Effects of Task Demands on Kinematics and EMG Signals during Tracking Tasks Using Multiscale Entropy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
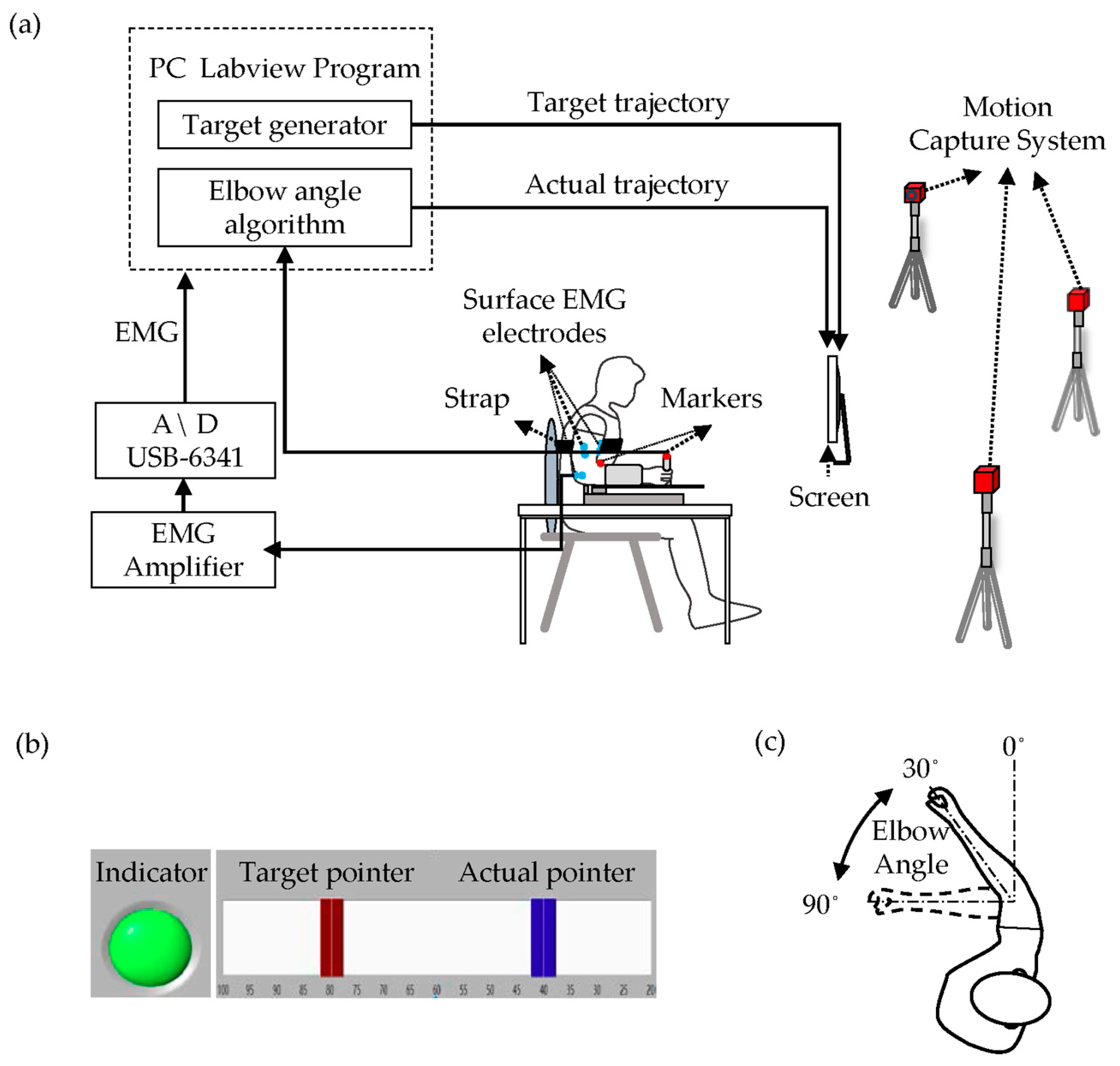
2.2. Apparatus and Procedures
2.3. Data and Statistical Analysis
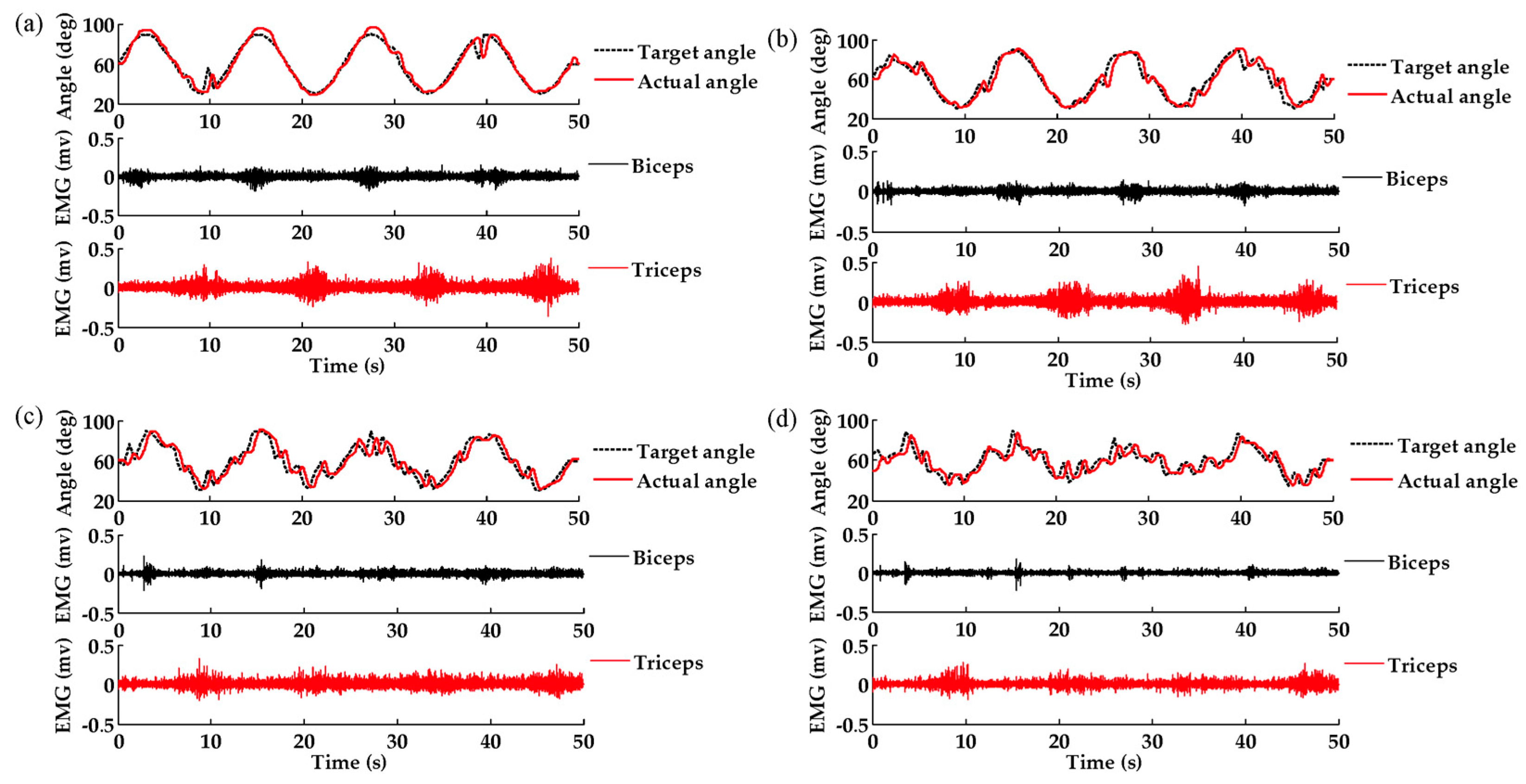
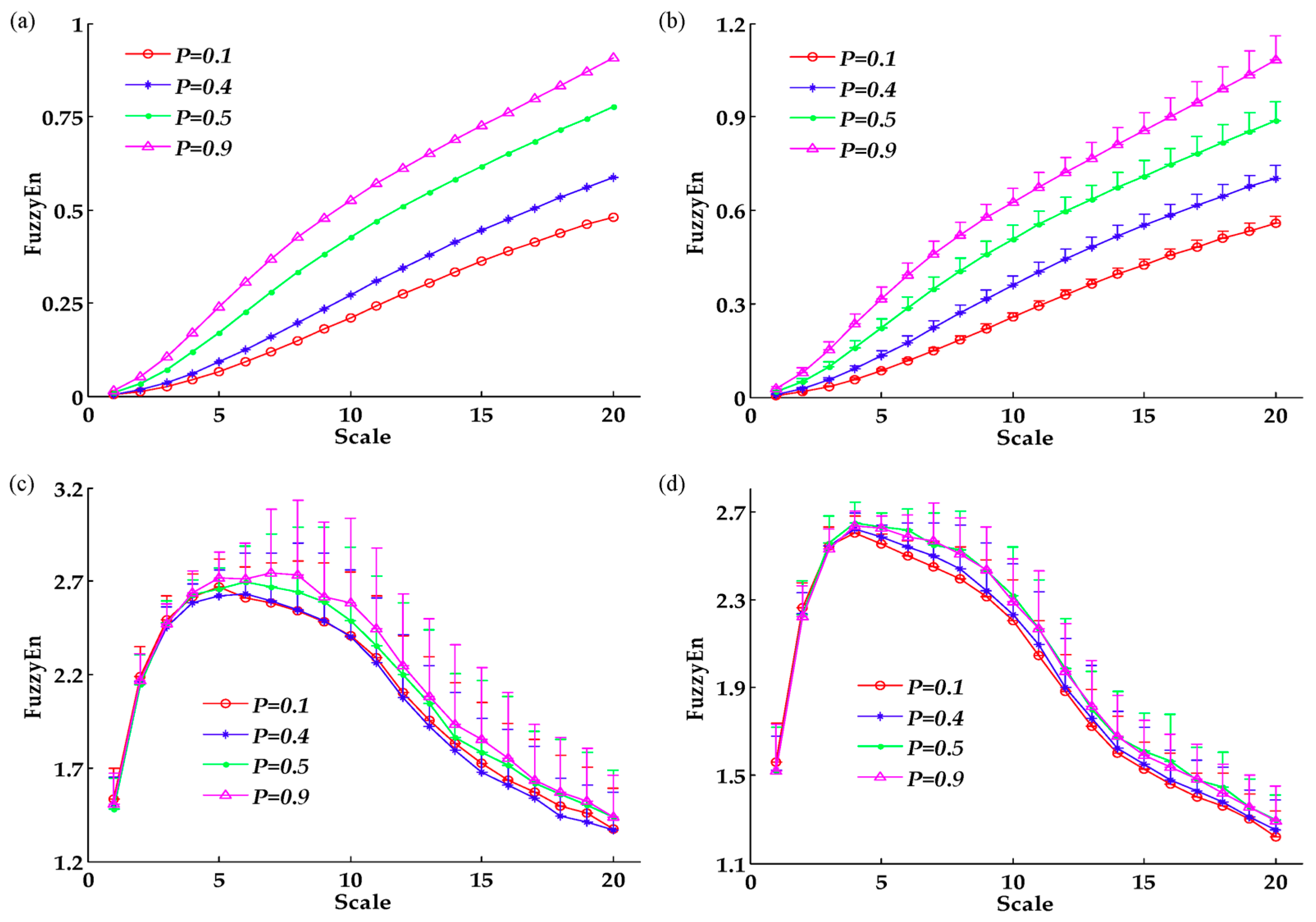
3. Results
4. Discussion
4.1. MSFuzzyEn Analysis of Trajectories and EMG Signals
4.2. Effects of Task Demands on Trajectories and EMG Signals
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlerf, J.E.; Ivry, R.B. Task goals influence online corrections and adaptation of reaching movements. J. Neurophysiol. 2011, 106, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Berthier, N.E.; Clifton, R.K.; Gullapalli, V.; Mccall, D.D.; Robin, D.J. Visual information and object size in the control of reaching. J. Mot. Behav. 1996, 28, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.L.; Newell, K.M. Motor entropy in response to task demands and environmental information. Chaos 2008, 18, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.L.; Newell, K.M. Entropy compensation in human motor adaptation. Chaos 2008, 18, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Selen, L.P.; van Dieën, J.H.; Beek, P.J. Impedance modulation and feedback corrections in tracking targets of variable size and frequency. J. Neurophysiol. 2006, 96, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-K; Park, J.-W.; Ryu, Y.U. Influence of movement speed on accuracy of tracking performance following stroke. J. Phys. Ther. Sci. 2011, 23, 141–144. [Google Scholar] [CrossRef]
- Miall, R.C.; Weir, D.J.; Stein, J.F. Intermittency in human manual tracking tasks. J. Mot. Behav. 1993, 25, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Hong, S.L.; Newell, K.M. Inverse relations in the patterns of muscle and center of pressure dynamics during standing still and movement postures. Exp. Brain Res. 2007, 181, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Barbado, M.D.; Sabido, S.R.; Veragarcia, F.J.; Gusi, F.N.; Moreno, F.J. Effect of increasing difficulty in standing balance tasks with visual feedback on postural sway and EMG: Complexity and performance. Hum. Mov. Sci. 2012, 31, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Fitts, P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1954, 121, 262–269. [Google Scholar] [CrossRef]
- Hyman, R. Stimulus information as a determinant of reaction time. J. Exp. Psychol. 1953, 45, 188. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 5, 3–55. [Google Scholar]
- Sm, P. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [PubMed]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of surface EMG signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Guo, J.Y.; Zheng, Y.P. Fuzzy approximate entropy analysis of chaotic and natural complex systems: Detecting muscle fatigue using electromyography signals. Ann. Biomed. Eng. 2010, 38, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Caldirola, D.; Bellodi, L.; Caumo, A.; Migliarese, G.; Perna, G. Approximate entropy of respiratory patterns in panic disorder. Am. J. Psychiatry 2004, 161, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Miller, C.; Vespa, P.; Bergsneider, M. Adaptive computation of approximate entropy and its application in integrative analysis of irregularity of heart rate variability and intracranial pressure signals. Med. Eng. Phys. 2008, 30, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; Mcgrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Chen, W.T.; He, W.X.; Liu, H. Complexity analysis of the biomedical signal using fuzzy entropy measurement. Appl. Soft Comput. 2011, 11, 2871–2879. [Google Scholar] [CrossRef]
- Costa, M.D.; Peng, C.K.; Goldberger, A.L. Multiscale analysis of heart rate dynamics: Entropy and time irreversibility measures. Cardiovasc. Eng. 2008, 8, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 92, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Navaneethakrishna, M.; Karthick, P.A.; Ramakrishnan, S. Analysis of biceps brachii semg signal using multiscale fuzzy approximate entropy. In Proceedings of the International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 7881–7884. [Google Scholar]
- Gu, K.H.; Dingwell, J.B. Differential changes with age in multiscale entropy of electromyography signals from leg muscles during treadmill walking. PLoS ONE 2016, 11, e0162034. [Google Scholar]
- Costa, M.; Peng, C.K.; Goldberger, A.L.; Hausdorff, J.M. Multiscale entropy analysis of human gait dynamics. Phys. A Stat. Mech. Appl. 2003, 330, 53–60. [Google Scholar] [CrossRef]
- Costa, M.D.; Goldberger, A.L. Generalized multiscale entropy analysis: Application to quantifying the complex volatility of human heartbeat time series. Entropy 2015, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jia, X.; Geocadin, R.G.; Thakor, N.V.; Maybhate, A. Multiscale entropy analysis of EEG for assessment of post-cardiac arrest neurological recovery under hypothermia in rats. IEEE Trans. Biomed. Eng. 2009, 56, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; Sun, R.; Tong, K.Y.; Song, R. Characterization of stroke- and aging-related changes in the complexity of EMG signals during tracking tasks. Ann. Biomed. Eng. 2015, 43, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Barkhaus, P.E.; Zhang, X.; Rymer, W.Z. Characterizing the complexity of spontaneous motor unit patterns of amyotrophic lateral sclerosis using approximate entropy. J. Neural Eng. 2011, 8, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.Y.; Su, M.C.; Wu, H.T.; Lin, M.C.; Tsai, I.; Sun, C.K. Multiscale entropy analysis of heart rate variability for assessing the severity of sleep disordered breathing. Entropy 2015, 17, 231–243. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Yu, W.; Wang, Z. Measuring complexity using FuzzyEn, ApEn, and SampEn. Med. Eng. Phys. 2008, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.I.; Trombly, C.A. Effects of task complexity on reaction time and movement kinematics in elderly people. Am. J. Occup. Ther. 2004, 58, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Ohtaki, Y.; Nagatomi, R.; Inooka, H. Estimation of the effect of cadence on gait stability in young and elderly people using approximate entropy technique. Meas. Sci. Rev. 2004, 4, 29–40. [Google Scholar]
- Kudoh, N.; Hattori, M.; Numata, N.; Maruyama, K. An analysis of spatiotemporal variability during prehension movements: Effects of object size and distance. Exp. Brain Res. 1997, 117, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E. Models of recruitment and rate coding organization in motor-unit pools. J. Neurophysiol. 1993, 70, 2470–2488. [Google Scholar] [PubMed]
- Zhou, P.; Rymer, W.Z. Factors governing the form of the relation between muscle force and the EMG: A simulation study. J. Neurophysiol. 2004, 92, 2878–2886. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Song, R. Effects of Task Demands on Kinematics and EMG Signals during Tracking Tasks Using Multiscale Entropy. Entropy 2017, 19, 307. https://doi.org/10.3390/e19070307
Wu Y, Song R. Effects of Task Demands on Kinematics and EMG Signals during Tracking Tasks Using Multiscale Entropy. Entropy. 2017; 19(7):307. https://doi.org/10.3390/e19070307
Chicago/Turabian StyleWu, Yuanyu, and Rong Song. 2017. "Effects of Task Demands on Kinematics and EMG Signals during Tracking Tasks Using Multiscale Entropy" Entropy 19, no. 7: 307. https://doi.org/10.3390/e19070307




