4. Microstructural Studies
Microstructural studies were carried out by means of SEM with microanalyzer. The studies involved surfaces located perpendicularly to the surface exposed to the electric arc.
Results of structural studies of the Cu–Cr system material after exposure to the electric arc have shown that three characteristic zones can be selected in that material:
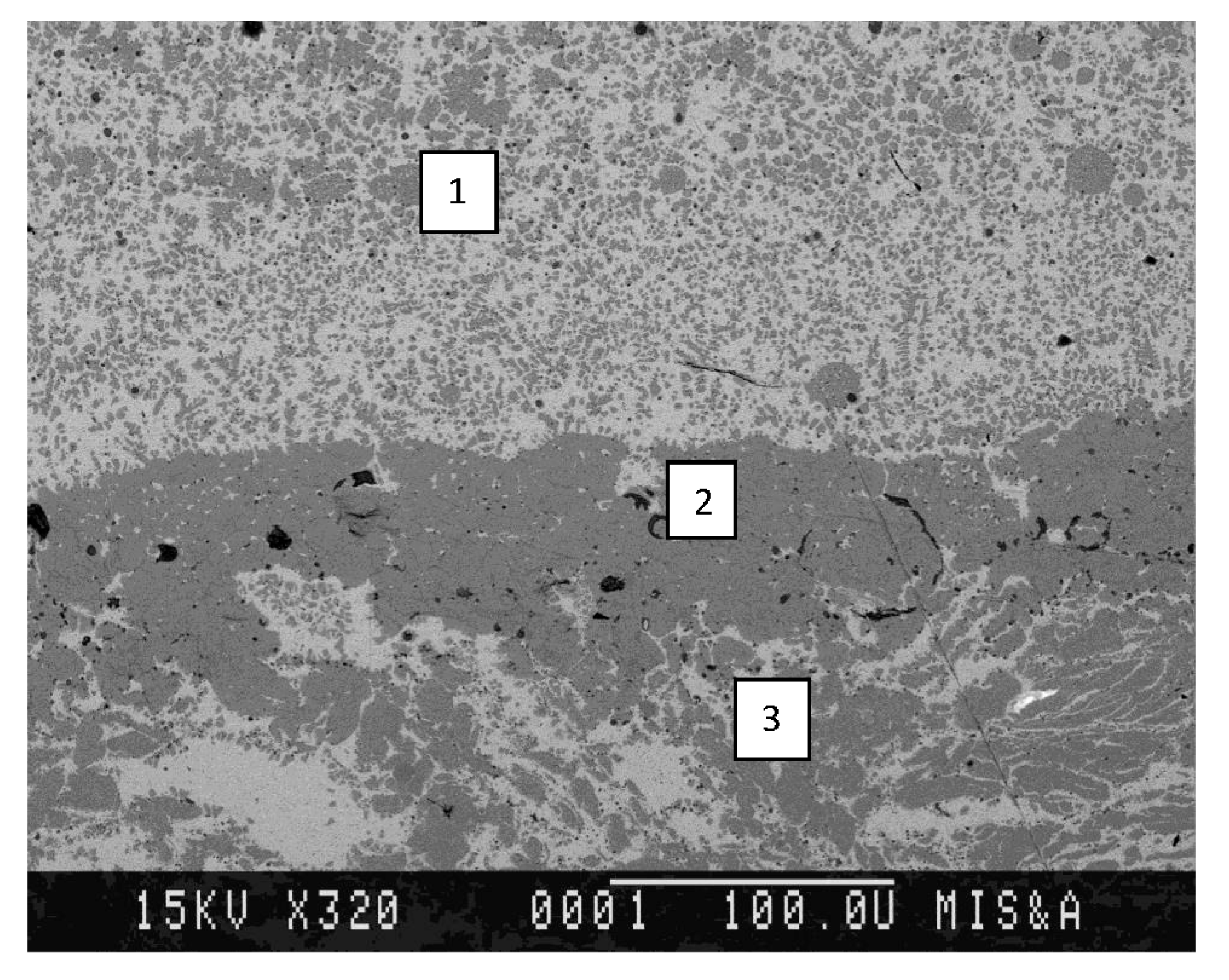
These three zones can be seen in
Figure 3, which shows the microstructure of the Cu–45%Cr material after exposure to the electric arc.
The surface exposed to the electric arc is located in the plane perpendicular to the plane of the figure, in its top section. The top section of
Figure 3 shows the zone that was crystallized after melting caused by exposure to the electric arc (exposure zone of the electric arc). The middle section of
Figure 3 shows the zone enriched with chromium. The bottom section of
Figure 3 shows the zone containing the original material structure (original material zone).
The exposure zone of the electric arc is a fine-dispersed mixture of copper and chromium, or of the respective solid solutions based thereof, with the uniform distribution of components. The depth of this zone ranged from 30 to 1000 µm depending on the position of a drop on the arc burning surface. This zone was formed as a result of melting caused by the electric arc and subsequent solidification. The zone has a clear boundary separating it from the rest of the material.
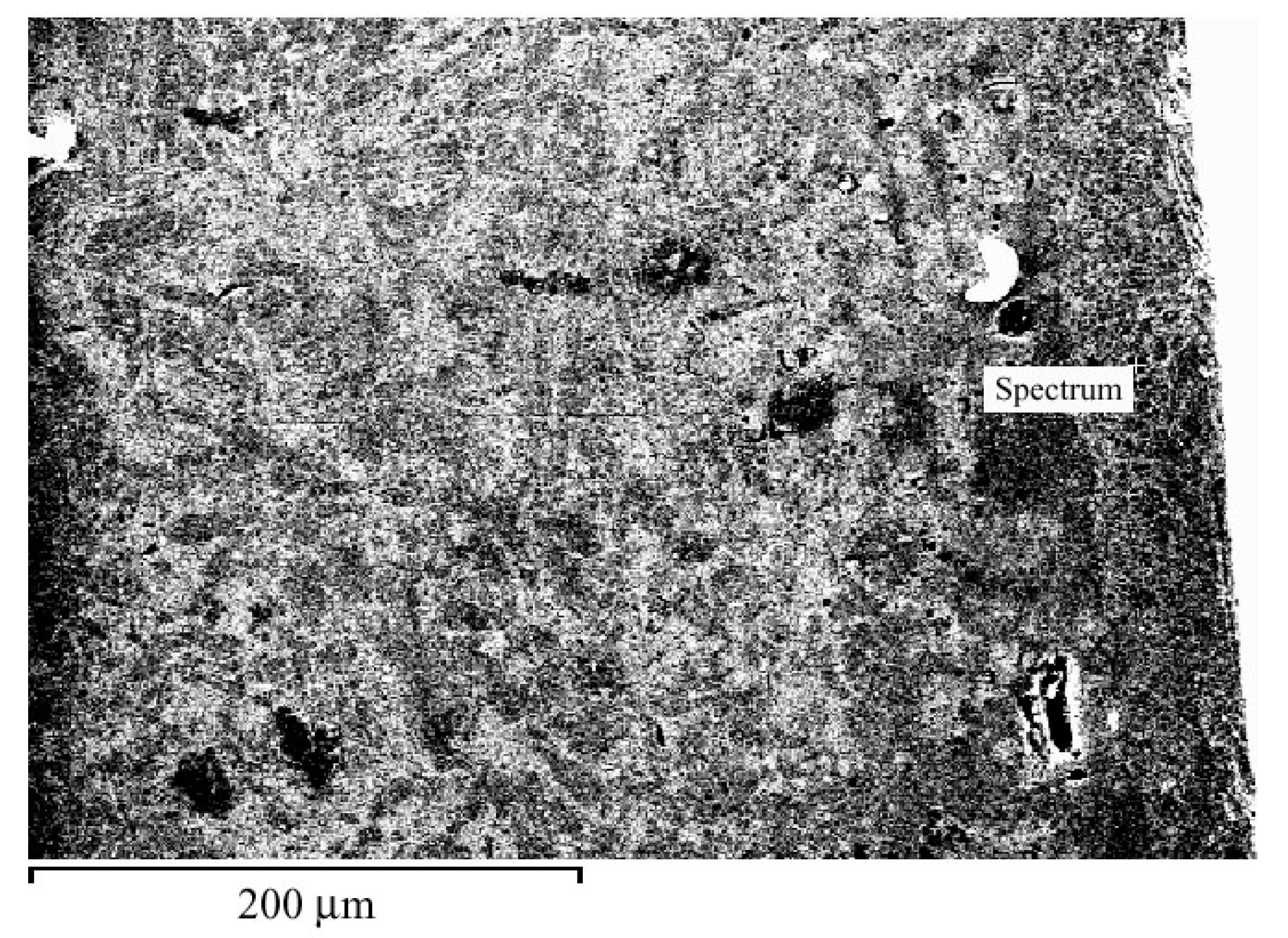
Figure 4 shows the zone enriched with chromium, in which the chemical composition was evaluated. This zone contains 93% Cr and 7% Cu.
The original material at the distance of 250 microns from the zone enriched with chromium contained 56% Cu and 44% Cr (
Figure 5). The arc exposure zone contained 50–57% Cr and 43–50% Cu (the area shown in
Figure 6 contains 53% Cr and 47% Cu).
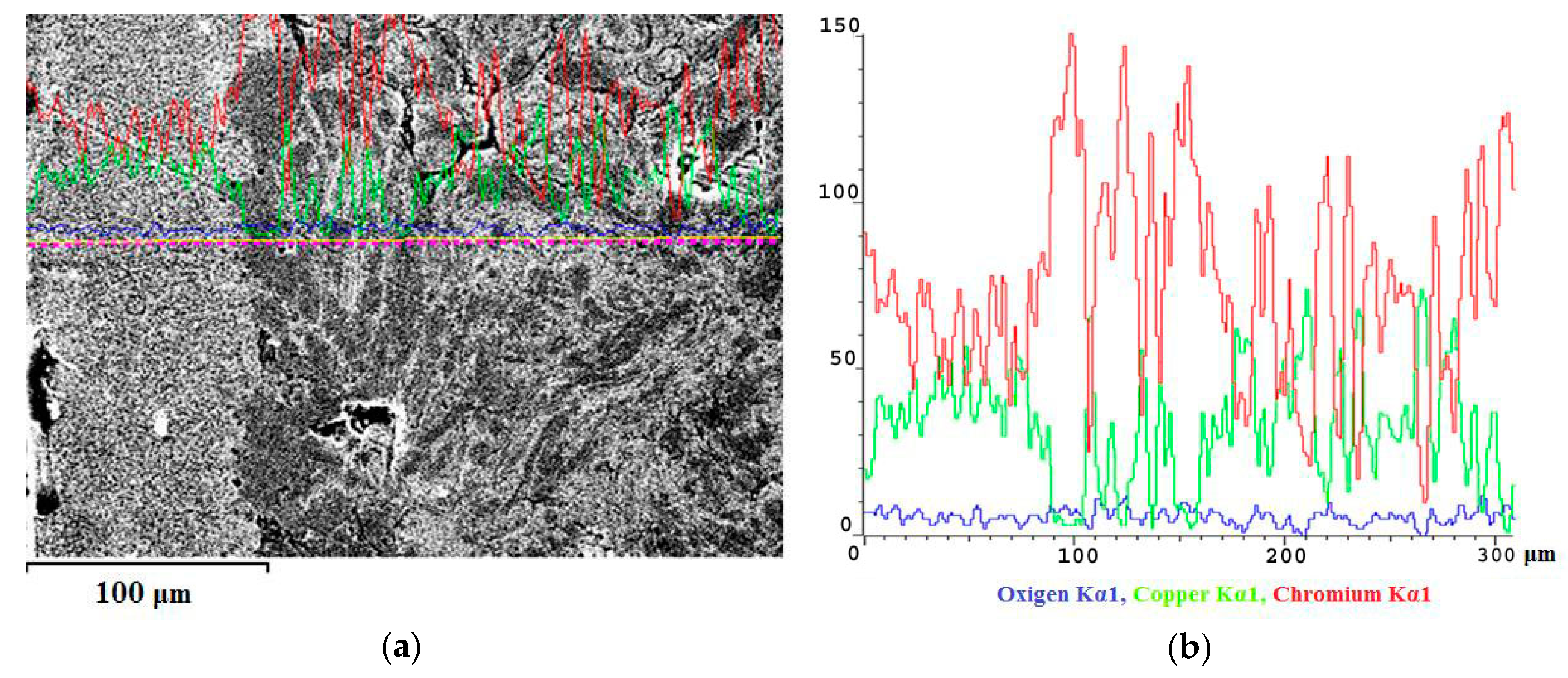
Figure 7 shows the dependence of the chromium, copper, and oxygen content on the distance from the surface of the arc exposure for Cu–45%Cr material. In
Figure 7, the arc exposure zone is located on the left. Its depth is approximately 90 microns. The zone enriched with chromium is located at the depth from 90 to 170 microns. The original material zone is located in the Figure on the right, at the depth from 170 to 300 microns.
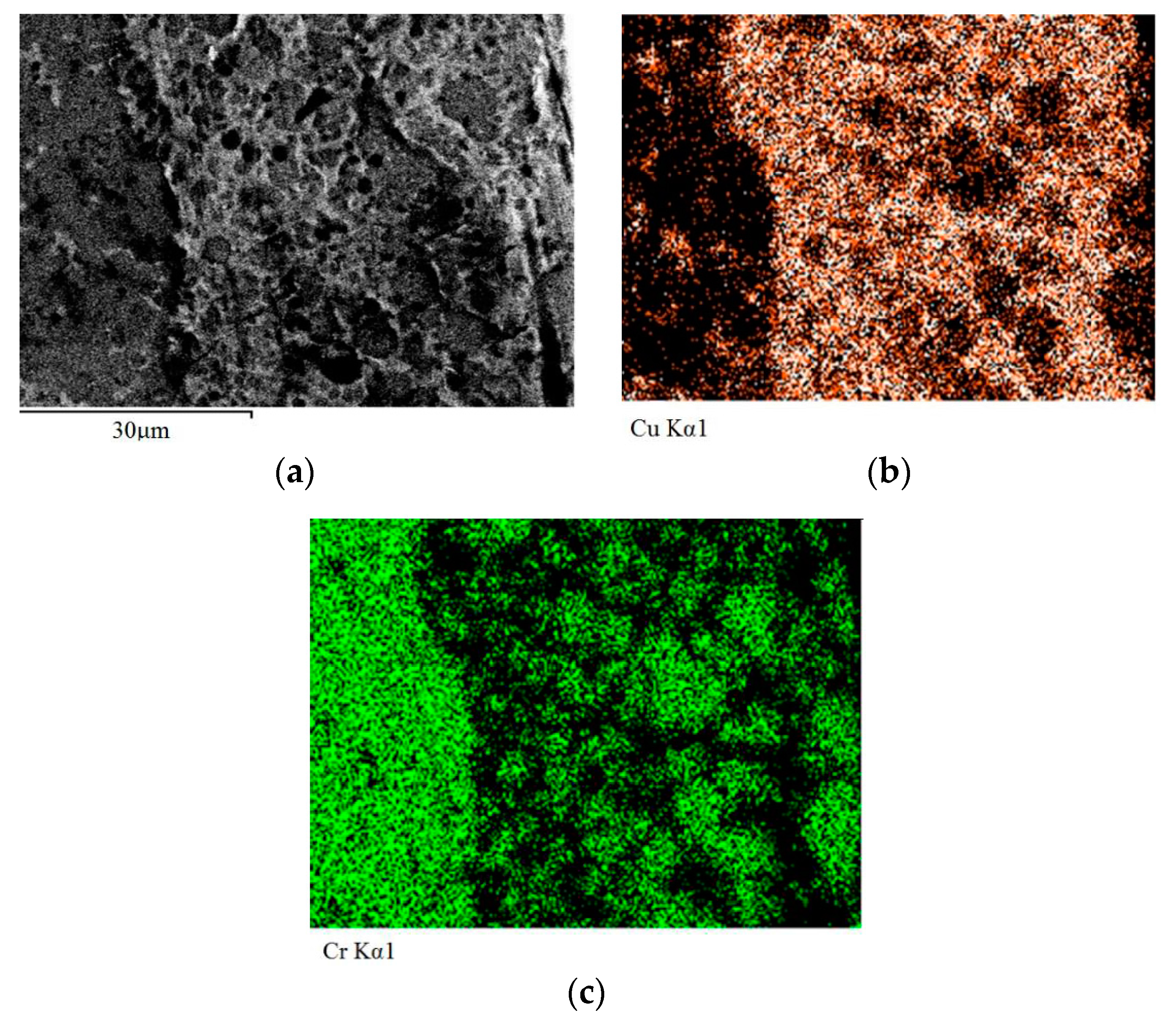
Figure 8a–c show distribution maps of copper and chromium in the zone enriched with chromium and in the adjacent arc exposure zone.
In
Figure 8а, the arc exposure zone is located on the right.
Figure 8 shows a sharp boundary between the arc exposure zone and the zone enriched with chromium
Figure 7 and
Figure 8 demonstrate that chromium content increases and copper content decreases near the exposure surface of the electric arc. Studies of the arc exposure surface have shown that it is enriched with chromium and, consequently, depleted of copper.
The electric arc exposure surface of and results of chemical analysis are shown in
Figure 9 and in
Table 2, respectively. The chemical analysis was carried out by means of SEM microanalyzer.
It follows from
Table 2 that the arc exposure surface is enriched with chromium and depleted of copper. A similar structure was observed in the Cu–30%Cr material after exposure to the electric arc.
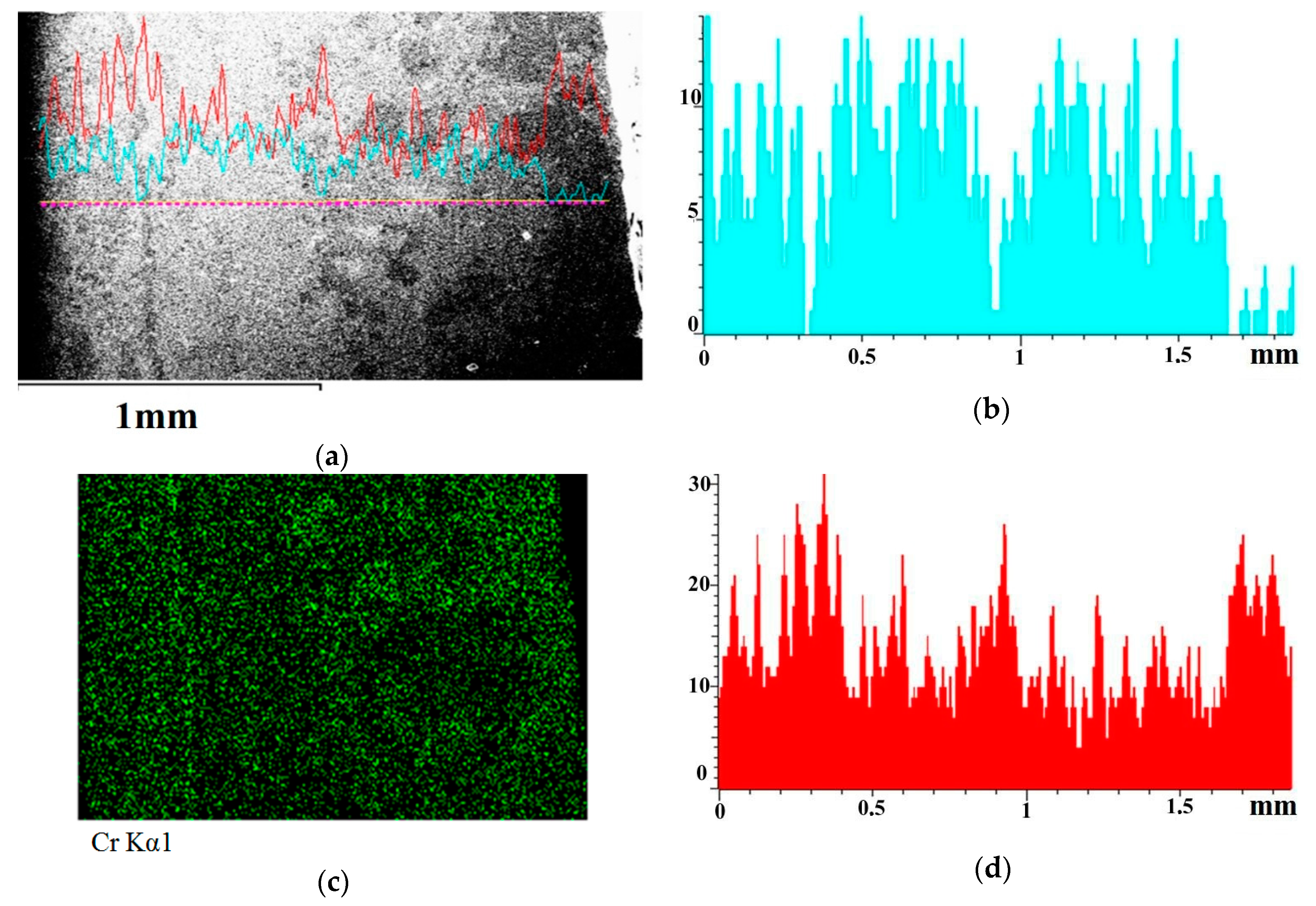
Figure 10 shows the structure of the Cu–30%Cr material adjacent to the arc exposure surface, depth distribution of chromium and copper as well as distribution maps of copper and chromium.
Figure 10a shows a relatively thin boundary between the original structure and the zone melted as a result of exposure to the arc. The boundary is a 30–50 µm wide streak enriched with chromium. This streak is marked with an arrow in
Figure 10a. It follows from
Figure 10a,c that the streak is enriched with chromium and that it contains virtually no copper. To the right of this strip, there is the arc exposure zone, approximately 1.8 mm wide, and to the left there is the original material. Near the arc exposure surface (in
Figure 10a—on the right), there is a 200–250 µm wide zone enriched with chromium. This is evidenced by the distribution maps of chemical elements. The chromium content (
Figure 10a) in the fine-dispersed part of the arc exposure zone is 43–57% by weight, the rest is copper.
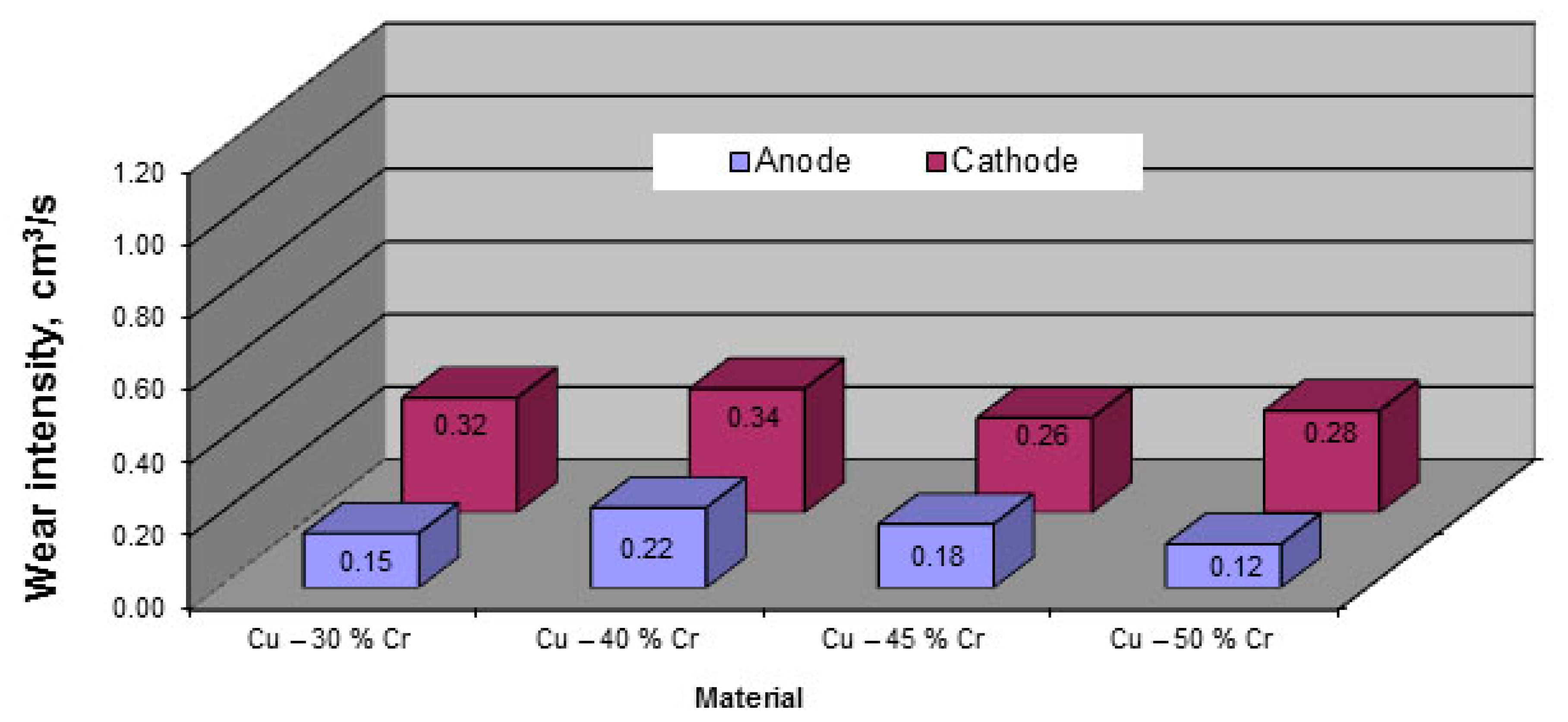
The results of microstructural studies of the Cu–30%Cr and Cu–45%Cr materials show that the electric arc exposure zone is a fine-dispersed mixture in which chromium and copper are uniformly distributed. The chromium content in the mixture ranges from 43 to 57%. This composition of solidified fine-dispersed mix is typical of all the tested materials. This mixture results from melting caused by exposure to the electric arc and subsequent solidification. The fine-dispersed mixture is restricted on the outside and inside with chromium rich zones (their chromium content exceeds 90%). The outer chromium rich zone separates the fine-dispersed mixture from the arc exposure surface. The inner chromium rich zone separates the fine-dispersed mixture from the original material. This structure and composition of the electric arc exposure zone are typical of all the tested materials.
According to the equilibrium state diagram Cu–Cr [
15,
16], solid particles of chromium (melting point 1863 °C) are the first to appear during crystallization of the Cu–Cr melt. In case of the molten Cu–45%Cr, crystallization of primary particles of chromium begins at the temperature of about 1720 °C, and in case of the molten Cu–30%Cr—at the temperature of about 1600 °C. The primary chromium particles depose from the melt until it reaches the eutectic temperature (1076.6 °C). At the eutectic temperature, there occurs simultaneous crystallization of secondary chromium and of the copper based solid solution containing maximum 1% chromium. The eutectic alloy contains about 1.5% chromium; the rest is the copper-based solid solution. Therefore, in the case of equilibrium crystallization, the structure of the solidified alloy consists of primary chromium particles that are distributed uniformly within the eutectic.
The solidified areas that were melted via exposure to the electric arc have two principal differences from an alloy of the Cu–Cr system that solidified under equilibrium conditions. The first difference is the non-uniform distribution of primary chromium. The primary chromium forms two walls. One chromium wall is located near the exposure surface of the electric arc, the second chromium wall separates the arc exposure zone from the original material. The second difference is the increased chromium content of the fine-dispersed mix in the arc exposure zone compared to the original alloy.
Overall, increasing of the chromium content in the exposure zone of the electric arc is explained by the fact that melting point of copper is lower than that of chromium, so evaporation and spattering of copper are more intense compared to chromium.
The formation of the chromium walls is explained by the fact that once burning the electric arc stops, the heat is removed from to the surface into the environment and inside the material. Therefore, the temperature near the surface and inside the material is lower than in the arc exposure zone. Chromium is the first to crystallize in the lower temperature areas. After formation of the chromium walls, the melt between these walls is crystallized.
The resulting structure in the exposure zone of the electric arc is characterized by a nonuniform distribution of chromium; hence, this structure has a lower entropy compared to the structure where chromium is distributed in a uniform manner. In view of this, it would be safe to assume that self-organization took place in the system [
14,
17].
The chromium wall located near the arc exposure surface contains about 26% oxygen (
Table 1). This wall therefore contains plenty of chromium oxide. Chromium oxide Cr
2O
3 has a high melting point (2435 °C), so it can resist the exposure to the electric arc better than the Cu–45%Cr alloy. The fine-dispersed mixture located between the two chromium walls contains more chromium than the original material. According to the equilibrium state diagram Cu–Cr, when the chromium content exceeds 50% by weight, the initial melting point of the alloy reaches the maximum value for alloys containing 0 to 80% chromium by weight (1767 °C). This is the temperature of eutectic transformation, so it remains unchanged as the chromium content changes from 50 to 80% by weight. The inner chromium wall protects the original material from thermal exposure of the electric arc. In case of repeated exposure to the electric arc, the melting of the resulting structure would require much more energy than during the first exposure. Therefore, alloys of the Cu–Cr system are adapted to exposure to the electric arc.
Similarly [
14], entropy change of the electric arc exposure zone consists of the following main components:
ΔSea—part of the entropy flow caused by exposure to the electric arc excluding the mass exchange with the environment, including the wear;
ΔSih—part of the entropy production excluding the mass transfer and chemical reactions (mostly heat flow and current);
ΔSim—part of the entropy production related to the mass transfer and chemical reactions;
Δ
Sem—part of the entropy flow related to the mass exchange with the environment.
ΔSeо—part of the entropy flow caused by the flow of oxygen from the environment into the arc exposure zone;
ΔSeCuCr—part of the entropy flow caused by the flow of chromium and copper from the electric arc exposure zone to the environment (the wear).
The last member in the right-hand section (1) is preceded by with a minus, since copper and chromium depart from the electric arc exposure zone into the environment with their entropy.
Entropy change of the electric arc exposure zone (Δ
S) is as follows:
Entropy change rate of the electric arc exposure zone is as follows:
The value (3) in stationary condition can be expressed as follows:
The first two members of the right-hand section of (5) depend on the power of the electric arc. If the arc power does not change, these members may be regarded as unchanged.
When the nonequilibrium structure shown in
Figure 3 is formed, its entropy will be less than the entropy of the equilibrium structure with uniform distribution of copper and chromium. All other things being equal, the entropy production of nonequilibrium structure formation (
Figure 1) will be less than the entropy production of equilibrium structure formation, i.e.,
The value consists of two main parts connected with the mass transfer and chemical reactions. During exposure to the electric arc, the chemical reaction of chromium oxidation takes place. Oxidation of chromium is a self-induced process producing positive entropy. The negative contribution into may be caused by entropy production of the mass transfer, since the resulting structure is nonequilibrium.
Due to additivity of entropy, the value
is proportional to wear intensity. It follows from (5) and (6) that during formation of the nonequilibrium structure (
Figure 1), material wear intensity when exposed to the electric arc will be less than in case of formation of the equilibrium structure.
Mention should be made of one more feature of formation of nonequilibrium structure under the influence of the electric arc. After formation of the nonequilibrium structure (
Figure 3), material loss intensity during repeated exposures to the electric arc will be several times less than during the first exposure. This is because, in case of repeated exposures, oxidation will only cover the part of chromium that will replenish the external wall. Therefore, oxidation will involve considerably less chromium than during the first exposure. Accordingly, entropy production related to oxidation will decrease. The same applies to entropy production of the mass transfer. Reduced entropy production in case of repeated exposures to the electric arc compared to the first exposure leads to a lower intensity of material loss in case of repeated exposures.
Therefore, adaptation of the Cu–Cr system alloys to the influence of the electric arc takes place via self-organization followed by formation of the nonequilibrium structure with non-uniform distribution of chromium.