Muscle Fatigue Analysis of the Deltoid during Three Head-Related Static Isometric Contraction Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Head-Related Static Isometric Contraction Tasks
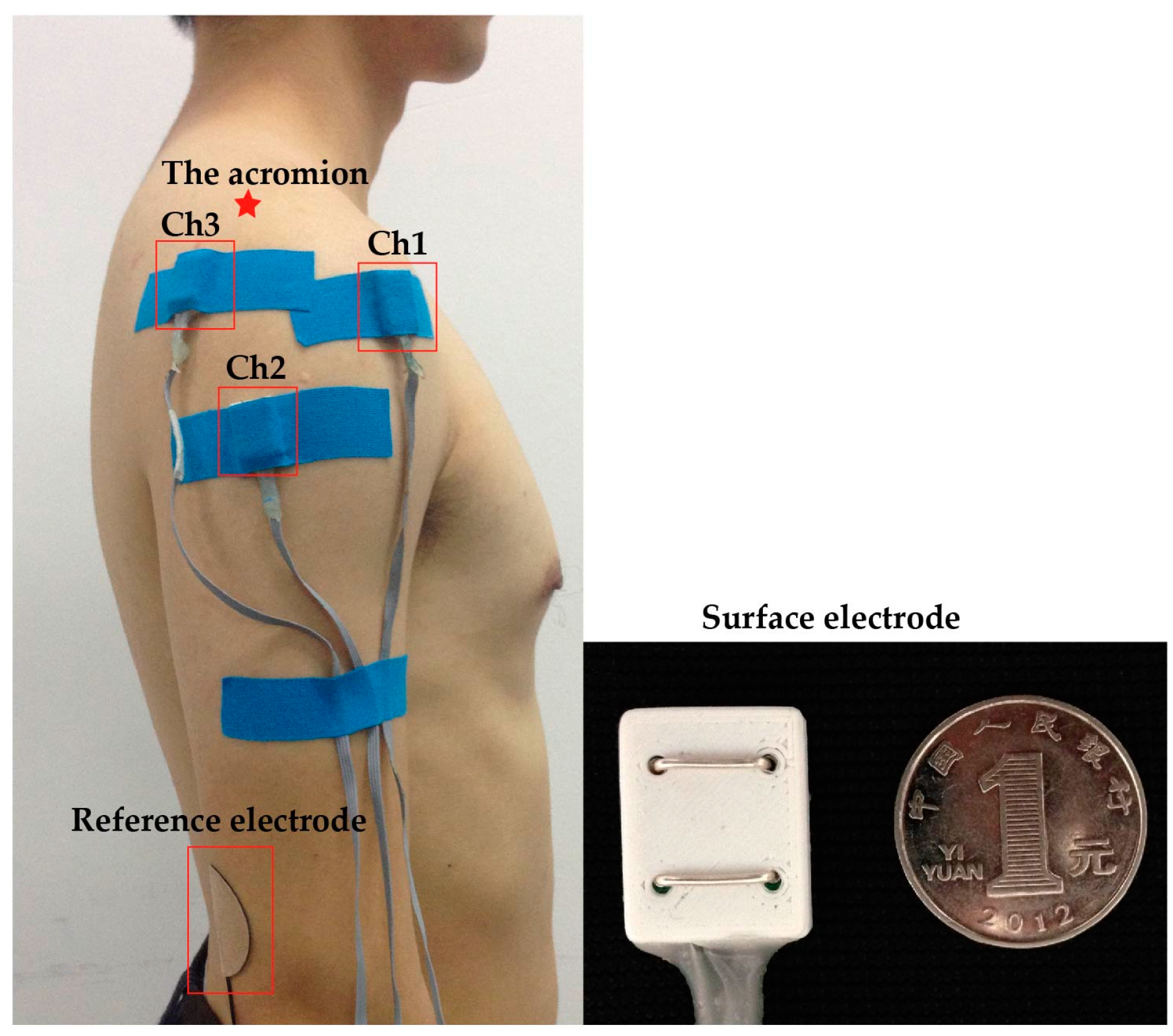
2.3. Data Collection
2.4. Signal Preprocessing
2.5. SEMG Parameters
2.6. The Performance of Fatigue Indexes in Quantification of Fatigue
2.7. Statistical Analysis
3. Results
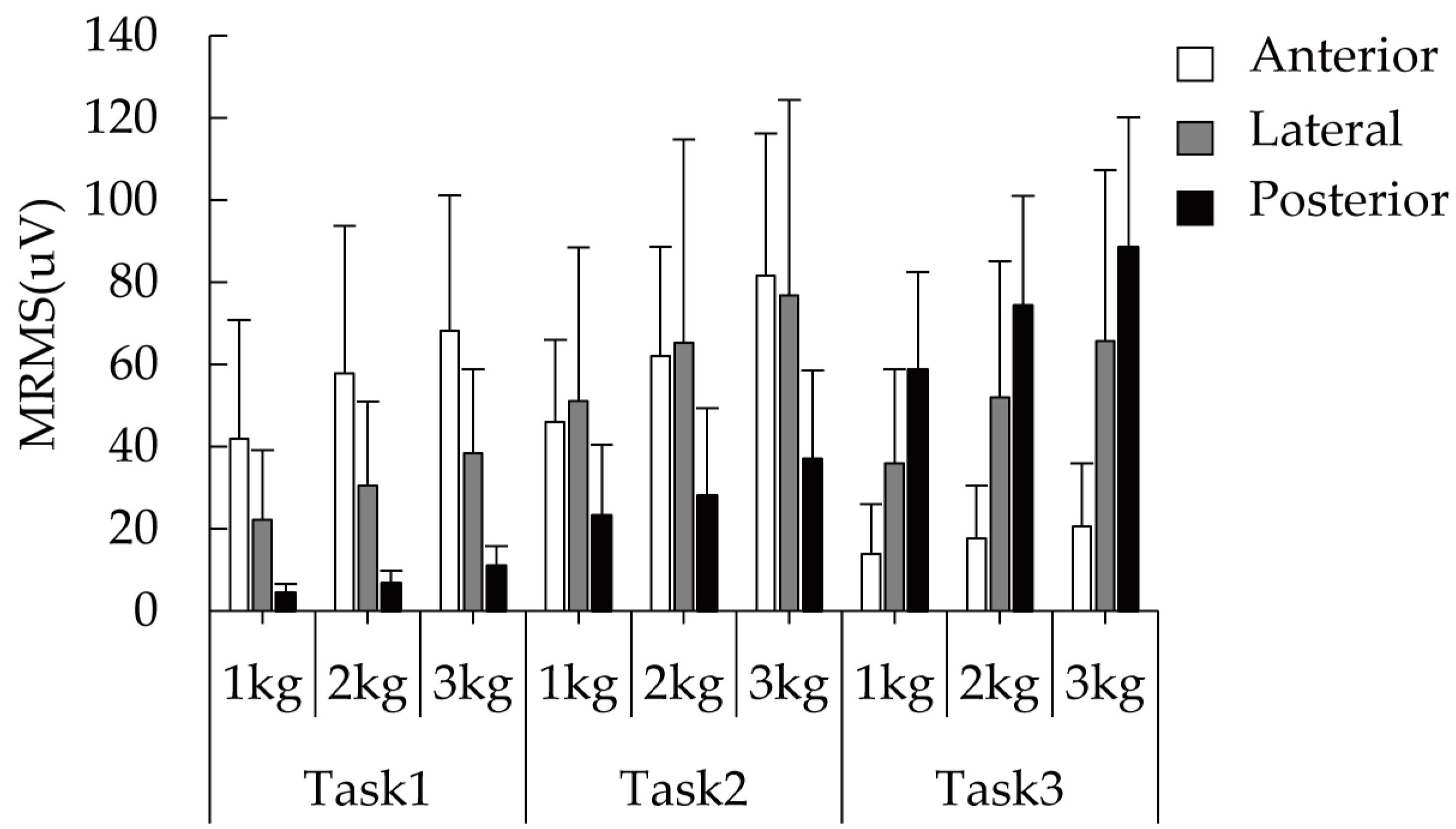
3.1. Activation Level Analysis of the Three Deltoid Heads during Three Head-Related Tasks
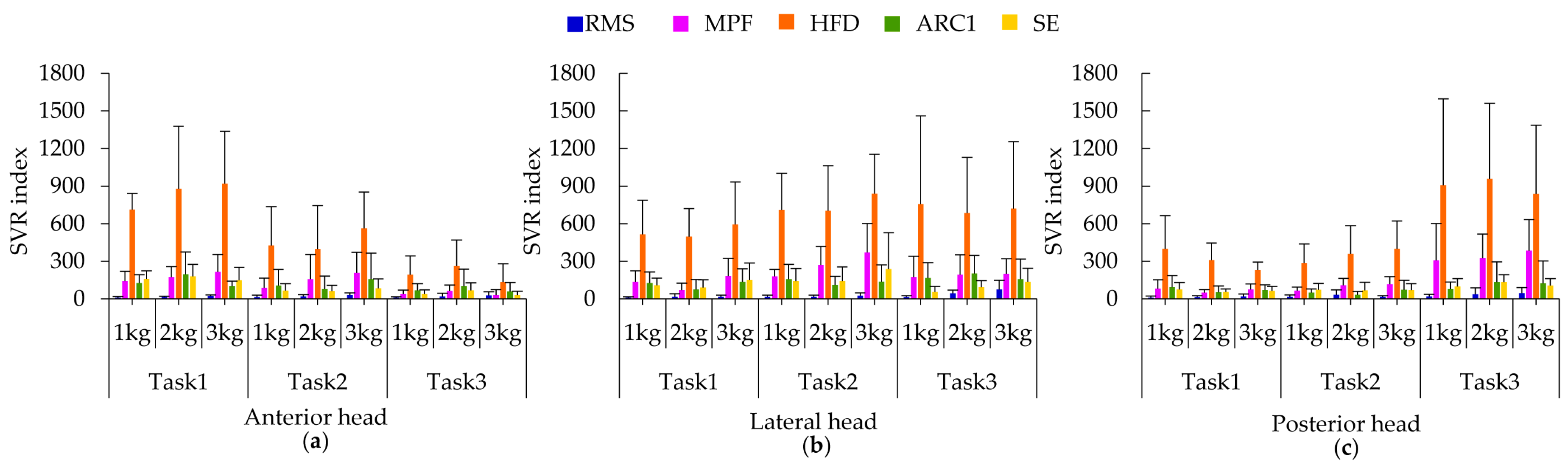
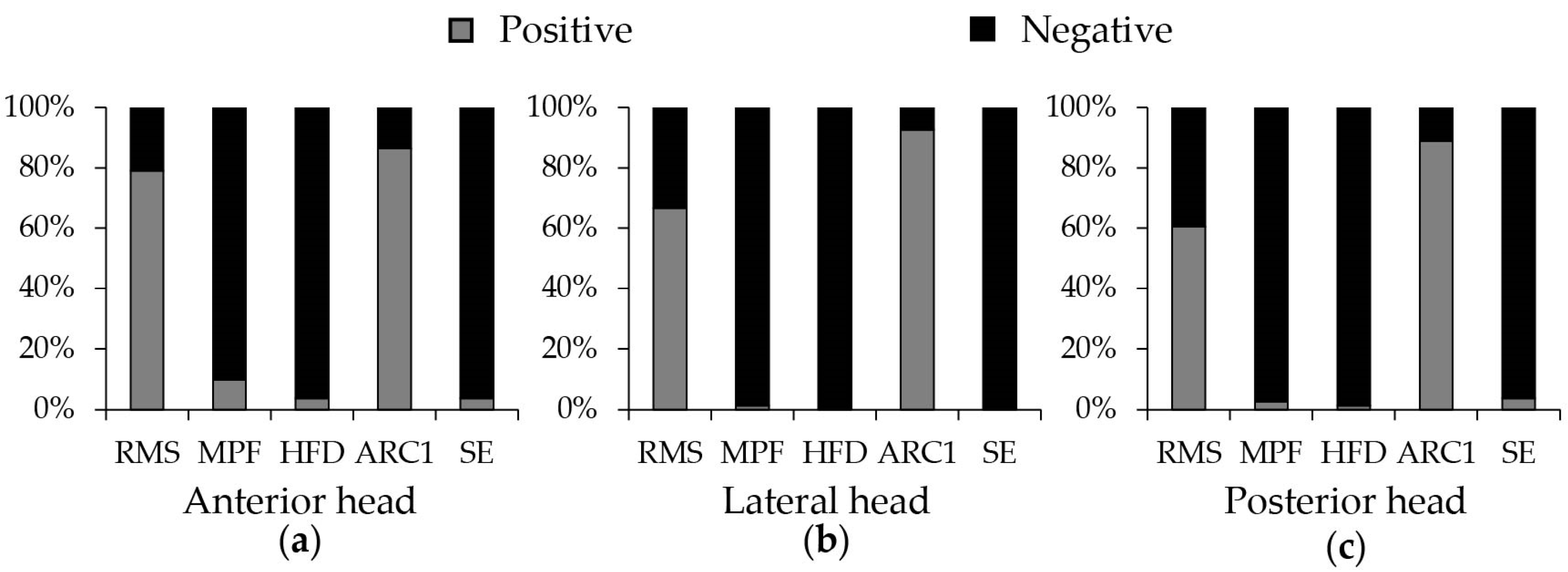
3.2. Performance of Five SEMG Parameters in Quantification of Fatigue
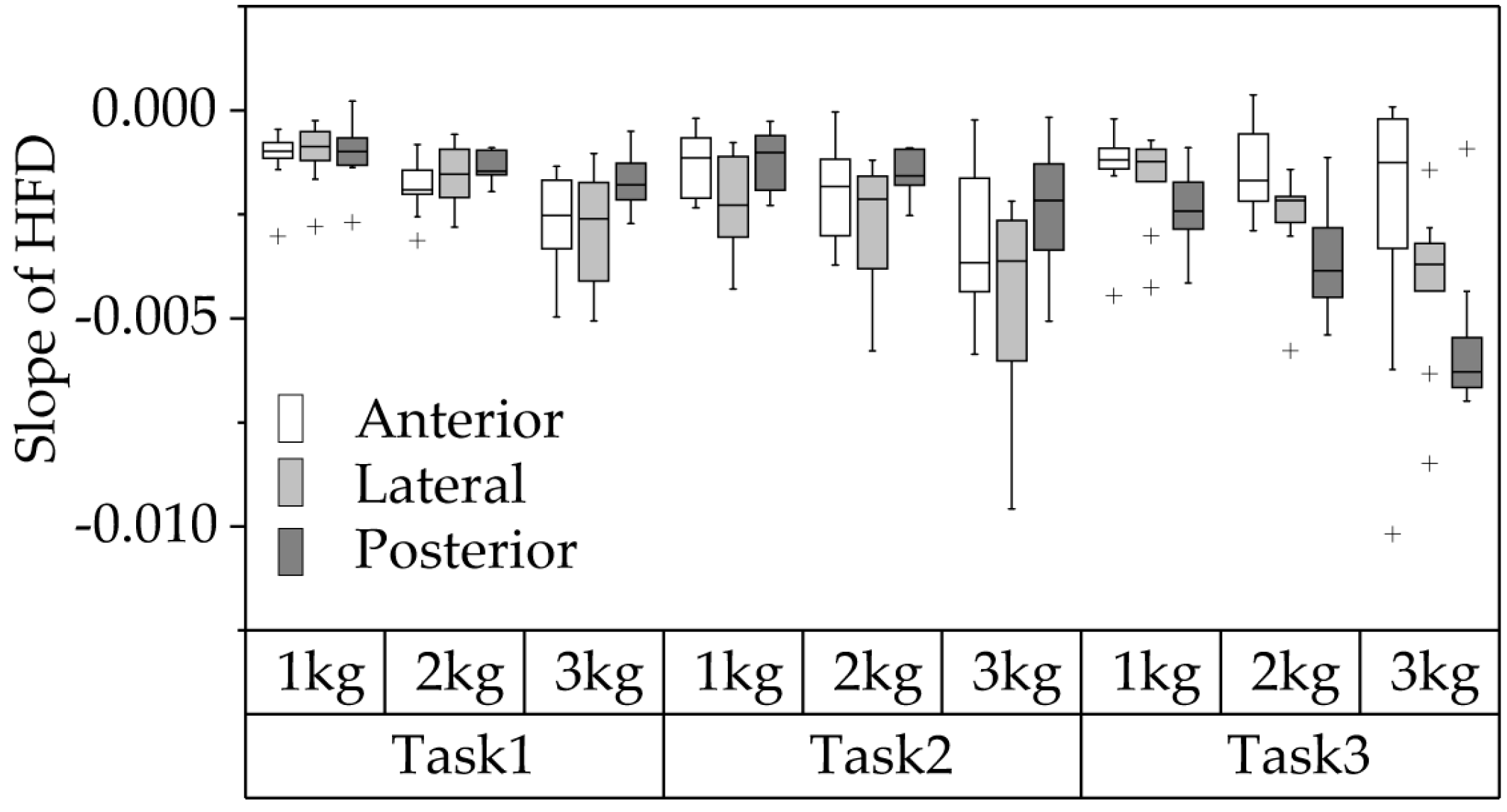
3.3. HFD-Based Fatigue Analysis of the Three Deltoid Heads
4. Discussion
4.1. The Performance in Quantification of Fatigue for Different SEMG Parameters
4.2. Possible Control Strategies of the CNS to Control the Three Deltoid Heads during Contraction Task
4.3. Possible Reasons for the Differences of the Fatiguing Characteristics among the Three Deltoid Heads
4.4. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edwards, R.H. Human muscle function and fatigue. Ciba Found. Symp. 1981, 82, 1–18. [Google Scholar] [PubMed]
- Gonzalez-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Rossiter, H.B.; Zoladz, J.A. Skeletal muscle fatigue and decreased efficiency: Two sides of the same coin? Exerc. Sport Sci. Rev. 2015, 43, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonkovic, S.; Ostojic, S. Surface emg based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Toebes, M.J.; Hoozemans, M.J.; Dekker, J.; van Dieen, J.H. Effects of unilateral leg muscle fatigue on balance control in perturbed and unperturbed gait in healthy elderly. Gait Posture 2014, 40, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Eken, M.M.; Dallmeijer, A.J.; Houdijk, H.; Doorenbosch, C.A. Muscle fatigue during repetitive voluntary contractions: A comparison between children with cerebral palsy, typically developing children and young healthy adults. Gait Posture 2013, 38, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Villafane, J.H.; Gobbo, M.; Peranzoni, M.; Naik, G.; Imperio, G.; Cleland, J.A.; Negrini, S. Validity and everyday clinical applicability of lumbar muscle fatigue assessment methods in patients with chronic non-specific low back pain: A systematic review. Disabil. Rehabil. 2016, 38, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, A.B.; Attaallah, M.; Saleh, A. The relation between muscle fatigue and some physiological and biomechanical parameters. J. Am. Sci. 2016, 12, 82–87. [Google Scholar]
- Becker, S.; Fröhlich, M.; Kelm, J.; Ludwig, O. Change of muscle activity as well as kinematic and kinetic parameters during headers after core muscle fatigue. Sports 2017, 5, 10. [Google Scholar] [CrossRef]
- Corben, J.S.; Cerrone, S.A.; Soviero, J.E.; Kwiecien, S.Y.; Nicholas, S.J.; McHugh, M.P. Performance demands in softball pitching: A comprehensive muscle fatigue study. Am. J. Sports Med. 2015, 43, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Jarmey, C. Muscles of the shoulder and upper arm. In The Concise Book of Muscles, 2nd ed.; Lotus Publishing and North Atlantic Books: Chichester, UK, 2008; pp. 96–97. [Google Scholar]
- Wickham, J.B.; Brown, J. The function of neuromuscular compartments in human shoulder muscles. J. Neurophysiol. 2012, 107, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Cao, S.; Zhang, X. Muscle-tendon units localization and activation level analysis based on high-density surface emg array and nmf algorithm. J. Neural Eng. 2016, 13, 066001. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kouzaki, M.; Moritani, T. Non-uniform surface electromyographic responses to change in joint angle within rectus femoris muscle. Muscle Nerve 2014, 50, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Gallina, A.; Merletti, R.; Vieira, T.M. Are the myoelectric manifestations of fatigue distributed regionally in the human medial gastrocnemius muscle? J. Electromyogr. Kinesiol. 2011, 21, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kouzaki, M.; Moritani, T. Region-specific myoelectric manifestations of fatigue in human rectus femoris muscle. Muscle Nerve 2013, 48, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Sundaraj, K.; Badlishah Ahmad, R.; Ahamed, N.U.; Islam, A.; Sundaraj, S. Muscle fatigue in the three heads of the triceps brachii during a controlled forceful hand grip task with full elbow extension using surface electromyography. J. Hum. Kinet. 2015, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.R.; Kumar, D.K.; Wheeler, K.; Arjunan, S.P. Estimation of Muscle Fatigue during Cyclic Contractions Using Source Separation Techniques. In Proceedings of the 2008 Digital Image Computing: Techniques and Applications (2009), Melbourne, Australia, 1–3 December 2009; pp. 217–222. [Google Scholar]
- Venugopal, G.; Navaneethakrishna, M.; Ramakrishnan, S. Extraction and analysis of multiple time window features associated with muscle fatigue conditions using semg signals. Expert Syst. Appl. 2014, 41, 2652–2659. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, M.C.; Haight, J.M. The sensitivity of autoregressive model coefficient in quantification of trunk muscle fatigue during a sustained isometric contraction. Int. J. Ind. Ergon. 2005, 35, 321–330. [Google Scholar] [CrossRef]
- Arjunan, S.P.; Kumar, D.K.; Naik, G. Computation and evaluation of features of surface electromyogram to identify the force of muscle contraction and muscle fatigue. Biomed. Res. Int. 2014, 2014, 197960. [Google Scholar] [CrossRef] [PubMed]
- Evans, N. Shoulders. In Bodybuilding Anatomy, 2nd ed.; Klug, J., Ed.; Human Kinetics: Champaign, IL, USA, 2015; pp. 1–43. [Google Scholar]
- Vøllestad, N.K. Measurement of human muscle fatigue. J. Neurosci. Methods 1997, 74, 219–227. [Google Scholar] [CrossRef]
- Hagberg, M. Work load and fatigue in repetitive arm elevations. Ergonomics 1981, 24, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for semg sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Seniam. Available online: http://www.seniam.org/ (accessed on 12 December 2015).
- Xie, H.B.; Guo, J.Y.; Zheng, Y.P. Fuzzy approximate entropy analysis of chaotic and natural complex systems: Detecting muscle fatigue using electromyography signals. Ann. Biomed. Eng. 2010, 38, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, P. Sample entropy analysis of surface emg for improved muscle activity onset detection against spurious background spikes. J. Electromyogr. Kinesiol. 2012, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Anmuth, C.J.; Goldberg, G.; Mayer, N.H. Fractal dimension of electromyographic signals recorded with surface electrodes during isometric contractions is linearly correlated with muscle activation. Muscle Nerve 1994, 17, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Troiano, A.; Naddeo, F.; Sosso, E.; Camarota, G.; Merletti, R.; Mesin, L. Assessment of force and fatigue in isometric contractions of the upper trapezius muscle by surface emg signal and perceived exertion scale. Gait Posture 2008, 28, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Garavito, F.; Gonzalez, J.; Cabarcas, J.; Chaparro, D.; Portocarrero, I.; Vargas, A. Emg signal analysis based on fractal dimension for muscle activation detection under exercice protocol. In Proceedings of the 2016 XXI Symposium on Signal Processing, Images and Artificial Vision (STSIVA), Bucaramanga, Colombia, 30 August–2 September 2016. [Google Scholar]
- Gonzalez-Izal, M.; Malanda, A.; Navarro-Amezqueta, I.; Gorostiaga, E.M.; Mallor, F.; Ibanez, J.; Izquierdo, M. Emg spectral indices and muscle power fatigue during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Izal, M.; Rodriguez-Carreno, I.; Malanda, A.; Mallor-Gimenez, F.; Navarro-Amezqueta, I.; Gorostiaga, E.M.; Izquierdo, M. Semg wavelet-based indices predicts muscle power loss during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.R.; MacIsaac, D.T. Emg-based muscle fatigue assessment during dynamic contractions using principal component analysis. J. Electromyogr. Kinesiol. 2011, 21, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Arjunan, S.P.; Naik, G.R. Measuring increase in synchronization to identify muscle endurance limit. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, M.; D’Antona, G.; Barbero, M.; Fisher, B.; Dieli-Conwright, C.M.; Clijsen, R.; Cescon, C. Evaluation of central and peripheral fatigue in the quadriceps using fractal dimension and conduction velocity in young females. PLoS ONE 2015, 10, e0123921. [Google Scholar]
- Mesin, L.; Cescon, C.; Gazzoni, M.; Merletti, R.; Rainoldi, A. A bi-dimensional index for the selective assessment of myoelectric manifestations of peripheral and central muscle fatigue. J. Electromyogr. Kinesiol. 2009, 19, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.D.; Botton, C.E.; Rodrigues, R.; Pinto, R.S.; Lima, C.S. Analysis of anterior, middle and posterior deltoid activation during single and multijoint exercises. J. Sports Med. Phys. Fit. 2015, 55, 714–721. [Google Scholar]
- Tang, L.; Li, F.; Cao, S.; Zhang, X.; Chen, X. Muscle synergy analysis for similar upper limb motion tasks. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 2014, 3590–3593. [Google Scholar] [PubMed]
- Arjunan, S.P.; Kumar, D.; Naik, G. Independence between two channels of surface electromyogram signal to measure the loss of motor units. Meas. Sci. Rev. 2015, 15, 152–155. [Google Scholar] [CrossRef]
- Naik, G.R.; Kumar, D.K.; Arjunan, S. Measure of Increase in Motor Unit Synchronisaton for Young and Old Using Semg. In Proceedings of the Biosignals and Biorobotics Conference (BRC), Manaus, Brazil, 9–11 January 2012; pp. 1–4. [Google Scholar]
- Maton, B. Human motor unit activity during the onset of muscle fatigue in submaximal isometric isotonic contraction. Eur. J. Appl. Physiol. 1981, 46, 271–281. [Google Scholar] [CrossRef]
- Al Harrach, M.; Carriou, V.; Boudaoud, S.; Laforet, J.; Marin, F. Analysis of the semg/force relationship using hd-semg technique and data fusion: A simulation study. Comput. Biol. Med. 2017, 83, 34–47. [Google Scholar] [CrossRef] [PubMed]


| Factors | Significance (p Values) | ||
|---|---|---|---|
| Anterior | Lateral | Posterior | |
| Task | 0.001 * | 0.007 * | 0.000 * |
| Load | 0.000 * | 0.000 * | 0.000 * |
| Factors | Significance (p Values) | ||
|---|---|---|---|
| Anterior | Lateral | Posterior | |
| Task | 0.782 | 0.031 * | 0.000 * |
| Load | 0.004 * | 0.000 * | 0.000 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Chen, X.; Cao, S.; Zhang, X. Muscle Fatigue Analysis of the Deltoid during Three Head-Related Static Isometric Contraction Tasks. Entropy 2017, 19, 221. https://doi.org/10.3390/e19050221
Cui W, Chen X, Cao S, Zhang X. Muscle Fatigue Analysis of the Deltoid during Three Head-Related Static Isometric Contraction Tasks. Entropy. 2017; 19(5):221. https://doi.org/10.3390/e19050221
Chicago/Turabian StyleCui, Wenxiang, Xiang Chen, Shuai Cao, and Xu Zhang. 2017. "Muscle Fatigue Analysis of the Deltoid during Three Head-Related Static Isometric Contraction Tasks" Entropy 19, no. 5: 221. https://doi.org/10.3390/e19050221






