1. Introduction
In the last decades, the study of energy systems has been focused on three main directions: the improvement of energy conversion efficiency, the use of clean technologies, and the employment of renewable energy sources like solar radiation, geothermal energy and waste heat from industrial processes [
1,
2]. To achieve this goal, new thermodynamic cycles have been proposed and some of them have been introduced in the market as competitive commercial alternatives to conventional heat to power cycles such as gas turbines and internal combustion engines. Some of these new thermodynamic cycles employ binary organic fluids mixtures due to their variable and low boiling temperatures during the boiling process, and their good thermal match with the heating fluid which produces an efficient utilization of the heat source [
3,
4,
5]. The new thermodynamic cycles have been proposed for both power production [
5,
6,
7,
8,
9], and combined power and refrigeration output [
4,
10]. Among these cycles, the Goswami cycle is one of the widely known combined cycles for simultaneous production of mechanical power and refrigeration supply. This combined cycle is the result of the combination of an ammonia-based organic Rankine cycle and an ammonia-water absorption refrigeration cycle. This cycle can be theoretically used at different scales, be integrated with different heat sources, e.g., solar energy, geothermal energy and waste heat and optimized for power, cooling, thermal or exergy efficiency, depending on the design priorities [
4].
Many studies have been conducted on the combined cooling and power system using ammonia–water as working fluid in mid-temperature applications. Some of these promising alternatives have combined the absorption refrigeration cycle with power cycles, mainly based on the Kalina cycle, to produce cooling and mechanical power. Zheng et al. [
11] proposed an absorption combined cycle where a rectifier replaced the original flash tank of the Kalina cycle. Sun et al. [
12] proposed a modified ammonia-water absorption cycle combined with a Rankine cycle for both power and refrigeration output to recover heat from a 350 °C heat source. The equivalent heat-to-power and exergy efficiencies of this cycle were 18.6% and 42.0% , respectively, and consumed 17.1% less heat than the absorption and the Rankine cycle separated. Later, Yu et al. [
13] presented a modified version of Zheng’s cycle with an ammonia refrigeration cycle. According to their results, Yu’s cycle was able to provide cooling and power simultaneously with adjustable ammounts. Zheng reported a 24.2% of thermal efficiency and a 37.3% of exergy efficiency , meanwhile Yu reported an improved thermal efficiency of 37.8%. In both studies, a turbine inlet temperature of 355 °C was adopted. Jin and Zheng [
14] took a similar approach of Zheng’s cycle and proposed a modified Kalina cycle coupled with a double effect absorption refrigeration cycle. The new combined cycle produced power and cooling with a co-generation efficiency of 41.2% for a heat source temperature of 465 °C.
In addition, other researchers have studied various combined ammonia-water cycles coupled with mid and low-grade temperature heat sources. Srinivas and Reddy [
15] presented a solar combined power and cooling cycle based on an absorption refrigeration system and a Kalina cycle, which was simulated for a solar field outlet temperature of 170 °C. They reported a specific net power output and cooling output of 62.56 kW/kg and 72 kW/kg, respectively. Also, reported results showed a 7–8.85% of heat to power efficiency and up to 7.4% for cooling efficiency. Junye et al.[
16] proposed a Kalina cycle with three operation pressures and three new components: a preheater, a water solution cooler and an absorber instead absorption condensers. Simulation results for a turbine inlet temperature of 300 °C showed a first law efficiency of 17.86%. A study from Ayou et al. [
17] compared the performance of the Goswami cycle and two new proposed cycles: a Single-stage combined absorption power and a refrigeration cycle with series flow (SSAPRC-S) and a Two-stage combined absorption power and refrigeration cycle with series flow (TSAPRC-S). This study, for an desorber temperature of 220 °C, showed that TSAPRC-S and SSAPRC-S achieved a thermal efficiency of 16.8% and 14.6%, respectively. According to this study, the TSAPRC-S and SSAPRC-S have a better performance than the Goswami cycle at 220 °C of desorber temperature. Rashidi et al. [
18] proposed two alternative systems of Kalina cycle coupled with an absorption chiller for low-grade heat sources. The first proposed system included two throttling valves and an evaporator to an ammonia-water based Kalina cycle to generate cooling. In the second system an ammonia-water Kalina cycle provided heat to a Lithium Bromide-water absorption chiller that provides cooling. After performing a parametric analysis, results showed that maximum thermal efficiency was 18.3% for the first system, and 25.9% for the second system. Another study conducted by Cao et al. [
19] compared two different alternatives for power and cooling production from low-grade heat sources: a modified Kalina cycle for power and cooling production, and a Kalina cycle coupled with a vapor compression cycle. They reported that the modified Kalina-based power and cooling cycle reached a thermal efficiency of 25.76%, 89.59 kW of power output and 5.58 kW of cooling, meanwhile the Kalina cycle coupled with a vapor compression cycle showed 23.44% of thermal efficiency and 77.81 kW of power output.
With regard to the Goswami cycle, several studies have been proposed to determine the relative advantage of this new technology compared to conventional heat to power applications [
20,
21,
22,
23,
24,
25,
26]. Xu et al. [
20] carried out a thermodynamic analysis to find the effect of some key parameters like ammonia mass fraction, boiler pressure and temperature on the thermal efficiency and the cooling capacity of the cycle. A second thermodynamic study was performed by Hasan et al. [
21] by using a Lorentz cascade arrangement. They found that second law efficiency reached a 65.8% at a heat source temperature of 420 K. Hasan found that, for the studied configuration, an increase in heat source temperature did not increase the second law efficiency. The experimental and theoretical analysis conducted by Martin and Goswami [
24] about the performance of the cycle led to a new measure of the effectiveness of cooling production. New configurations of the Goswami cycle have also been proposed and analyzed from the first and second law point of view [
25,
26]. A comprehensive exergy analysis of two alternative configurations of the cycle was presented by Fontalvo et al. [
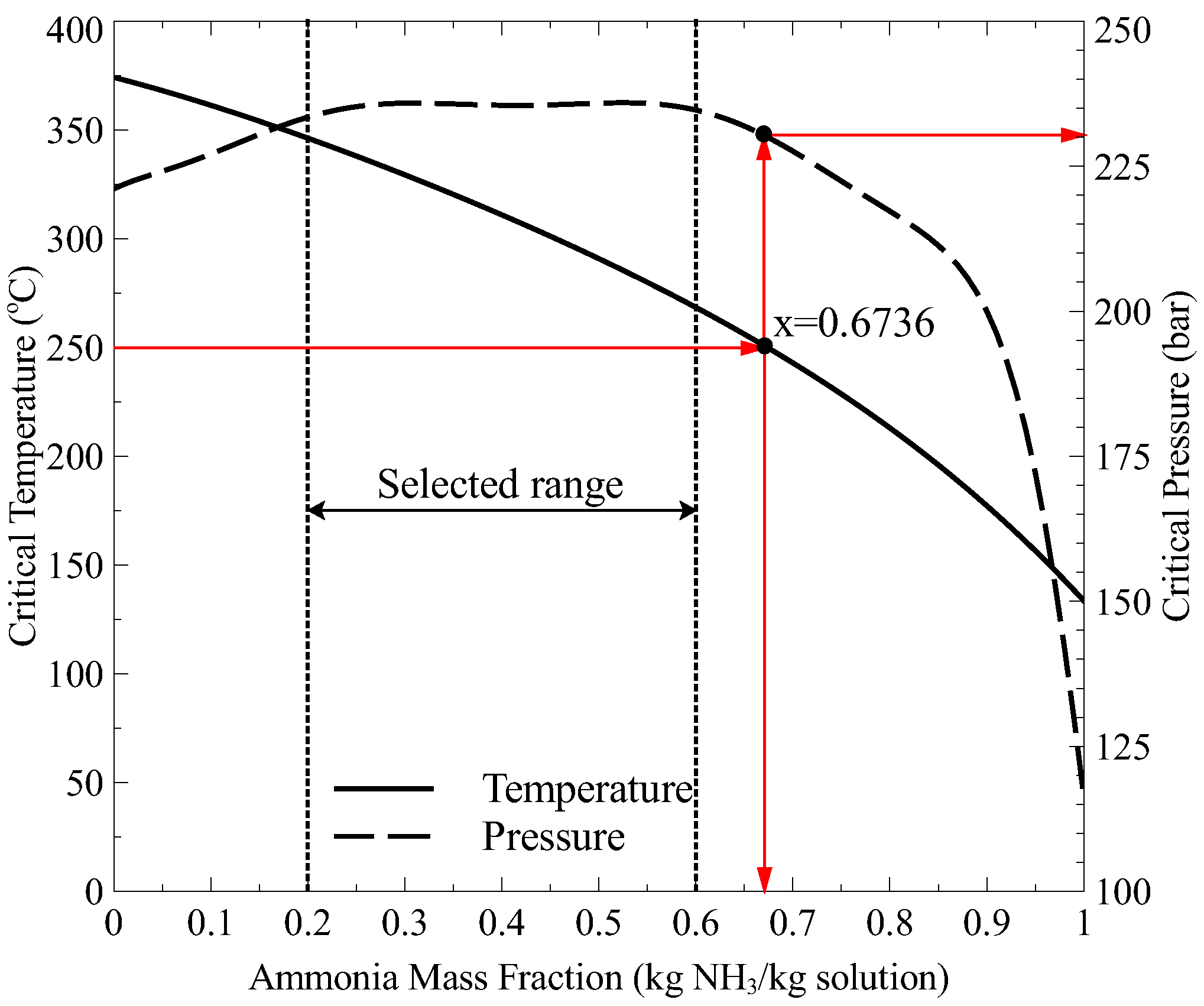
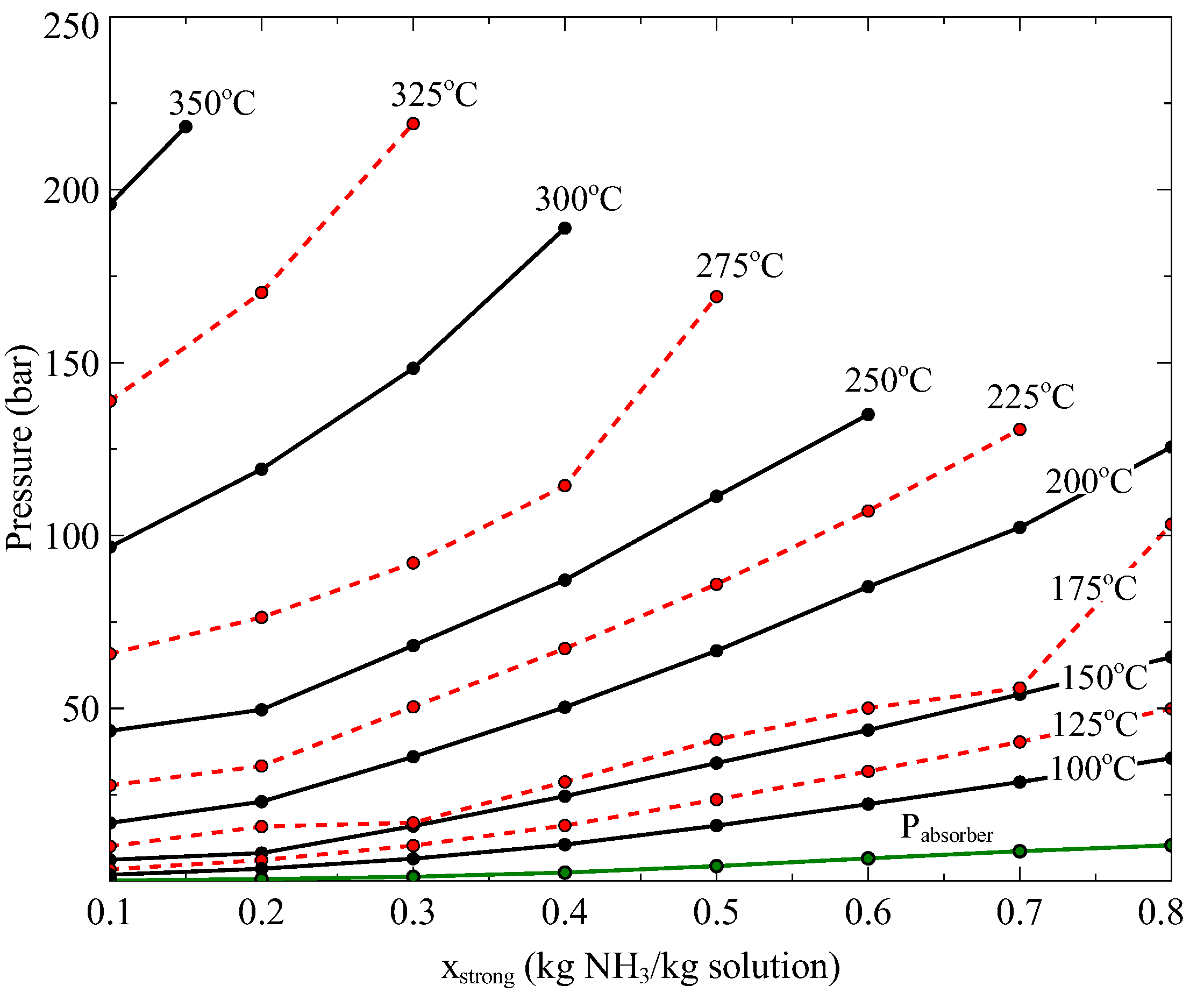
27]. They determined the effect of some key parameters on the exergy destruction and calculated the contribution of each component of the cycle, showing that the absorber and the boiler had the highest contribution. However, these studies were carried out at heat source temperatures below 420 K. Therefore, the performance of the Goswami cycle has not been studied for higher heat source temperatures, which can be found in solar thermal energy or geothermal sources. The integration of the Goswami cycle with medium heat source temperatures leads to a more complex thermodynamic analysis, since at boiler temperatures above 150 °C the strong solution concentration has a certain range in which an ammonia-water mixture can exist as consequence of the variability of the critical point (temperature and pressure) of the ammonia-water mixture.
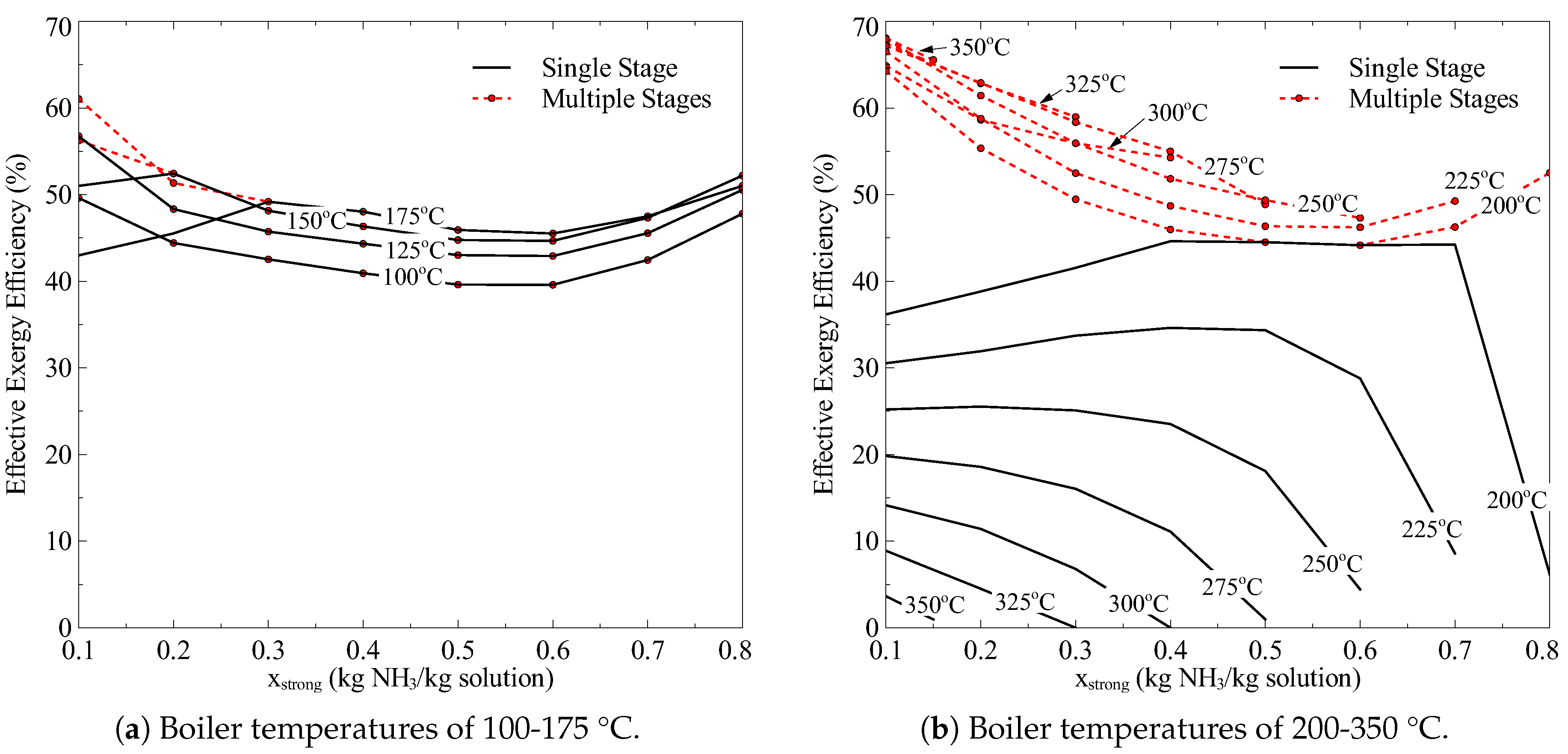
This study presents a theoretical thermodynamic analysis of the Goswami cycle for low-Grade and Mid-Grade heat sources. In addition, some modifications to the original Goswami cycle are proposed to improve its thermodynamic integration with Mid-Grade heat sources and overcome some operating restrictions in its components. The aim of this paper is to find the optimum operating conditions and configurations which maximize the performance of the proposed cycle operating at low and medium heat source temperatures, with a boiling temperature in the range of 100 °C to 350 °C. The effect of the different thermodynamic parameters such as: boiling temperature, pressure ratio, reheating, turbine stages on the thermal and exergy efficiencies is analyzed.
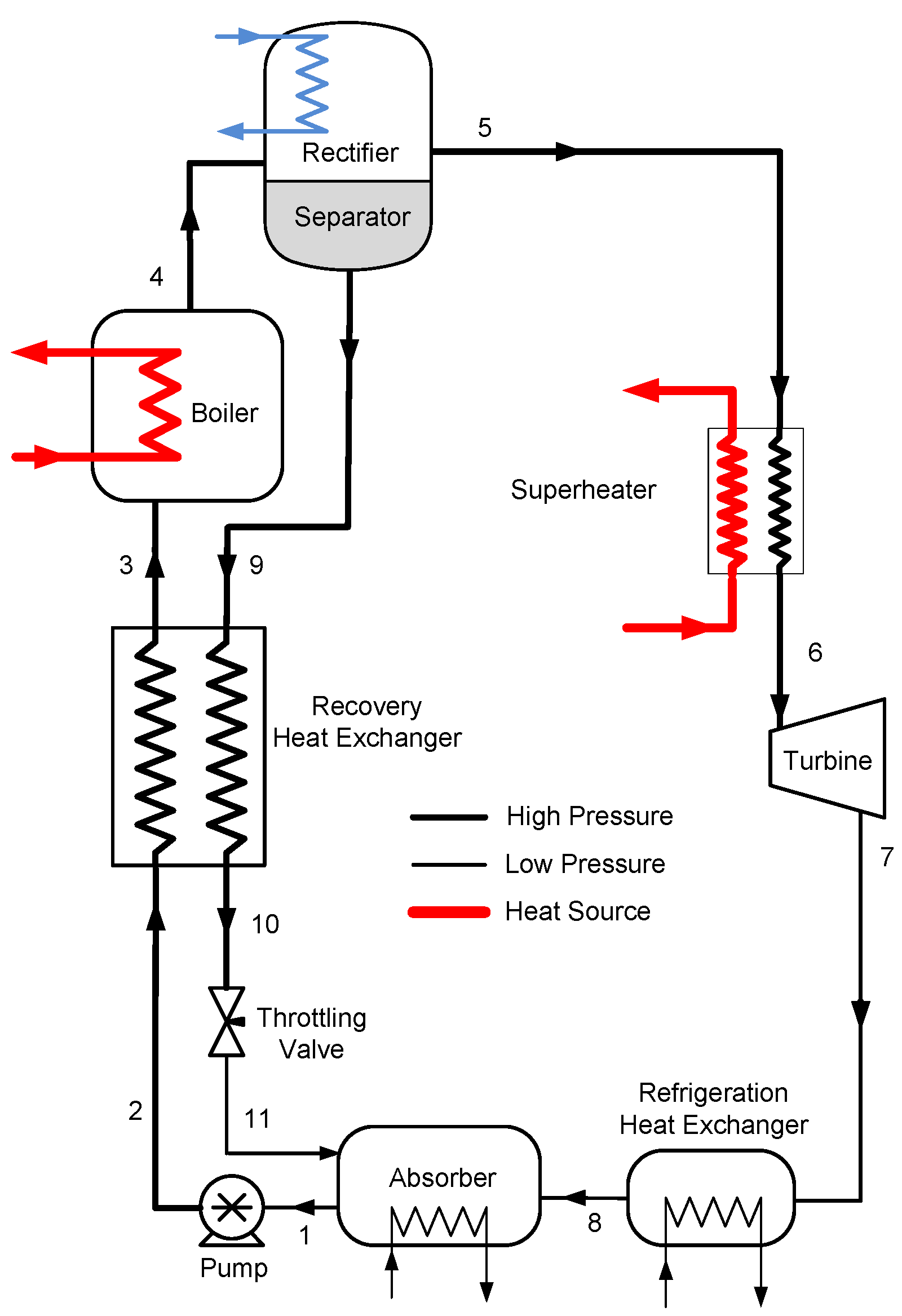
3. Alternative Configurations
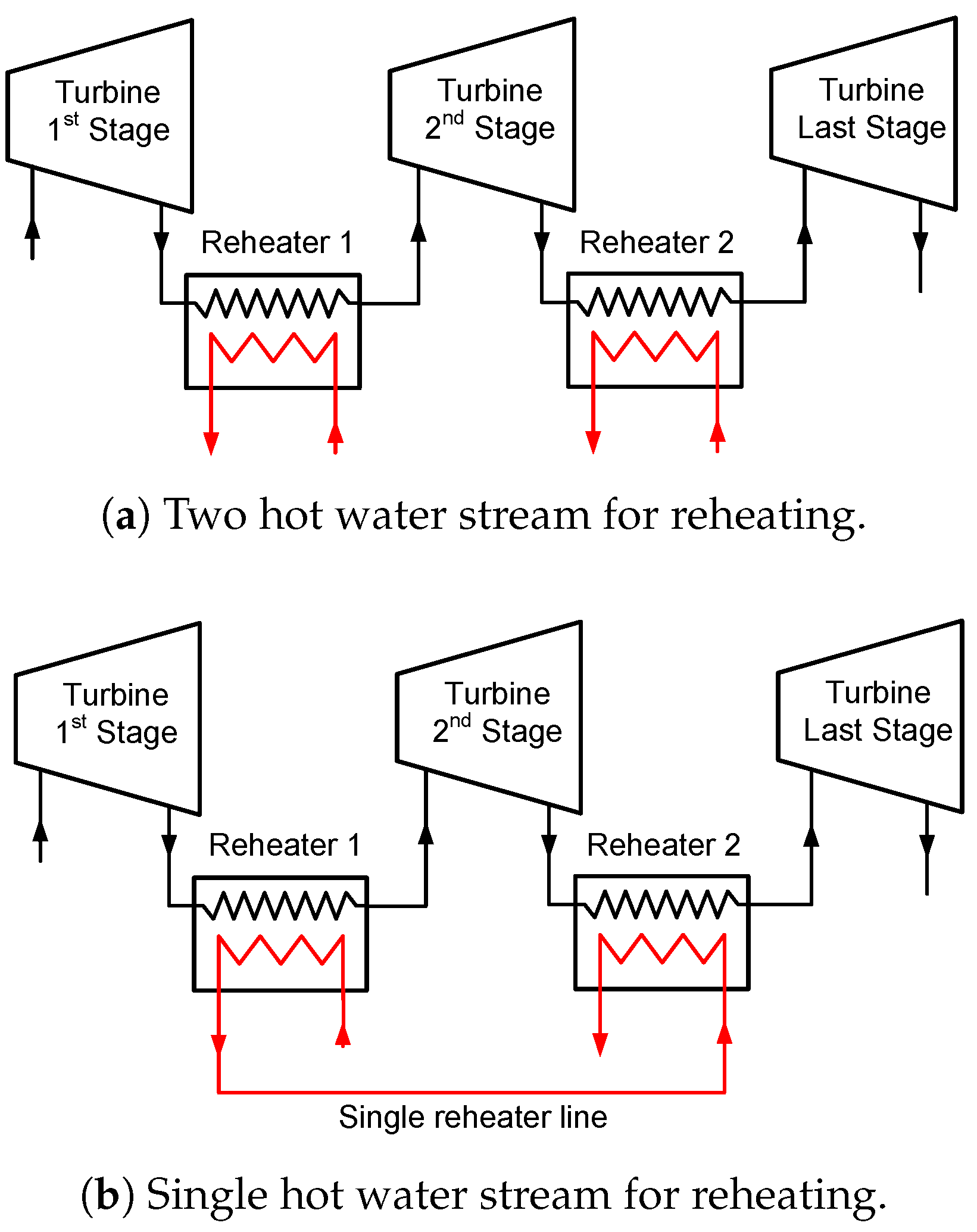
If the reheating temperature is held constant, the question arises whether the high temperature vapor can be used as waste heat to increase the overall cycle efficiencies. The high temperature vapor can be used as a heat source for a bottoming cycle or a heat recovery system.
Combined Cycle and Vapor Heat Recovery Configurations
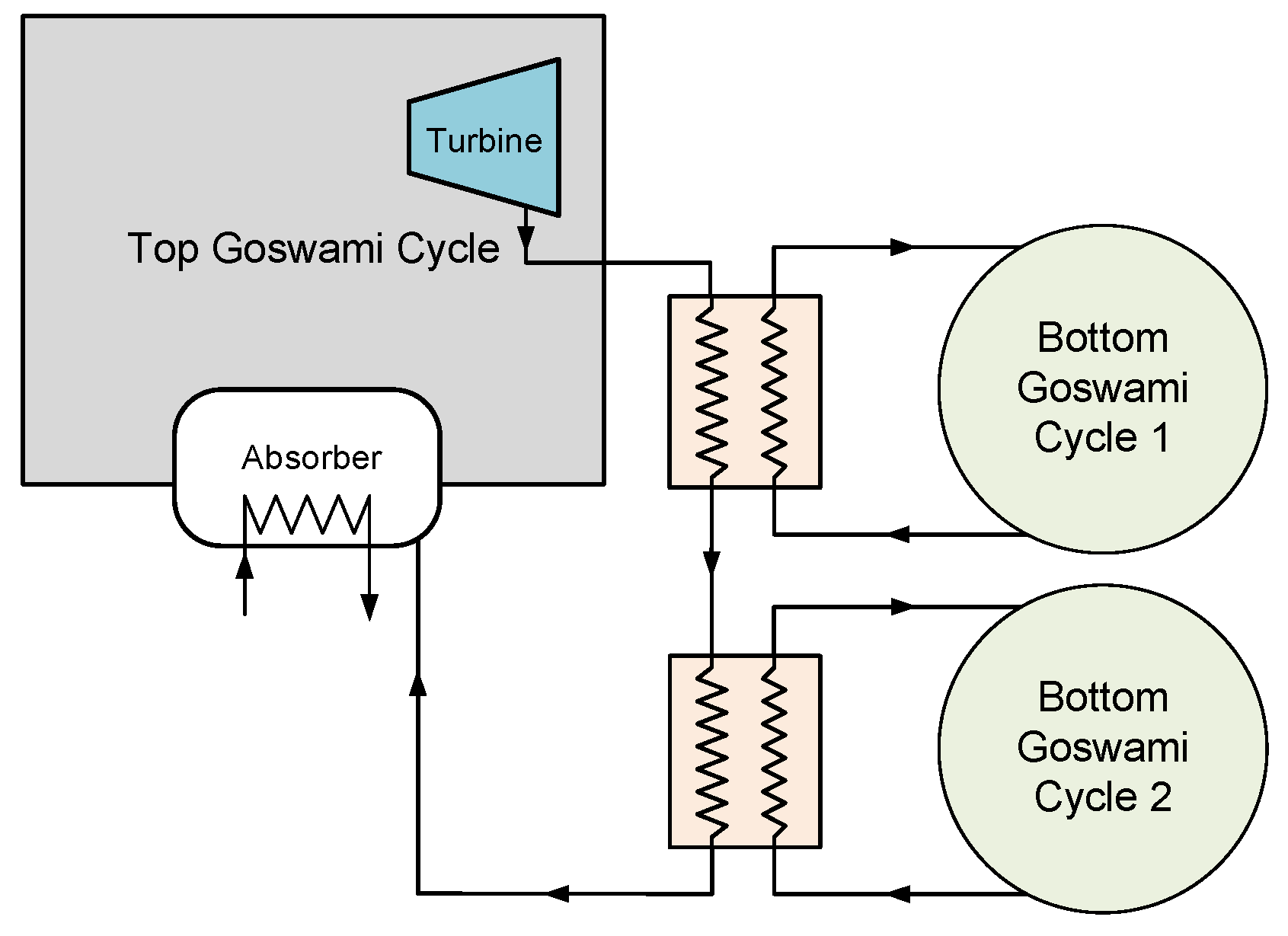
The combined cycle has a top and bottom Goswami cycles. As shown in
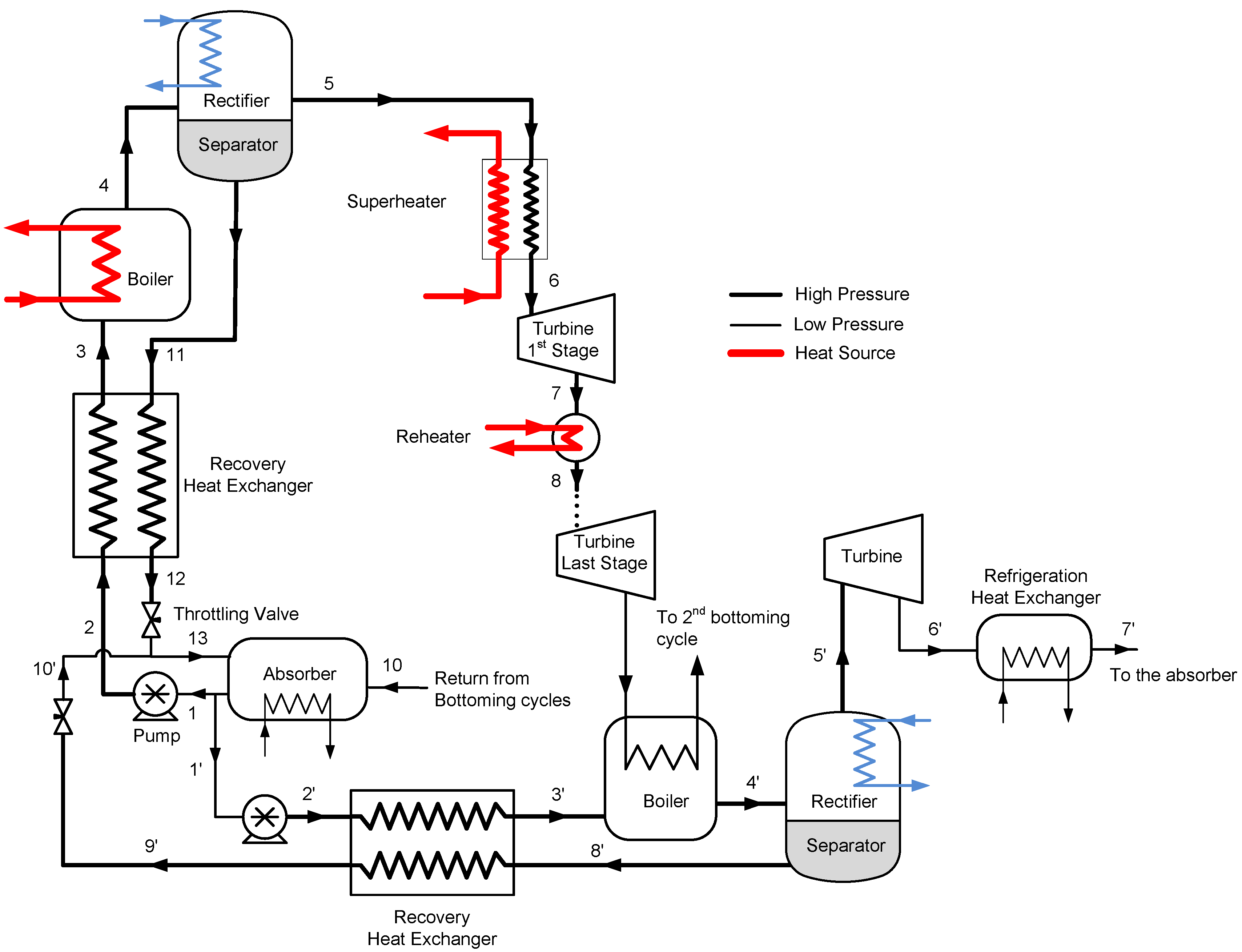
Figure 5, the turbine exhaust of the top Goswami cycle can be utilized by heating the bottom Goswami cycles working fluid. The simulation of the combined cycle is a complex problem as the optimization of the bottom cycles is also required. The detailed description of the top cycle and the first bottoming cycle are shown in
Figure 6. The operating conditions at maximum effective exergy efficiency are used to simulate the top cycle, therefore the mass flow rate and temperature of the vapor are known. The boiler temperature, system high pressure, and strong solution concentration of the bottom cycle will define the temperature of state 8’ as shown in
Figure 6, which enters the recovery heat exchanger. The temperature of state 8’ is independent of the strong solution mass flow rate. By performing recovery heat exchanger calculations, the temperature of state 3’ is determined. Then, the boiler heat exchanger calculations are performed and bottom cycle strong solution mass flow rate is found.
The entropy generation in a certain control volume cannot be lower than zero, based on the second law of thermodynamics, and this constraint is applied to all heat exchangers as well as boiler heat exchangers. In the previous analysis, the heat source mass flow rate of the top cycle is calculated based on the pinch point assumption, and then the entropy generation is calculated for the heat exchanger. If the entropy generation term is less than zero, which is an impossible process, the heat source mass flow rate is increased to satisfy the entropy generation constraint. In this case, the top cycle vapor mass flow rate is constant, therefore the bottom cycle mass flow rate is calculated based on the pinch point assumption, and then if the entropy generation term is negative, the pinch point value is increased until the entropy generation is higher than zero. Whenever the pinch point increases, the mass flow rate of the bottom cycle decreases, and the temperature of the top cycle vapor after the heat exchanger might be still high. For this reason, when the top cycle vapor temperature is above 150 °C, two bottom cycles are required to cool down the high temperature vapor of the top cycle to lower than 100 °C.
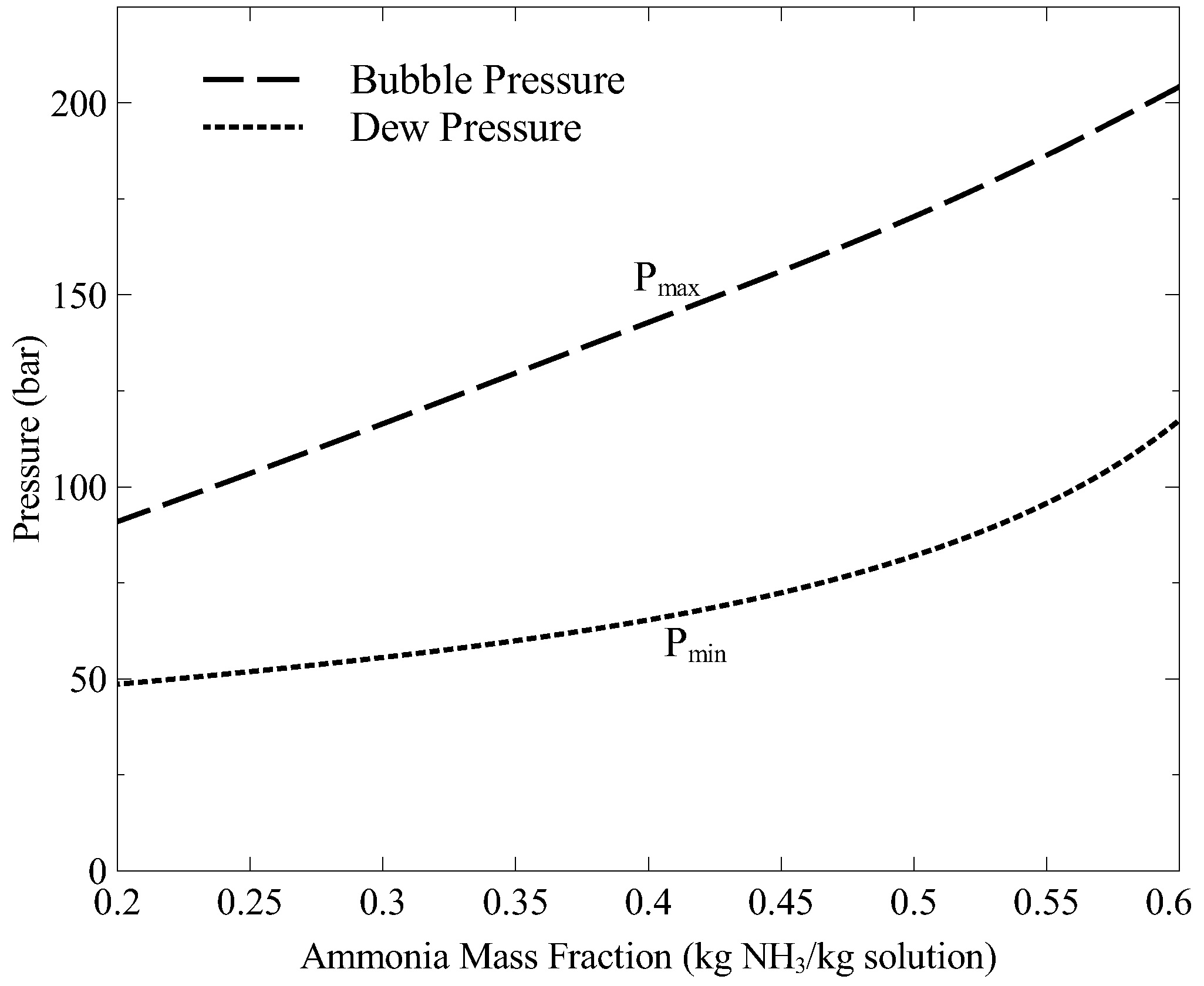
In order to search the maximum work output from the bottoming cycle, the system high pressure is varied between the bubble and dew point pressures for the corresponding boiler temperature and strong solution concentration.
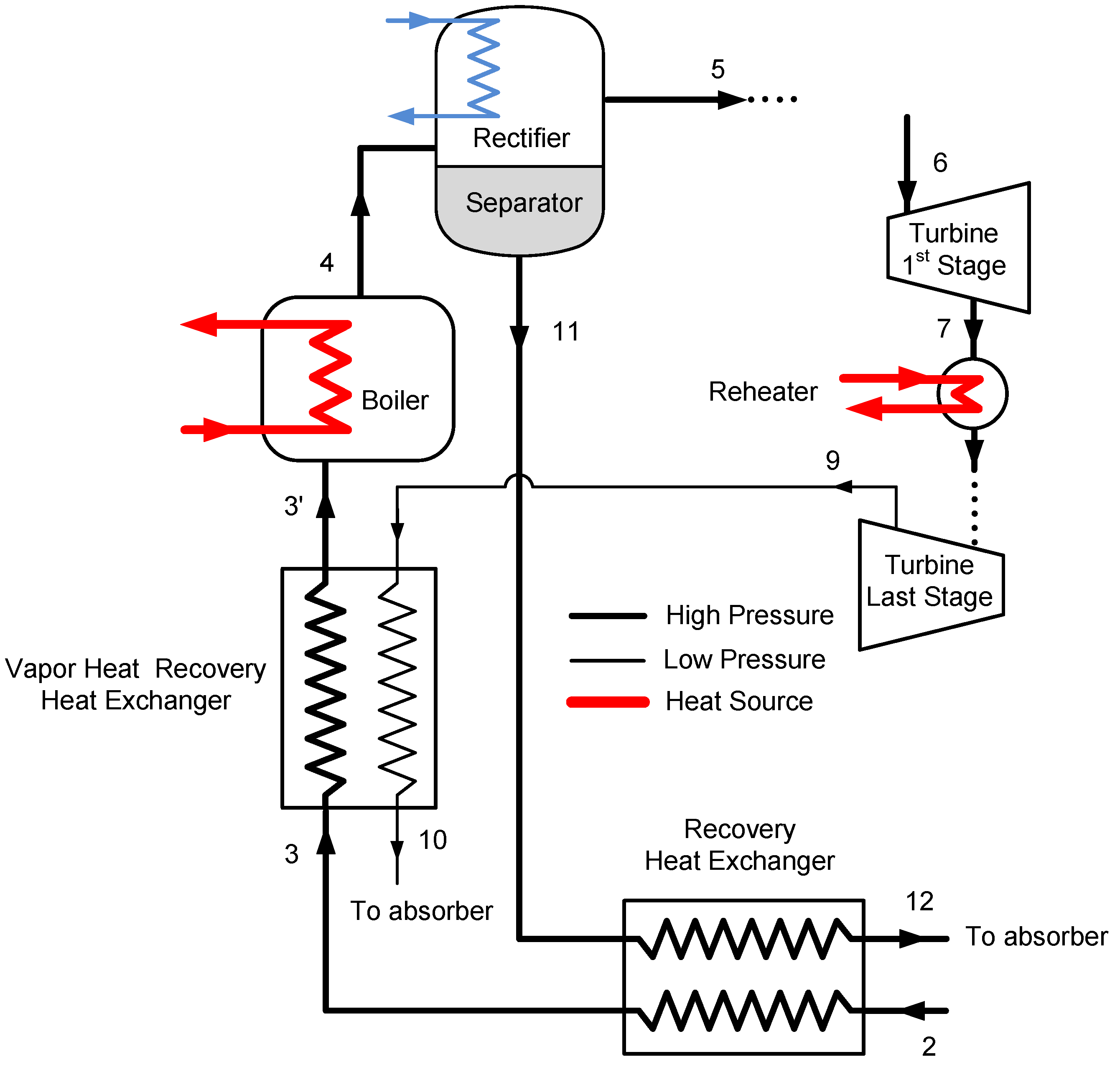
The vapor heat recovery system is shown in
Figure 7. The Goswami cycle system is simpler than the Kalina cycle distillation and condensation subsystems, it has two heat recovery heat exchanger, one separator and a pump as shown in
Figure 7. The strong solution is reheated first by the liquid weak solution return from the separator. Then, it is reheated by the high temperature turbine exit vapor, and then it enters the boiler heat exchanger. As described above, entropy generation constraint is also imposed on the vapor heat recovery exchanger.