1. Introduction
Modern sensor technology makes it possible to monitor vital signs continuously in everyday life, with high precision and without causing much discomfort. This will lead to very personalized, powerful and preventive medical treatment. Sleep medicine is one of the fields where such development is already apparent. While the technical basis is now available, automatic evaluation of the big data series is lagging behind. Classical methods of time series analysis usually require clean data, and preprocessing routines can easily remove information. New methods have to be developed and tested.
Use of order patterns in time series is such a new methodology. Permutation entropy, introduced in [
1], has been applied not only to geophysical, financial and machine data but also in biomedical context [
2,
3,
4,
5,
6]. Here, we are concerned with EEG (electroencephalographic) sleep data only, to which permutation entropy was applied by Ouyang et al. [
7], Kuo and Liang [
8], Nicolaou and Georgiou [
9], and others. We shall introduce a new version of permutation entropy that can be supported by a statistical model which allows for calculating significance, and show how the settings of parameters can be optimized to recognize sleep stages from short time series of a single EEG channel. The presented methodology can be used for other medical applications, such as (see [
6,
10] for further references):
In the next section, we define mathematical concepts and compare the new version of permutation entropy with the usual one. A statistical discussion of significance limits for permutation entropy seems to appear here for the first time. In
Section 3, we apply our method to a classical database of sleep medicine by Terzano et al. [
16], which is available on physionet [
17]. The sleep stages annotated by experts apparently coincide with the entropy that is measured on a continuous scale. While the annotation was done by medical doctors on the basis of multichannel data, we use only one EEG channel, and no preprocessing or special treatment of the data, just the simple entropy formula. Segments of 30 s do suffice to evaluate accurately the depth of sleep. While the experts study graphic elements on a scale of several seconds, like delta waves, as recommended by the official guidelines [
18], our method analyses the invisible microstructure in high-resolution measurements. EEG data were recorded with 512 Hz, and patterns of length between 4 and 40 ms were studied. In
Section 4, we explain how we found optimal parameters, and
Section 5 summarizes our main points.
2. Distance to White Noise—A New Version of Permutation Entropy
We consider a time series with
T values, denoted
In our application, the typical length
T will vary between 500 and 20,000. Any three consecutive values
can form one of the six order patterns, or permutations, shown in
Figure 1. We can also consider three values
with a time distance
We say the points represent pattern 231, for instance, if
In the context of EEG data, ties
are very rare. They can be counted as < and will be neglected in the present study. The initial time point
t runs from 1 to
The delay parameter
d can vary between 1 and
and has the same meaning as in classical autocorrelation. Usually,
consecutive values are considered for permutation entropy, and there are
patterns. In this paper, however, we focus on the case
for the following reasons:
we want to keep things simple;
for we understand the meaning of each pattern;
there is a nice statistical theory for patterns of length 3 [
19,
20,
21];
results for are good when we consider various delay parameters So far, most authors consider only and different
It should also be mentioned that the statistics of order pattern frequencies are excellent, even for short time series like
when we have only six patterns. For
for instance, we have
patterns and need a very long time series to estimate all of those pattern frequencies. Permutation entropy will still work since it is an average over all patterns. Here, we prefer the simple setting. Let us explain how frequencies are estimated for a pattern
We count the number of all appearances of the pattern and divide by the number of places where the pattern can occur:
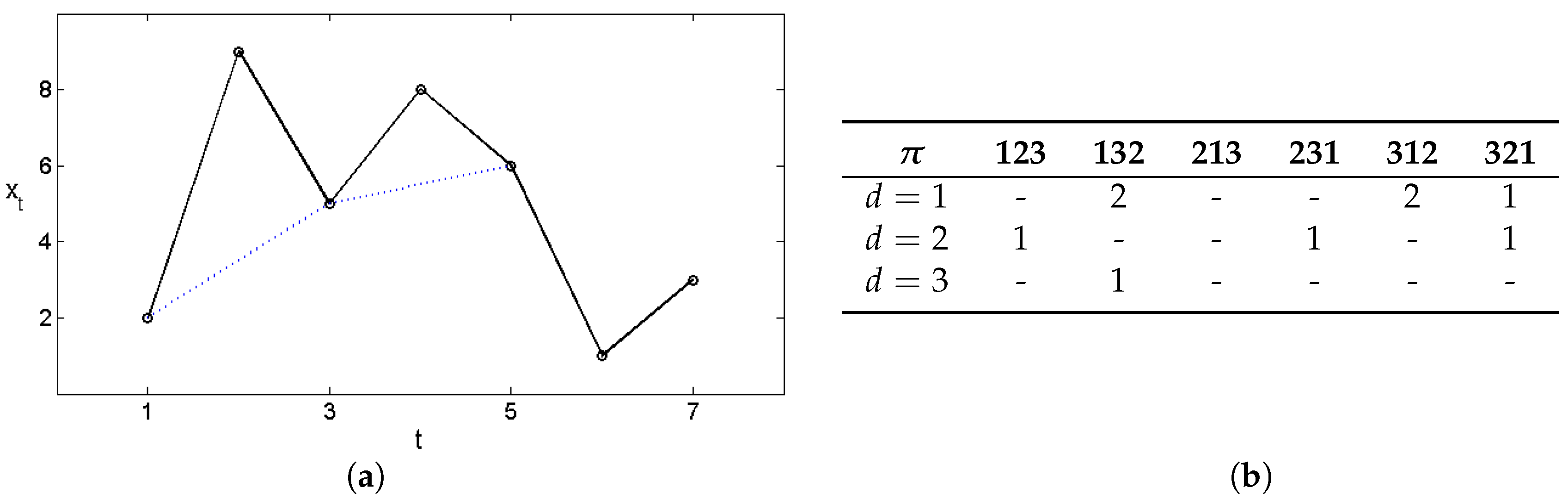
To understand the method, consider the short time series
shown in
Figure 2. The table collects the frequencies. As a result, we have
and
, and could draw this as a kind of autocorrelation function.
Frequencies of single patterns have been studied by several authors [
4]. For statistical reasons, we prefer to study only certain sums and differences of pattern frequencies [
20,
21]. The permutation entropy is the Shannon entropy of the distribution of all patterns. It is defined for the set
of all
patterns of length
m [
1]. For our case
the sum involves only the six terms indicated in
Figure 1. However, the delay parameter
d can vary again between 1 and
so that permutation entropy also becomes a kind of autocorrelation function:
Entropy as a measure of disorder is a basic concept in physics. Permutation entropy was introduced as complexity measure for time series.
H assumes its smallest value zero for a monotone series, and its maximum
for white noise, where there is no dependence among the values. White noise means that all possible permutations appear with the same probability
. Here, we use a new version of permutation entropy, called ”distance to white noise” and defined for
as
We just take the squared Euclidean distance of the observed pattern frequencies from the uniform pattern frequencies
in the space of all pattern distributions. Thus, the smallest value of
is zero and means complete independence of values. Large
means much dependence among the values of the time series. This is easy to understand, and we cannot become confused by terms like “complexity”, “chaos”, and “disorder”. The sum in Equation (
3), as well as in Equation (
2), contains
terms, which means six terms for
The equality on the right side of Equation (
3) follows from
There are several reasons to call a version of permutation entropy:
Equation (
3) says that we get
from
H by replacing
with the simpler function
and adding a constant so that the minimum is zero—not a big change!
Up to a linear transformation,
is the quadratic Taylor approximation of
H at white noise ([
19], see below for
).
For a discrete probability space
with probabilities
the quantity
is called Renyi entropy of order 2, or correlation entropy [
22], and
is called Tsallis entropy of order 2 or Kendall information content [
23].
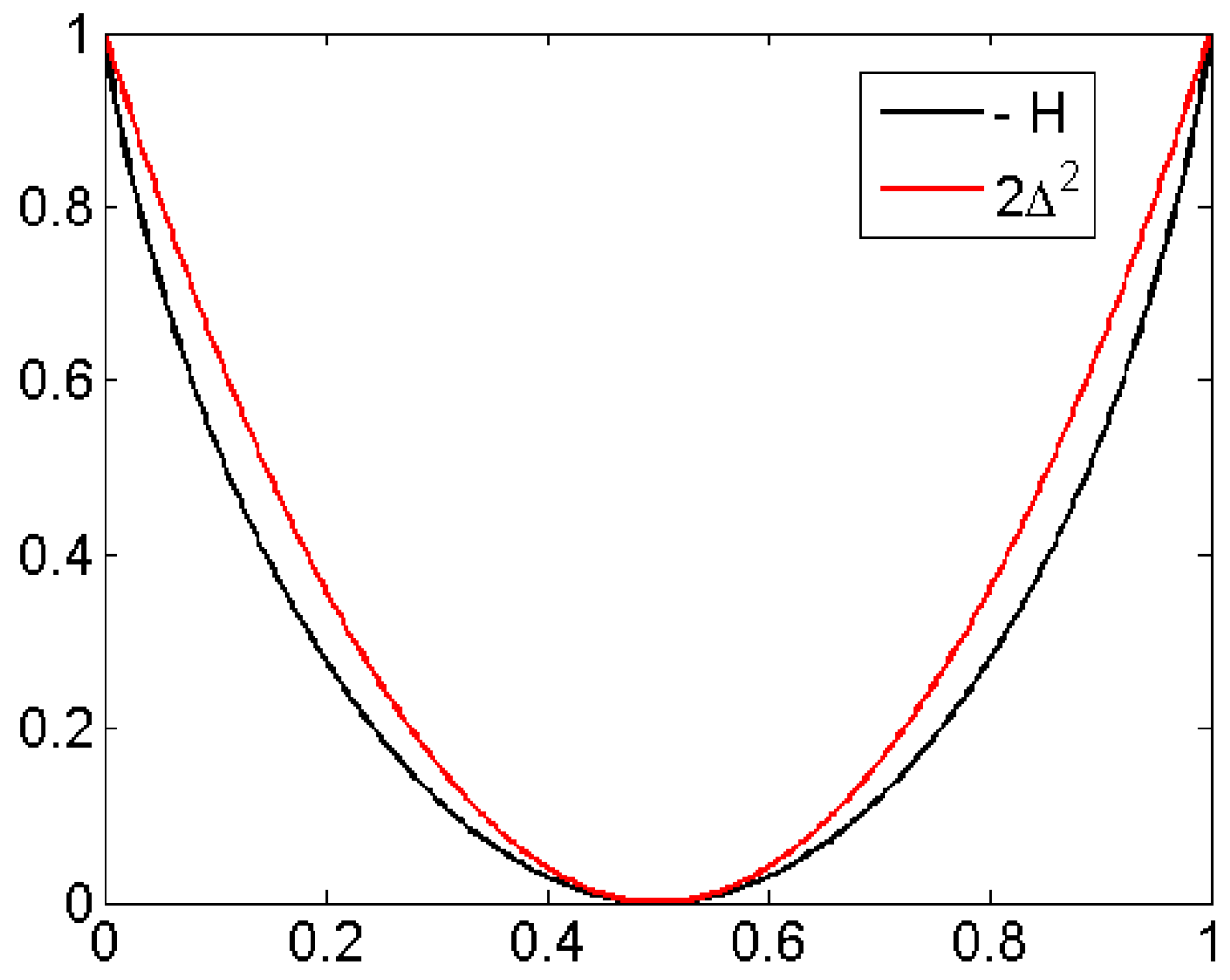
For the case of two probabilities
p and
(length 2 patterns 12 and 21),
Figure 3 shows the functions
and
They do not differ much, and agree asymptotically at the point
The same holds for the six probabilities
of patterns of order 3. At the point
for all
which corresponds to white noise, it can be shown that
is the quadratic Taylor approximation of
H [
19]. Note that EEG data, compared to other time series like ECG (electrocardiogram), are very erratic, close to white noise so that
H and
will lead to similar results. This will be demonstrated in Figure 10.
It turns out that
has better statistical properties than
In [
19], it was shown that
can be separated into different components according to an equation
which allows a more detailed study with a kind of ANOVA method. For EEG data, only the
component is important and will be discussed below.
We need to know the statistics of permutation entropy in order to check whether certain extreme values of H are mere coincidence or really indicate a certain effect: good order or large disorder. Although a few hundred papers deal with permutation entropy, this statistical aspect seems to be discussed here for the first time.
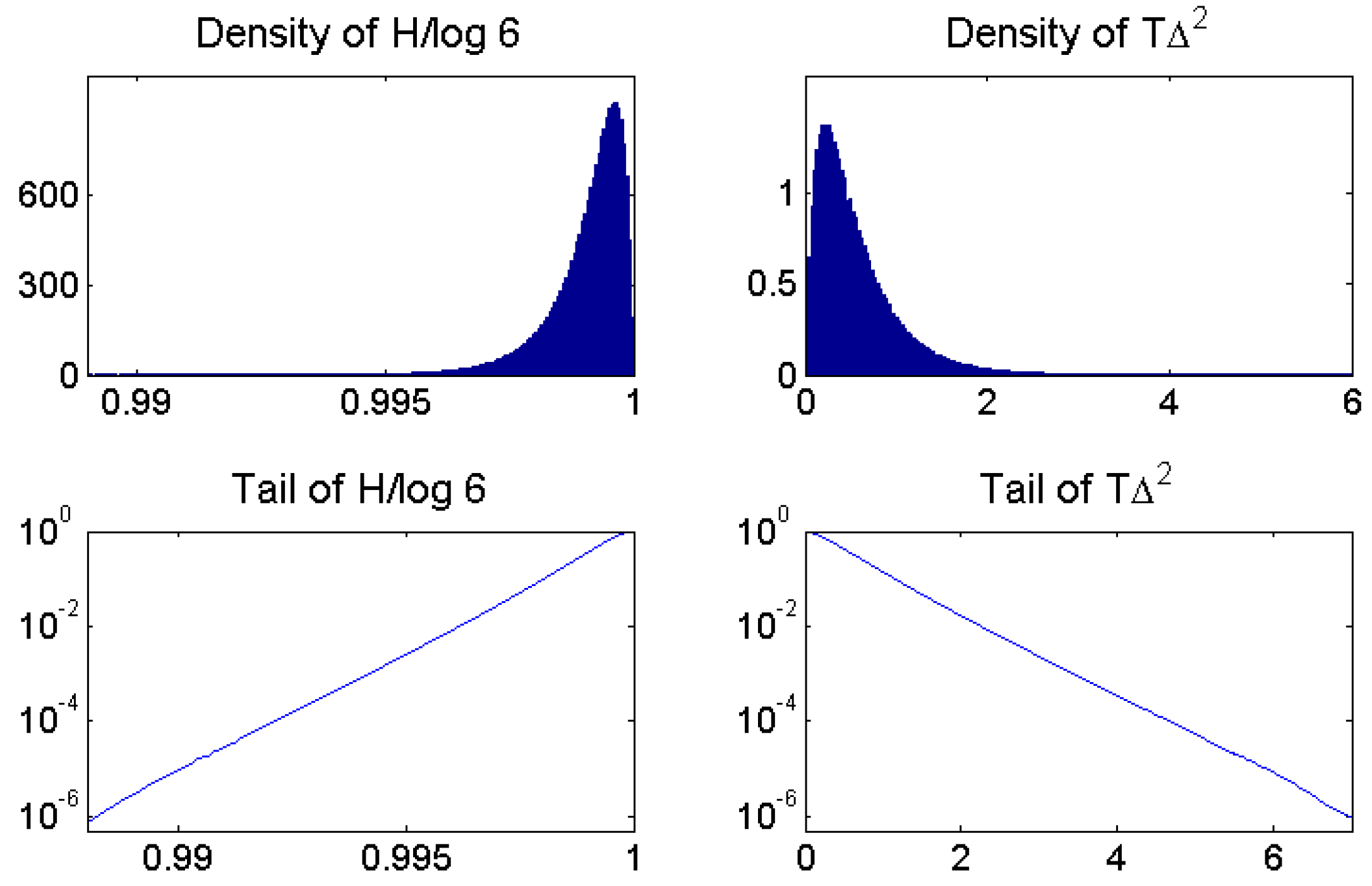
For statistical inference, we always need a null model. In our case, the null hypothesis is that the data are white noise: completely independently chosen random numbers from the same distribution. The type of distribution does not matter for ordinal patterns. We can take the uniform distribution on In a computer simulation, we now take a large number say 10 million, time series of length all made of independent random numbers. We determine the permutation entropy for each sample series, getting N possible values These N values vary near the maximum value , which is the theoretical value of permutation entropy of length 3 patterns for white noise. In each sample series, the value is somewhat smaller, however.
It is reasonable to consider
to get a standard scale with maximum value 1.
Figure 4 shows the density of all
N sample series. We see that all standardized
H-values vary between 0.99 and the maximum value 1. The conclusion is that when we observe a time series of length
in practice, and
is less than 0.99, we can be sure that this is not a random deviation from white noise! Even 0.995 would be a significant observation, since the tail probability or
p-value of 0.995 shown in the lower panel of
Figure 4 is about
This means that only
of our 10 million simulations of white noise gave a standardized
H value below 0.995. The value 0.99 is more significant, however, since only 100 samples gave a still smaller
which corresponds to a
p-value of
The tail probability of
in
Figure 4 is almost a linear function in semilogarithmic representation, so it is easy to approximate numerically. There are two problems, however. First, the scale between 0.99 and 1 is not very intuitive. It can lead to confusion between quantile values of the
H-statistics and the
p-values themselves. Second, and worse, the dependence on
T has not been considered. It is clear that the simulation will vary more when we have smaller
that is, shorter time series. The mathematical formula for this dependence is not obvious.
On the right-hand side of
Figure 4,
was simulated for the 10 million sample series. Its distribution very much resembles a
distribution known from classical statistics. In contrast to
extreme values are on the right. The quantiles are spread over a wider range, as seen in the lower panel and in
Table 1. Even more importantly, we have drawn
instead of
Since distance to white noise is a kind of variance, it can be shown to scale with
and the curves of
almost coincide for
T larger 1000. Thus, the critical values of
in
Table 1 are almost universal while the critical values of
are valid for
only. To give just one example, the value 4.68 for the 0.01% threshhold of
is 4.67 for
and 4.69 for
while the
-quantile 0.9921 of standardized
H will change to 0.9843 and 0.9961, respectively. Still more extreme quantiles are harder to simulate and less stable.
To conclude our statistical discussion, let us note that this is just a beginning. More modelling is needed. The white noise hypothesis is not so exciting and would not make sense for heart or respiration data. For EEG data and, in particular, for sleep stages; however, white noise is a reasonable null hypothesis, as we shall see below.
3. as a Measure of Sleep Depth
We briefly explain the basic idea for our classification of sleep stages. The brain of a healthy awake adult fulfils a large variety of functions, and each EEG channel covers the activity of millions of neurons in the cortex. Thus, normally, the signal will be almost white noise. With the onset of sleep, neuronal activity will become weaker and less diverse, and global rhythms are taking over. Global phenomena become visible for a human observer of the data as delta waves, sleep spindles, K-complexes, as described in the official guidelines for sleep scoring [
18]. However, long before global phenomena become visible, they manifest themselves in statistical properties of the fine structure of high-resolution data. For our application, the main change is an increase of the frequency of patterns 123 and 321, compared to the other patterns of
Figure 1.
In other words, the number of local minima and maxima will decrease. Since a change of standardized H from 1 to 0.99 is already highly significant, such a statistical tendency can be swiftly determined with permutation entropy. Using the language of Fourier analysis, we would state that high frequencies become weaker. However, the idea of brain signal as a composition of sine waves is not quite correct. Such waves may develop only partially, for less than a quarter than a wavelength. In such cases, they are detected by permutation entropy before the frequency spectrum shows any changes. Moreover, order pattern statistics is much more stable and less susceptible to data artefacts than the Fourier frequency statistics.
The deeper the sleep, the more our EEG signal will deviate from white noise. Thus,
should be a good measure for sleep depth.
Figure 5 shows how well this idea works. We have chosen the classical CAP sleep database of Terzano et al. [
16] for different reasons: it is freely available at physionet [
17]. It contains a number of EEG measurements taken with sample rate 512 Hz while many other datasets contain 128 Hz measurements or low-pass filtered signals. The data quality is good and expert sleep annotation files are provided, still including sleep stage S4, which was later abandoned [
18].
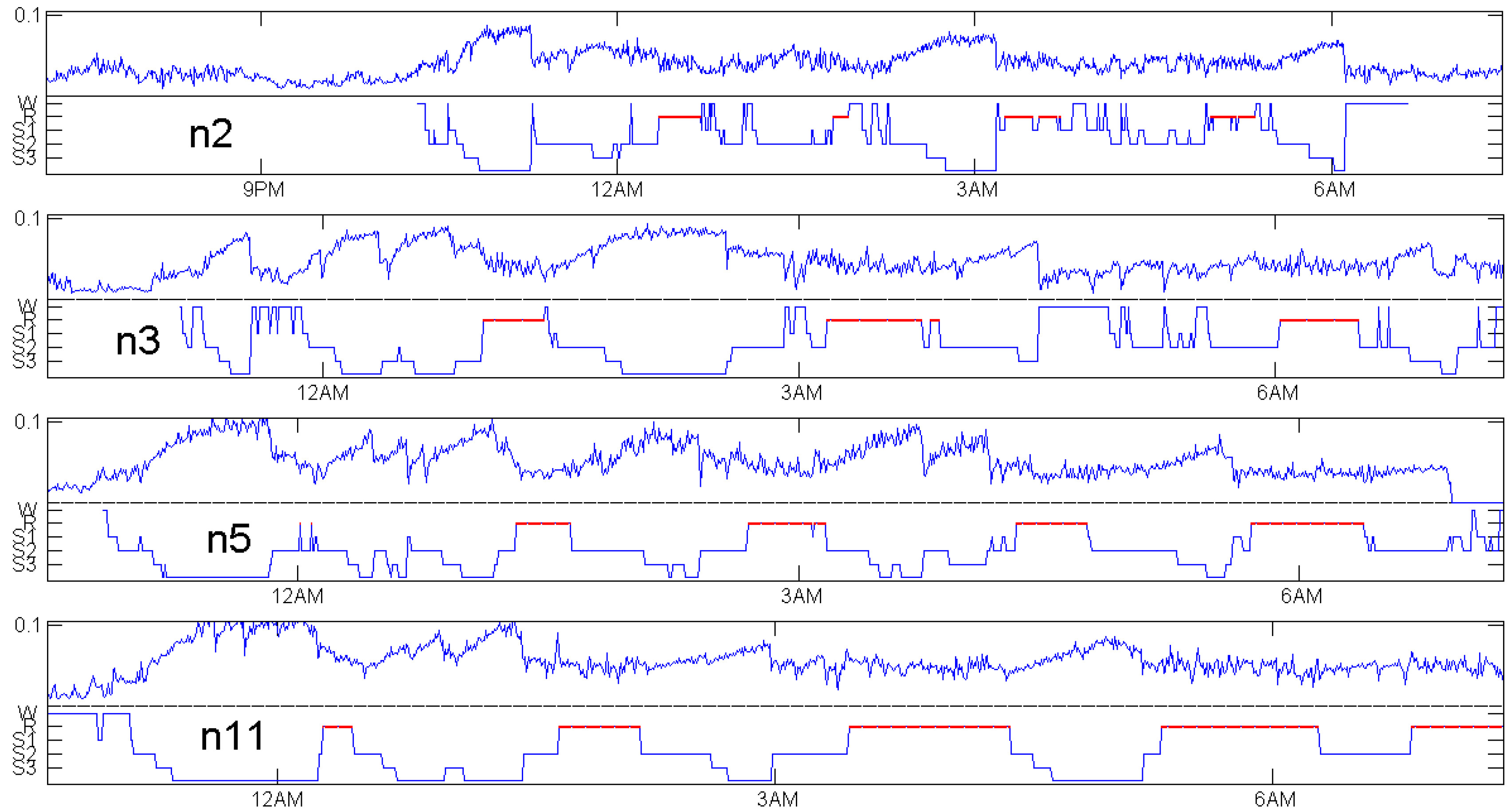
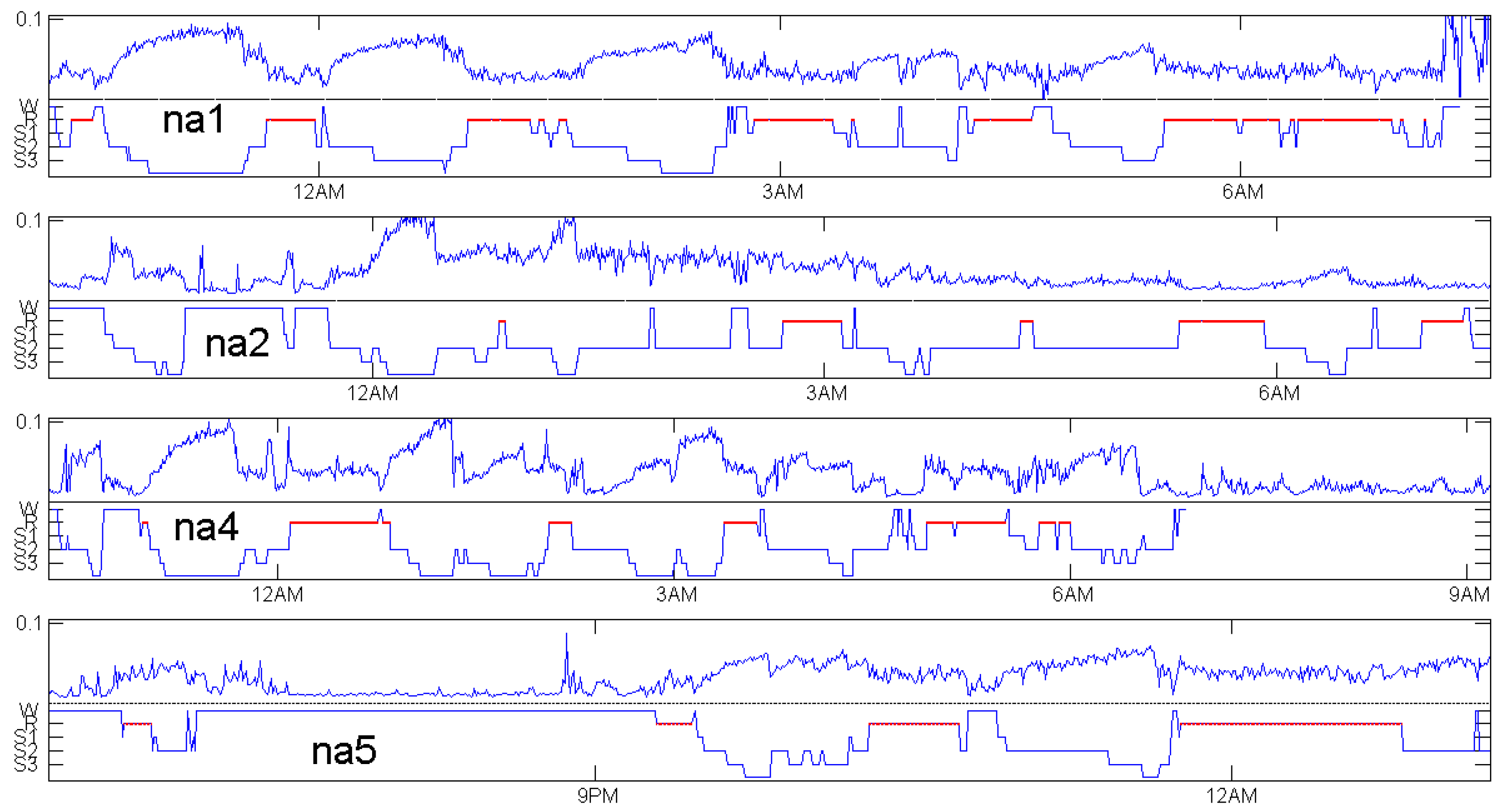
For four healthy subjects,
Figure 5 shows the expert annotation as a step function on the lower part and
as a noisy function on the upper part of the respective panel. It turns out that we almost have mirror symmetry. Whenever the sleep stage increases,
increases, and vice versa. The calculation of
was done with the data as they were provided, without any preprocessing or selection of “clean segments”. Non-overlapping windows of 30 s in length were used to calculate each value.
REM (rapid eye movement) phases, indicated by red lines in every annotation of
Figure 5, are not considered here.
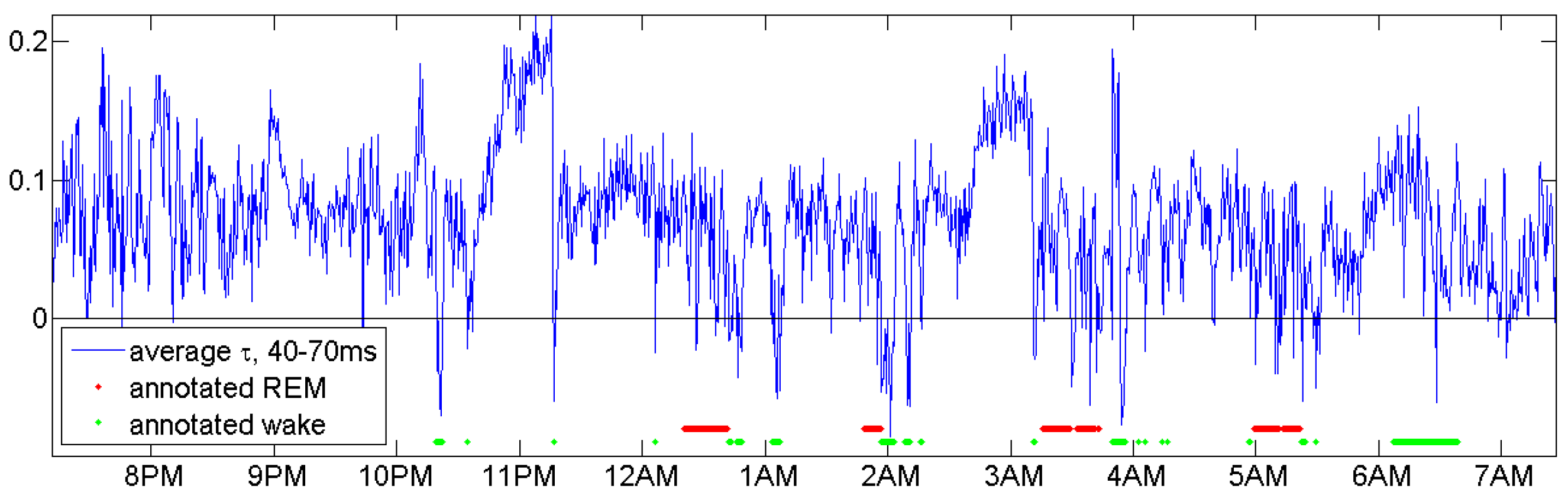
Figure 6 below shows that with modified delays, a function
related with
and defined in Equation (
5) below can indicate REM phases, but does not classify them accurately. We should admit that this is not a systematic study, which would need tight cooperation with medical experts and could be better done with recent measurements. Moreover, if our primary interest was accurate classification, we had to use the whole power of multivariate datasets. Here, the challenge was to get maximum information from a single EEG channel.
The choice of patients for
Figure 5 was based on availability of an EEG channel with 512 Hz frequency in the data, not at all on the quality of the coincidence. There were only four healthy controls with a 512 Hz EEG channel.
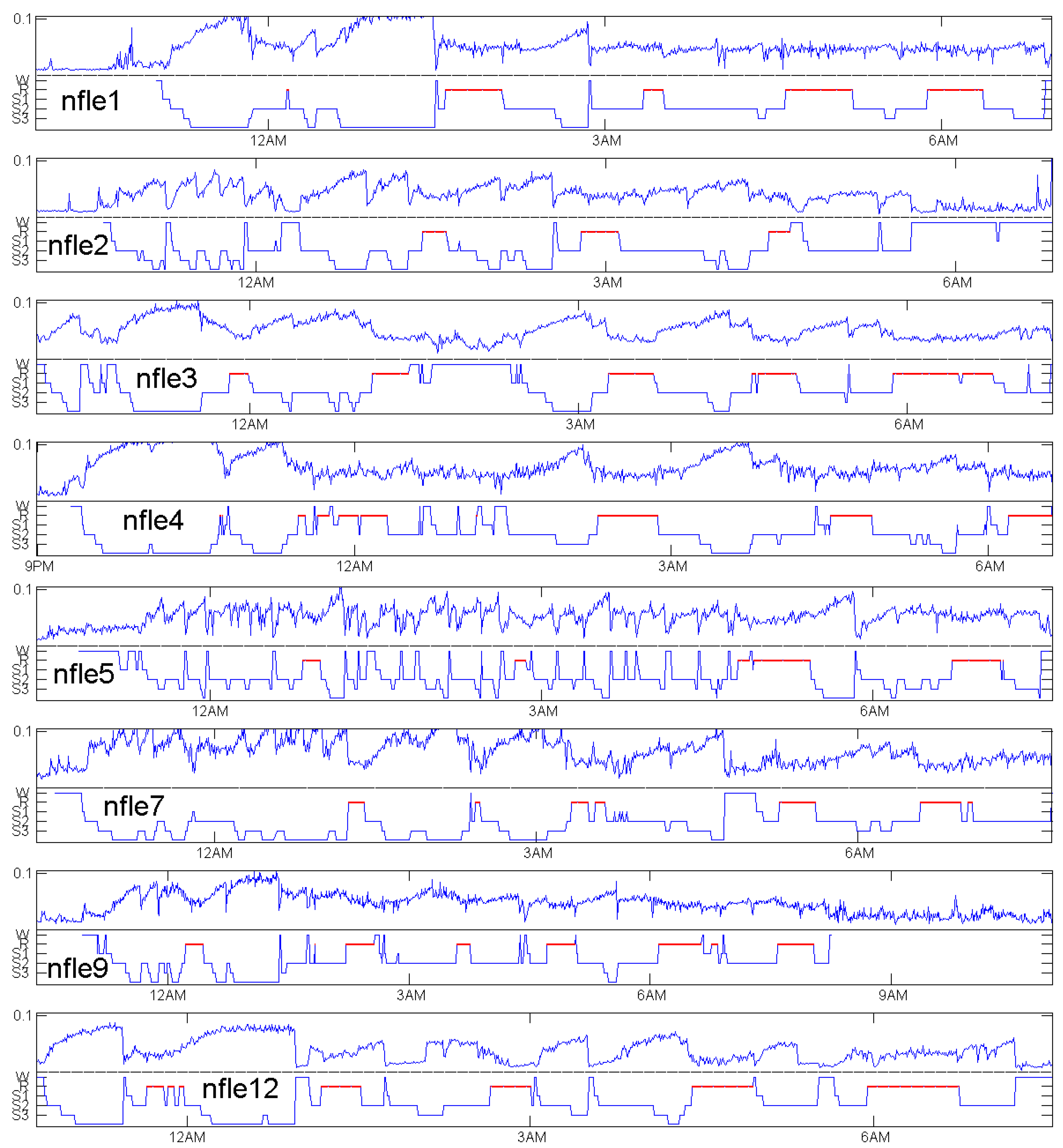
Figure 7,
Figure 8 and
Figure 9 show patients with insomnia, narcolepsy and nocturnal frontal lobe epilepsy with high-resolution EEG channel from the CAP sleep database of Terzano et al. [
16] available at physionet [
17]. The coincidence of annotation and
was always excellent, though there were more artefacts. The standard EEG channel was Fp2–F4. On three occasions, another channel was provided, and gave similar results.
For the healthy subjects in
Figure 5, maximum values of
do not differ much: they are around 0.1. We do not know whether it makes sense to compare sleep depth of different persons just by
Maybe individual factors do influence
We even had no data to check changes of
when one subject is measured repeatedly. We think
will not depend much on measurement details, but this was not verified. In
Figure 7, showing patients with insomnia, the overall level of
is much lower than for the controls in
Figure 5. This indicates that
can also detect certain sleep disorders, by taking the average
and grouped box plots over several hours. In a similar way, average
can be used to compare the sleep of one subject in several nights or under different conditions. There are numerous ways to exploit the permutation entropy.
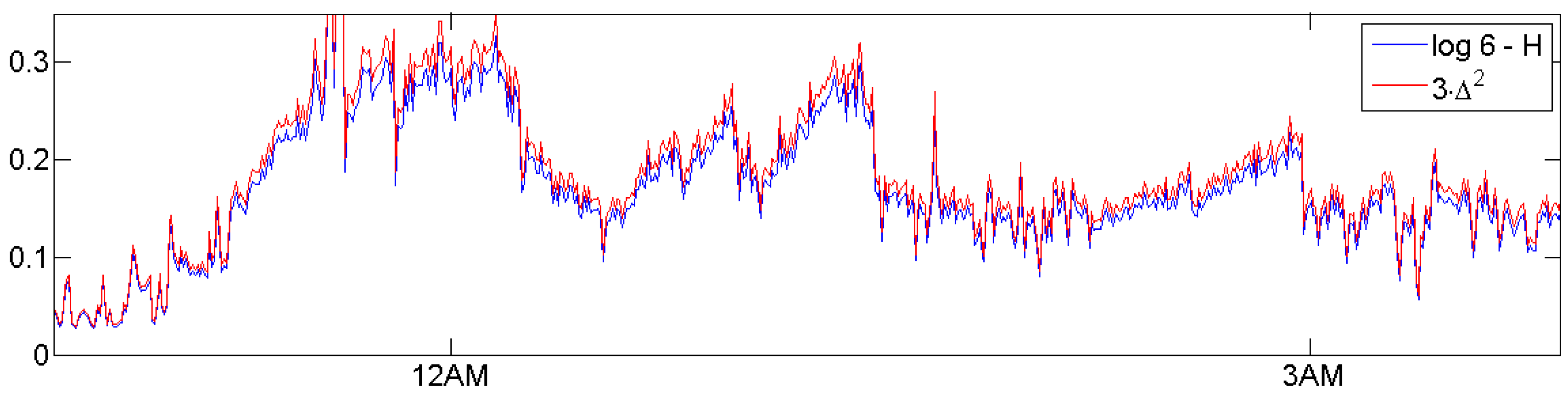
Actually, it does not matter whether we take
or the original permutation entropy
H as a measure of sleep depth. As
Figure 10 shows, they do almost coincide after a linear scale change. Since our data are fairly near to white noise, this can be proved by Taylor’s formula, as mentioned in
Section 2. We chose
since it has a more natural scale, a nice interpretation, and a familiar statistics. What really matters is the choice of delays
d over which we average
or
respectively. Now, we explain how we choose those parameters.
4. The Choice of Optimal Parameters
Compared to Fourier analysis and other complicated tools such as ‘detrended fluctuation analysis’, permutation entropy is a simple method. It does not depend too much on long-term experience. Essentially, with a bit of care, one cannot go wrong with it. Nevertheless, we have to think about some details in order to optimize performance.
Window length. Since we decided to study patterns of length
only two parameters can be chosen: the length of the sliding window and the delay
For the window, a length of 30 s seemed to be most appropriate. On one hand, 512 Hz sampling means that we get 15,360 values within 30 s, which provided excellent statistics for order patterns even in the presence of gross artefacts. On the other hand, we got 120 instances of
per hour, each obtained independently of all other data, while expert annotation usually keeps in mind the previous sleep stage. As
Figure 5,
Figure 7,
Figure 8 and
Figure 9 confirm, there are few outliers, indicating that
is a reliable and robust measure of sleep depth. For artefact-free data, shorter windows can be used. It is possible to consider overlapping windows, which was not needed in this study.
Delays. For the choice of delay, some experiments were done. To minimize statistical error, taking the average over several
d is better than a single
An average over all possible
d between 1 (two milliseconds) and 1000 (two seconds) does not make sense, however. We have to decide whether small or large
d will give the most informative
According to the official guidelines for sleep annotation [
18], we should care mostly for delta and theta waves, that is,
Our experiments were based on another idea: we looked for parameter regions that are generally far from white noise. When there is already some smoothness in the data, a wave is more likely to emerge than in complete disorder.
In real measurements, true white noise is unlikely to appear. The majority of our
values was well above the bound
which marks the significance level of
in
Table 1. Smaller values occur mainly for large
d where measurements at
t and
have nothing to do with each other (theta and delta waves are exceptions). For small
however, there are always dependencies among the values
and
due to some slowly changing conditions in the environment of the measuring device. Thus, for small
d, there is a kind of smoothness that causes patterns 123 and 321 to occur more often than the other patterns. However, if
d is very small, the smooth component of
will be dominated by noise. This argument says that it is best to take small
but not too small
It seems a general rule for the choice of delays in such applications.
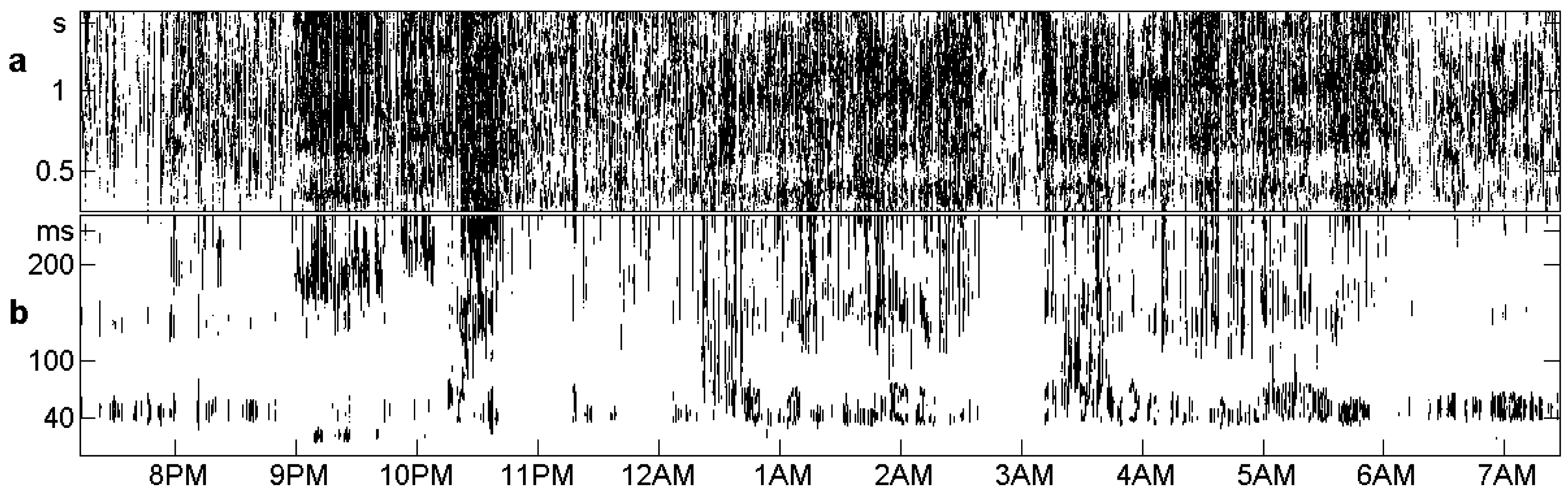
Figure 11 shows large and small values of
for all windows and all
d between 1 and 768, for the control n2 shown already in the top panel of
Figure 5. Values smaller than
are called small, and indicated by a dark dot while greater values are left white. Moreover, the
d scale is divided into an upper part with
s, ..., 1.5 s and a lower part
ms, ..., 0.25 s, which is magnified in order to show details. The chosen threshold is three times the significance level of
of
Theoretically, it should correspond to a tiny
p-value (cf.
Section 2), but as real data are not white noise, this threshold seems appropriate [
19].
In the upper part of
Figure 11, the average
is 0.0017, and 47% of the places have small
These black spots are spread rather uniformly, so there is little chance to get information from this range of
d. In the lower part, the average
is 0.013, and only 10% of the places have small
There is some structure related to the sleep annotations in
Figure 5. There may be different choices of an interval for
After some experiments, we took the bottom region,
d below 40 ms, which is almost completely white. The smallest value
ms was excluded since variations within 2 ms are more due to the electronic equipment than to the brain, as we knew from our own measurements. Thus, the
for
Figure 5,
Figure 7,
Figure 8,
Figure 9 and
Figure 10 was taken as an average over the values
corresponding to
ms.
Checking oscillations. Periodic phenomena have a large influence on the statistics of order patterns. As explained in [
20], the persistence
assumes large negative values for
and
when
p is the period of a periodic component. For our parameters, distance to white noise consists mainly of the persistence part, Equation (
4) turns into
Thus, it is natural to ask whether our
was caused by certain oscillations.
In EEG measurements, a danger is contamination with mains hum, the 50 Hz frequency of the power supply. The corresponding is Since is not particularly small, there seems to be no such contamination. We should also check for alpha waves in the range 8 up to 12 Hz, although they are not likely to appear in the channel Fp2–F4. The corresponding is a d between 40 ms and 60 ms and is outside the range of our average. We conclude that our distance to white noise is not caused by oscillations.
This section should demonstrate ways to find good parameters. We do not claim that we made the best choice.
Figure 6 shows that for the same person n2 an average of
for
d between 40 and 70 ms indicates sleep stages as large
-values and REM phases by negative
-values.
5. Discussion and Conclusions
Several authors used permutation entropy as a tool for EEG analysis, both in sleep medicine [
7,
8,
9] as well as in epilepsy [
12,
13,
14] and anaesthesia research [
15]. One advantage is the robustness of ordinal parameters like
and
with respect to motion artefacts and low-frequency perturbations, which often appear in EEG data. While in correlation and spectral analysis, an outlier will cause an error proportional to its size, in ordinal pattern statistics, an outlier is counted as any other value.
In this note, we tried to improve the methodology by introducing distance to white noise, which can be supported by a statistical model. It was shown how good parameters can be determined. As a result, we defined an average
for time spans between 4 and 40 ms, which can be considered as a measure of sleep depth on a continuous scale, very similar to the discrete sleep stages annotated by experts or by automatic scoring. A remarkable coincidence was shown in
Figure 5,
Figure 7,
Figure 8 and
Figure 9 for 20 subjects in the classical CAP sleep database of Terzano et al. [
16]. A single EEG channel and short windows of 30 s gave a reliable estimate of sleep depth. Patients with insomnia had much smaller
levels than healthy controls.
Although these results have to be checked with other, more recent databases, it could be confirmed that permutation entropy is a very effective tool for distinguishing sleep stages. In the present study, only length 3 patterns were used. The distance between the points, the so-called delay was varied in a wide range, so that permutation entropy and distance to white noise become functions like classical autocorrelation. Such a function is more meaningful than permutation entropy of patterns of length for delay 1.
On a general level, it was shown that the fine structure of high-resolution measurements can contain invisible information. Routine low-resolution measurement, downsampling or low-pass filtering can destroy this information, while ordinal methods have the capacity to exploit the microstructure of signals. They need to be developed further.