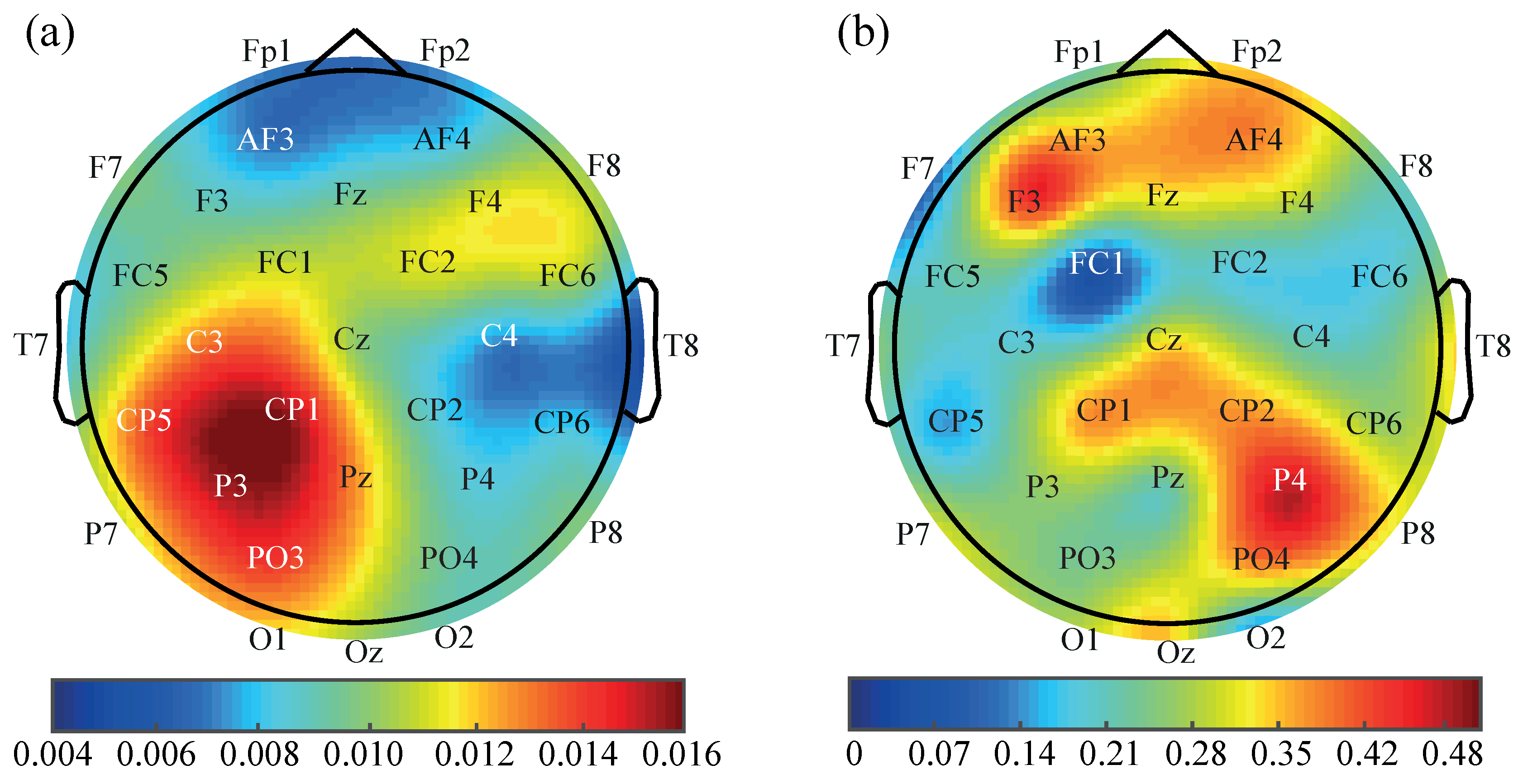
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanei, S. Adaptive Processing of Brain Signals; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Micoulaud-Franchi, J.A.; McGonigal, A.; Lopez, R.; Daudet, C.; Kotwas, I.; Bartolomei, F. Electroencephalographic neurofeedback: Level of evidence in mental and brain disorders and suggestions for good clinical practice. Neurophysiol. Clin. 2015, 45, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Complexity of spontaneous brain activity in mental disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.; Manthalkar, R.; Joshi, Y. Effect of meditation on emotional response: An EEG-based study. Biomed. Signal Process. Control 2017, 34, 101–113. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power spectral density and coherence analysis of Alzheimerś EEG. Cogn. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Tran, Y.; Naik, G.R.; Nguyen, T.N.; Ling, S.H.; Craig, A.; Nguyen, H.T. Classification of EEG based-mental fatigue using principal component analysis and Bayesian neural network. In Proceedings of the 2016 IEEE 38th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4654–4657. [Google Scholar]
- Chai, R.; Ling, S.H.; San, P.P.; Naik, G.R.; Nguyen, T.N.; Tran, Y.; Craig, A.; Nguyen, H.T. Improving EEG-Based Driver Fatigue Classification Using Sparse-Deep Belief Networks. Front. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Ji, X.; Huang, D. Classification of EEG signals based on autoregressive model and Wavelet packet decomposition. Neural Proc. Lett. 2017, 45, 365–378. [Google Scholar] [CrossRef]
- Vavadi, H.; Ayatollahi, A.; Mirzaei, A. A wavelet-approximate entropy method for epileptic activity detection from EEG and its sub-bands. J. Biomed. Sci. Eng. 2010, 3, 1182. [Google Scholar] [CrossRef]
- Simons, S.; Abasolo, D.; Hughes, M. Investigation of Alzheimer’s Disease EEG Frequency Components with Lempel-Ziv Complexity. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering, Dubrovnik, Croatia, 7–11 September 2014; Springer: Berlin, Germany, 2015; pp. 46–49. [Google Scholar]
- Akar, S.A.; Kara, S.; Agambayev, S.; Bilgic, V. Nonlinear analysis of EEG in major depression with fractal dimensions. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 7410–7413. [Google Scholar]
- Kalev, K.; Bachmann, M.; Orgo, L.; Lass, J.; Hinrikus, H. Lempel-Ziv and multiscale Lempel-Ziv complexity in depression. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 4158–4161. [Google Scholar]
- Micheloyannis, S.; Pachou, E.; Stam, C.J.; Breakspear, M.; Bitsios, P.; Vourkas, M.; Erimaki, S.; Zervakis, M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr. Res. 2006, 87, 60–66. [Google Scholar] [CrossRef]
- Padma Shri, T.; Sriraam, N.; Bhat, V. Characterization of EEG signals for identification of alcoholics using ANOVA ranked approximate entropy and classifiers. In Proceedings of the 2014 International Conference on Circuits, Communication, Control and Computing (I4C), Bengaluru, India, 21–22 November 2014; pp. 109–112. [Google Scholar]
- Andrzejak, R.G.; Lehnertz, K.; Mormann, F.; Rieke, C.; David, P.; Elger, C.E. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys. Rev. E 2001, 64, 061907. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, L.; Wang, J.; Wang, R.; Yu, H.; Cao, Y.; Liu, J. Characterization of complexity in the electroencephalograph activity of Alzheimer’s disease based on fuzzy entropy. Chaos 2015, 25, 083116. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J. Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clin. Neurophysiol. 2005, 116, 2266–2301. [Google Scholar] [CrossRef] [PubMed]
- Jenke, R.; Peer, A.; Buss, M. Feature Extraction and Selection for Emotion Recognition from EEG. IEEE Trans. Affect. Comput. 2014, 5, 327–339. [Google Scholar] [CrossRef]
- Calvo, R.A.; D’Mello, S.K. Affect Detection: An Interdisciplinary Review of Models, Methods, and Their Applications. IEEE Trans. Affect. Comput. 2010, 1, 18–37. [Google Scholar] [CrossRef]
- Valenza, G.; Lanata, A.; Scilingo, E.P. The Role of Nonlinear Dynamics in Affective Valence and Arousal Recognition. IEEE Trans. Affect. Comput. 2012, 3, 237–249. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, M.; Wang, Y.; Yang, J.; Zhang, J. Recognition of emotions using multimodal physiological signals and an ensemble deep learning model. Comput. Methods Progr. Biomed. 2017, 140, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J. Operator functional state classification using least-square support vector machine based recursive feature elimination technique. Comput. Methods Progr. Biomed. 2014, 113, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.A.; Allen, J.J.B. Handbook of Emotion Elicitation and Assessment; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Gomes, M.; Oliveira, T.; Silva, F.; Carneiro, D.; Novais, P. Establishing the relationship between personality traits and stress in an intelligent environment. In Proceedings of the International Conference on Industrial, Engineering and Other Applications of Applied Intelligent Systems, Kaohsiung, Taiwan, 3–6 June 2014; pp. 378–387. [Google Scholar]
- Ekman, P. An argument for basic emotions. Cogn. Emot. 1992, 6, 169–200. [Google Scholar] [CrossRef]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Alberdi, A.; Aztiria, A.; Basarab, A. Towards an automatic early stress recognition system for office environments based on multimodal measurements: A review. J. Biomed. Inform. 2016, 59, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Bong, S.Z.; Murugappan, M.; Yaacob, S. Methods and approaches on inferring human emotional stress changes through physiological signals: A review. IJMEI 2013, 5, 152–162. [Google Scholar] [CrossRef]
- Minguillon, J.; Lopez-Gordo, M.A.; Pelayo, F. Stress Assessment by Prefrontal Relative Gamma. Front. Comput. Neurosci. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- What Is Stress? American Institute of Stress. Available online: http://www.stress.org (accessed on 15 February 2017).
- Pickering, T.G. Mental stress as a causal factor in the development of hypertension and cardiovascular disease. Curr. Hypertens. Rep. 2001, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mönnikes, H.; Tebbe, J.J.; Hildebrandt, M.; Arck, P.; Osmanoglou, E.; Rose, M.; Klapp, B.; Wiedenmann, B.; Heymann-Mönnikes, I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig. Dis. 2001, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecien, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD). Role of Brain-Gut Axis. Curr. Neuropharmacol. 2016, 14, 892–900. [Google Scholar] [CrossRef]
- Bender, R.E.; Alloy, L.B. Life stress and kindling in bipolar disorder: Review of the evidence and integration with emerging biopsychosocial theories. Clin. Psychol. Rev. 2011, 31, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Martínez-Rodrigo, A.; Pastor, J.M.; Castillo, J.C.; Lozano-Monasor, E.; López, M.T.; Zangróniz, R.; Latorre, J.M.; Fernández-Sotos, A. Smart environment architecture for emotion detection and regulation. J. Biomed. Inform. 2016, 64, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Khalilzadeh, M.A.; Changiz, S. Emotional stress recognition system for affective computing based on bio-signals. J. Biol. Syst. 2010, 18, 101–114. [Google Scholar] [CrossRef]
- Khosrowabadi, R.; Quek, C.; Ang, K.K.; Tung, S.W.; Heijnen, M. A Brain-Computer Interface for classifying EEG correlates of chronic mental stress. In Proceedings of the 2011 International Joint Conference on Neural Networks (IJCNN), San Jose, CA, USA, 31 July–5 August 2011; pp. 757–762. [Google Scholar]
- Bastos Filho, T.F.; Ferreira, A.; Atencio, A.C.; Arjunan, S.P.; Kumar, D. Evaluation of feature extraction techniques in emotional state recognition. In Proceedings of the 4th International Conference on Intelligent Human Computer Interaction (IHCI), Kharagpur, India, 27–29 December 2012; pp. 1–6. [Google Scholar]
- García-Martínez, B.; Martínez-Rodrigo, A.; Cantabrana, R.Z.; García, J.M.P.; Martínez, R.A. Application of Entropy-Based Metrics to Identify Emotional Distress from Electroencephalographic Recordings. Entropy 2016, 18, 221. [Google Scholar] [CrossRef]
- Lake, D.E.; Moorman, J.R. Accurate estimation of entropy in very short physiological time series: The problem of atrial fibrillation detection in implanted ventricular devices. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H319–H325. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Unakafov, A.; Unakafova, V. Ordinal patterns, entropy, and EEG. Entropy 2014, 16, 6212–6239. [Google Scholar] [CrossRef]
- Zanin, M.; Zunino, L.; Rosso, O.; Papo, D. Permutation entropy and its main biomedical and econophysics applications: A review. Entropy 2012, 14, 1553–1577. [Google Scholar] [CrossRef]
- Amigó, J.M.; Keller, K.; Unakafova, V.A. Ordinal symbolic analysis and its application to biomedical recordings. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2015, 373, 20140091. [Google Scholar] [CrossRef] [PubMed]
- Bandt, C.; Pompe, B. Permutation entropy: a natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Escudero, J. Amplitude-aware permutation entropy: Illustration in spike detection and signal segmentation. Comput. Methods Progr. Biomed. 2016, 128, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Koelstra, S.; Mühl, C.; Soleymani, M.; Lee, J.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. DEAP: A Database for Emotion Analysis using Physiological Signals. IEEE Trans. Affect. Comput. 2012, 3, 18–31. [Google Scholar] [CrossRef]
- Morris, J.D. Observations SAM: The Self-Assessment Manikin—An efficient cross-cultural measurement of emotional response. J. Advert. Res. 1995, 35, 63–68. [Google Scholar]
- Pomer-Escher, A.G.; de Souza, M.D.P.; Filho, T.F.B. Methology for analysis of stress level based on asymmetry patterns of alpha rhythms in EEG signals. In Proceedings of the 5th ISSNIP-IEEE Biosignals and Biorobotics Conference: Biosignals and Robotics for Better and Safer Living (BRC), Salvador, Brazil, 26–28 May 2014; pp. 1–5. [Google Scholar]
- Klem, G.H.; Lüders, H.O.; Jasper, H.; Elger, C. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1999, 52, 3–6. [Google Scholar]
- Jadhav, P.; Shanamugan, D.; Chourasia, A.; Ghole, A.; Acharyya, A.; Naik, G. Automated detection and correction of eye blink and muscular artefacts in EEG signal for analysis of Autism Spectrum Disorder. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 1881–1884. [Google Scholar]
- Bhardwaj, S.; Jadhav, P.; Adapa, B.; Acharyya, A.; Naik, G.R. Online and automated reliable system design to remove blink and muscle artefact in EEG. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 6784–6787. [Google Scholar]
- Reis, P.M.R.; Hebenstreit, F.; Gabsteiger, F.; von Tscharner, V.; Lochmann, M. Methodological aspects of EEG and body dynamics measurements during motion. Front. Hum. Neurosci. 2014, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, B.; Chen, B.; Keil, A.; Príncipe, J. Weighted-permutation entropy: a complexity measure for time series incorporating amplitude information. Phys. Rev. E 2013, 87, 022911. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, A.; Xu, N.; Xue, J. Increment entropy as a measure of complexity for time series. Entropy 2016, 18, 22. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [PubMed]
- Jung, Y.; Jianhua, H. A K-fold averaging cross-validation procedure. J. Nonparametr. Stat. 2015, 27, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Liang, L.; Li, S.; Wang, R.; Yu, H.; Wang, J.; Wei, X. Complexity extraction of electroencephalograms in Alzheimer’s disease with weighted-permutation entropy. Chaos 2015, 25, 043105. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Wang, J.; Dng, B.; Wei, X. Complexity of resting-state EEG activity in the patients with early-stage Parkinson’s disease. Cogn. Neurodyn. 2017, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Bruzzo, A.A.; Gesierich, B.; Santi, M.; Tassinari, C.A.; Birbaumer, N.; Rubboli, G. Permutation entropy to detect vigilance changes and preictal states from scalp EEG in epileptic patients. A preliminary study. Neurol. Sci. 2008, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Thul, A.; Lechinger, J.; Donis, J.; Michitsch, G.; Pichler, G.; Kochs, E.F.; Jordan, D.; Ilg, R.; Schabus, M. EEG entropy measures indicate decrease of cortical information processing in Disorders of Consciousness. Clin. Neurophysiol. 2016, 127, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Arica, N.; Ergul, E.; Tan, O. Classification of obsessive compulsive disorder by EEG complexity and hemispheric dependency measurements. Int. J. Neural Syst. 2015, 25, 1550010. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Khalilzadeh, M.A.; Naghibi-Sistani, M.B.; Homam, S.M. Emotional stress recognition using a new fusion link between electroencephalogram and peripheral signals. Iran. J. Neurol. 2015, 14, 142–151. [Google Scholar] [PubMed]
- Begić, D.; Hotujac, L.; Jokić-Begić, N. Electroencephalographic comparison of veterans with combat-related post-traumatic stress disorder and healthy subjects. Int. J. Psychophysiol. 2001, 40, 167–172. [Google Scholar] [CrossRef]
- Natarajan, K.; Acharya, U.R.; Alias, F.; Tiboleng, T.; Puthusserypady, S.K. Nonlinear analysis of EEG signals at different mental states. Biomed. Eng. OnLine 2004, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, J.; Wu, B.W.Y.; Zhang, Z.; Chang, C.; Hung, Y.S.; Fung, P.C.W.; Sik, H.H. Entrainment of chaotic activities in brain and heart during MBSR mindfulness training. Neurosci. Lett. 2016, 616, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Heller, W.; Nitschke, J.B. The Puzzle of regional brain activity in and anxiety: The importance of subtypes and comorbidity. Cogn. Emot. 1998, 12, 421–447. [Google Scholar]
- Davidson, R.J. Affect, cognition, and hemispheric specialization. In Emotions, Cognition, and Behavior; Cambridge University Press: Cambridge, UK, 1984; p. 320. [Google Scholar]
- Manna, A.; Raffone, A.; Perrucci, M.G.; Nardo, D.; Ferretti, A.; Tartaro, A.; Londei, A.; Del Gratta, C.; Belardinelli, M.O.; Romani, G.L. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010, 82, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. The neurobiology of Meditation and its clinical effectiveness in psychiatric disorders. Biol. Psychol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nauta, W.J. Neural associations of the frontal cortex. Acta Neurobiol. Exp. 1972, 32, 125–140. [Google Scholar]
- Amigó, J.; Zambrano, S.; Sanjuán, M. Permutation complexity of spatiotemporal dynamics. EPL (Europhys. Lett.) 2010, 90, 10007. [Google Scholar] [CrossRef]
- Morabito, F.C.; Labate, D.; La Foresta, F.; Bramanti, A.; Morabito, G.; Palamara, I. Multivariate multi-scale permutation entropy for complexity analysis of Alzheimer’s disease EEG. Entropy 2012, 14, 1186–1202. [Google Scholar] [CrossRef]
- Papana, A.; Kyrtsou, C.; Kugiumtzis, D.; Diks, C. Simulation study of direct causality measures in multivariate time series. Entropy 2013, 15, 2635–2661. [Google Scholar] [CrossRef]
| Entropy | EEG | ROC Analysis | |||
|---|---|---|---|---|---|
| Metric | Channel | Value | Se (%) | Sp (%) | Acc (%) |
| P3 | 5.62 | 78.17 | 48.46 | 64.11 | |
| PO3 | 8.14 | 62.85 | 59.00 | 61.42 | |
| PEn | P8 | 2.16 | 65.74 | 55.79 | 61.03 |
| CP5 | 5.95 | 66.47 | 53.32 | 60.28 | |
| FC1 | 1.85 | 67.23 | 52.55 | 60.19 | |
| P3 | 2.71 | 78.83 | 50.05 | 65.39 | |
| FC2 | 1.34 | 73.74 | 48.42 | 61.89 | |
| F8 | 1.85 | 71.54 | 50.83 | 61.81 | |
| AAPEn | PO3 | 6.53 | 58.42 | 64.87 | 61.43 |
| CP5 | 5.31 | 70.13 | 50.86 | 61.07 | |
| P8 | 2.07 | 65.77 | 56.64 | 60.92 | |
| FC1 | 1.64 | 70.12 | 50.01 | 60.64 | |
| Work | Experiment | Features | Classifier | Accuracy |
|---|---|---|---|---|
| Bastos-Filho et al. [39] (2012) | 32 subjects | Statistical features, PSD and HOC | K-nearest neighbor (K-NN) | Stat.: 66.25% |
| 4 EEG channels | PSD: 70.1% | |||
| Videoclips | HOC: 69.6% | |||
| Hosseini et al. [37] (2010) | 15 subjects | FD, CD and wavelet entropy | Linear discriminant analysis (LDA) and SVM | LDA: 80.1% SVM: 84.9% |
| 5 EEG channels | ||||
| IAPS | ||||
| García-Martínez et al. [40] (2016) | 32 subjects | SEn, QSEn and DEn | Decision tree | 75.29% |
| 32 EEG channels | ||||
| Videoclips | ||||
| This study | 32 subjects | QSEn, PEn and AAPEn | SVM | 81.31% |
| 32 EEG channels | ||||
| Videoclips |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martínez, B.; Martínez-Rodrigo, A.; Zangróniz, R.; Pastor, J.M.; Alcaraz, R. Symbolic Analysis of Brain Dynamics Detects Negative Stress. Entropy 2017, 19, 196. https://doi.org/10.3390/e19050196
García-Martínez B, Martínez-Rodrigo A, Zangróniz R, Pastor JM, Alcaraz R. Symbolic Analysis of Brain Dynamics Detects Negative Stress. Entropy. 2017; 19(5):196. https://doi.org/10.3390/e19050196
Chicago/Turabian StyleGarcía-Martínez, Beatriz, Arturo Martínez-Rodrigo, Roberto Zangróniz, José Manuel Pastor, and Raúl Alcaraz. 2017. "Symbolic Analysis of Brain Dynamics Detects Negative Stress" Entropy 19, no. 5: 196. https://doi.org/10.3390/e19050196






