3.1. A Variational Formulation for Time-Dependent Nonlinear Nonholonomic Systems
In this section, we present an abstract variational framework for the treatment of open thermodynamic systems later. The variational formulation consists in computing the critical curve of an action functional by imposing two kinds of constraints: a variational constraint which imposes constraints on the variations of the curve, and a kinematic constraint which imposes constraints on the critical curves. These two constraints are related in a very specific way. This setting extends the variational formulation proposed in [
2,
3,
12] for closed thermodynamic systems. In the particular case of linear constraints, the variational formulation recovers the Lagrange-d’Alembert principle for nonholonomic mechanics, see, e.g., [
13]. A general variational setting for mechanics with nonlinear constraints is described in [
14,
15]. For the variational formulation of time-dependent nonholonomic mechanics with affine constraints, see [
16].
Let Q be a configuration manifold with local coordinates and let be the extended configuration manifold which includes the time variable . We consider the vector bundle over with local coordinates and the vector bundle over with local coordinates .
Consider a time-dependent constraint
of the form
Such a constraint defines for each
an affine subspace
. By considering, for each
, the linear subspace
associated to
, we get the time-dependent variational constraint
Given
, we define the associated nonlinear nonholonomic kinematic constraint
by
We note that the function
and
may depend explicitly on the time
t.
Let
L be a time-dependent Lagrangian defined on
and let
be an external force field assumed to be fiber preserving, i.e.,
. We consider the following Lagrange-d’Alembert principle with time-dependent nonlinear constraints and external force field:
with respect to variations
, i.e.,
with
, and where the curve
satisfies the the nonlinear constraint
, i.e,
By a direct computation, using Lagrange multipliers
, we get that a curve
is critical for the variational formulations (
2)–(
4) if and only if it is a solution of the time-dependent nonlinear nonholonomic Lagrange-d’Alembert equations with external forces:
Associated to the Lagrangian
, we define the energy
on
as
Along a solution of the evolution Equation (
5), we get the energy balance equation
As we shall show later, the variational formulation for discrete open thermodynamic systems falls into the abstract variational setting (
2)–(
4). In this case, the system (
5) yields the complete evolution equations for the thermodynamic system, in which the nonlinear constraint gives the entropy equation, in agreement with the second law of thermodynamics. The energy balance Equation (
6) yields the first law of thermodynamics.
3.2. Foundations of the Variational Formulation for Discrete Open Thermodynamic Systems
In order to introduce our variational formulation, we shall first consider a particular case of simple discrete system, namely, the case of a system with a single chemical component
N in a single compartment with constant volume
V. Recall that, since the system is assumed to be simple, there is a single entropy
S attributed to the whole system. Non-simple systems will be considered in
Section 4. We assume that there is no heat and work exchanges, except the ones associated to the transfer of matter. We also ignore all the mechanical effects, which will be included later in
Section 3.3. In this particular situation, the energy of the system is given by the internal energy written as
, since
is constant. The balance of mole and the balance energy, i.e., the first law, are respectively given by
see (
1), where
is the molar enthalpy at the
a-th port and where
,
, and
are respectively the molar internal energy, the pressure and the molar volume at the
a-th port. From these equations and the second law, one obtains the equations for the rate of change of the entropy of the system as
where
is the molar entropy at the
a-th port and
I is the rate of internal entropy production of the system given by
with
the temperature and
the chemical potential. For our variational treatment, it is useful to rewrite the rate of internal entropy production as
where we defined the entropy flow rate
and also used the relation
. The thermodynamic quantities known at the ports are usually the pressure and the temperature
,
, from which the other thermodynamic quantities, such as
or
are deduced from the state equations of the gas.
Remark 1. There are several representations for the rate of internal entropy production given in (8), namely, we can rewrite I as as well as Variational formulation. Let
W,
, and
be the molar and thermal displacements and the internal entropy of the system respectively, each of whose interpretation will be explained later. Given the molar flow rates
, the entropy flow rates
, the temperatures
, and the chemical potentials
at the ports
, we consider the following variational formulation:
where the curves satisfy the nonlinear nonholonomic constraint
and with respect to variations subject to the constraint
with
.
Remark 2. We call the internal entropy production (10) the kinematic constraint, where the internal entropy production, in general, is described not only by the phenomenological relations obtained experimentally but also by the pressures, temperatures and the molar flow rates at the external ports. This equation is generally a nonlinear nonholonomic constraint imposed on the curve of the state variables in the variational formulation, see (4). We will later explain in details how the variational formulations (9)–(11) arises as a special instance of the abstract variational formulations (2)–(4). Remark 3. In the variational formulations given in (9)–(11), we employed the new variables, Γ and W, such that and , where we interpreted the variable Γ as the thermal displacement and the variable W as the molar displacement. These are examples of thermodynamic displacements, as introduced in [2,3], whose rate of change coincide with the affinity of the irreversible process. In particular, note that Γ is a monotonically increasing real function of time t since the temperature T takes positive real values. Note also that the notion of thermal displacement was first used by [17] and in the continuum setting by [18]. Refer to the Appendix of [19] for an historical account. The variables Γ and W are conjugate variables with S (or Σ) and N, respectively. Moreover, we used another new variable Σ, which is not the same object as the entropy of the system S but it denotes the internal entropy of the system. In fact, indicates the internal entropy production, with , sometimes denoted as in the standard literature (see, for instance, Equation (8) on page 21 in [20]), while the total rate of entropy production of the system, , does not need to be positive if the system is not adiabatically closed. Comments and structure of the variational formulation. Before applying the variational formulation to derive the equations, we first comment on the variables and functions involved as well as on the structure of this formulation. The variational formulations (
9)–(
11) gives conditions on the curve
that ultimately gives a system of ODEs for this curve. This curve involves the thermodynamic quantities associated to the system, namely, the number of moles
, the total entropy
and the internal entropy
of the system, as well as the thermodynamic displacements
,
whose interpretation will be explained below. Recall that the knowledge of a fundamental state equation of the system, such as
, allows one to deduce all the other thermodynamic relations.
The thermodynamic quantities associated to the ports , are denoted with an exponent a, such as and . They satisfy the same relations as the corresponding quantities of the system, because they represent the same species, i.e., are obtained from the same state equation. Given a port a, if matter is flowing into the system through that port from the exterior, then we will assume that the thermodynamic quantities are known from the experimenter, i.e., they are prescribed functions of time such as and , which characterise the thermodynamic properties of the matter flowing into the system. On the other hand, if matter is flowing out of the system through that port, then the associated thermodynamic quantities coincide with the thermodynamic quantities of the system, such as, , . In this case, these quantities are not assumed to be known, they are computed via the evolution equations. If matter is flowing in, we shall assume that the molar flow rate is a prescribed function of time, i.e., . If matter is flowing out of the system then is usually computed from the other variables and the physical properties of the system. In the system considered here, since there is no additional heating from the exterior, the entropy flow rate is given in terms of the molar flow rate as , where is the molar entropy at the a-th port.
We note that the variational constraint (
11) follows from the phenomenological constraint (
10) by formally replacing the rates by the corresponding virtual displacements, i.e.,
, and by removing all the terms that depend uniquely on the ports
, i.e., the terms
and
. Such a systematic correspondence between the phenomenological and variational constraints, was already verified for the variational formulation of closed systems, see [
2,
3]. It is extended here to the case of open systems. Note that the action functional in (
9) has the same form as that in the case of closed systems, see [
2]. As will be shown later, this systematic relation between the two constraints still holds in the more general cases of discrete open systems considered below.
Application of the variational formulation. By taking the variation of the integral in (
9) and using the variational constraint (
11), we get the following evolution equations:
From the first and third equations in (
12), temperature is obtained as the rate of the thermal displacement
while the chemical potential as the rate of the molar displacement
W, since the temperature and the chemical potential of the system are defined by
and
.
By inserting the conditions (
12) into the phenomenological constraint (
10), we get the desired entropy equation in terms of
T and
, namely
in accordance with (
7) and (
8). From the entropy equation and the last relation in (
12), we have the rate of internal entropy production of the system as
To summarize, from the variational formulations (
9)–(
11), we have obtained the following evolution equations for the open system with
A ports:
From this system, the first law is computed as
Equations (
14) yield a system of two ODEs for the two variables
and
, for given molar flow rates
. The evolution of the other variables, such as
,
,
, can be computed from
and
by using the state equation. In some situations, the molar flow rates are chosen in order the system to respect some properties, such as, for example a constant pressure
.
Example 1 (Filling an Insulated Tank with Pressurized Gas).
In order to illustrate our formalism, we consider an insulated tank containing an amount of N moles of ideal gas at temperature T and pressure p. We assume that gas with temperature and pressure , which are given prescribed functions of time t, is entering the tank at a single port with the molar flow rate , ([21], Section 8). From the fundamental state equation of the ideal gas , all the thermodynamic relations can be obtained. For instance, we have where R is the universal gas constant, , are the heat capacity coefficients at constant volume and constant pressure of the ideal gas, and the index indicates reference values. The constants R, , and are expressed in units . Note that , , denote the molar quantities associated to U, V, S. The above variational formulation yields the equations of motion where, for an ideal gas, I is computed as This expression recovers the entropy production for this system, see, e.g., ([21] Equation (8.74)). It is associated to the irreversible process of mixing ideal gas at different temperature and pressure. By rewriting the entropy production as we see that for all T, , and for . The second law thus consistently requires that . 3.3. Variational Formulation of Open Simple Systems with Mechanical Interactions
We shall now extend the previous variational formulation to the case of an open system including exchange of mechanical momenta. Such a system is described by two kind of mechanical variables. Mechanical variables describe the motion of the mechanical devices involved in the system, while are mechanical variables which indicate the motion of the species of the system. Through each of the A ports, we have the flow rates , , and , , corresponding to exchange of matter, mechanical momentum, and entropy. We assume that the species flows through the a-th port at velocity . We also assume that the mechanical components are subject to friction and external forces, respectively, denoted by , and , .
Variational formulation. We consider the following variational formulation:
where the curves satisfy the nonlinear nonholonomic constraint
and with respect to variations subject to the constraint
with
,
,
.
Application of the variational formulation. By taking the variation of the integral in (
16) and using the variational constraint (
18), we get the following conditions:
Extending the earlier definitions, we define the temperature and the chemical potential of the system in tersm of the Lagrangian as
and
. By inserting the conditions (
19) into the phenomenological constraint (
17), we get the following entropy equation
To summarize, from the variational formulations (
16)–(
18), we have obtained the following evolution equations for the open system with mechanical interaction and
A ports:
This system of ODEs completely determines the time evolution of all the variables. In absence of thermodynamic effects, it reduces to the Euler-Lagrange equations of classical mechanics.
Defining the total energy of the system as
we have the energy balance
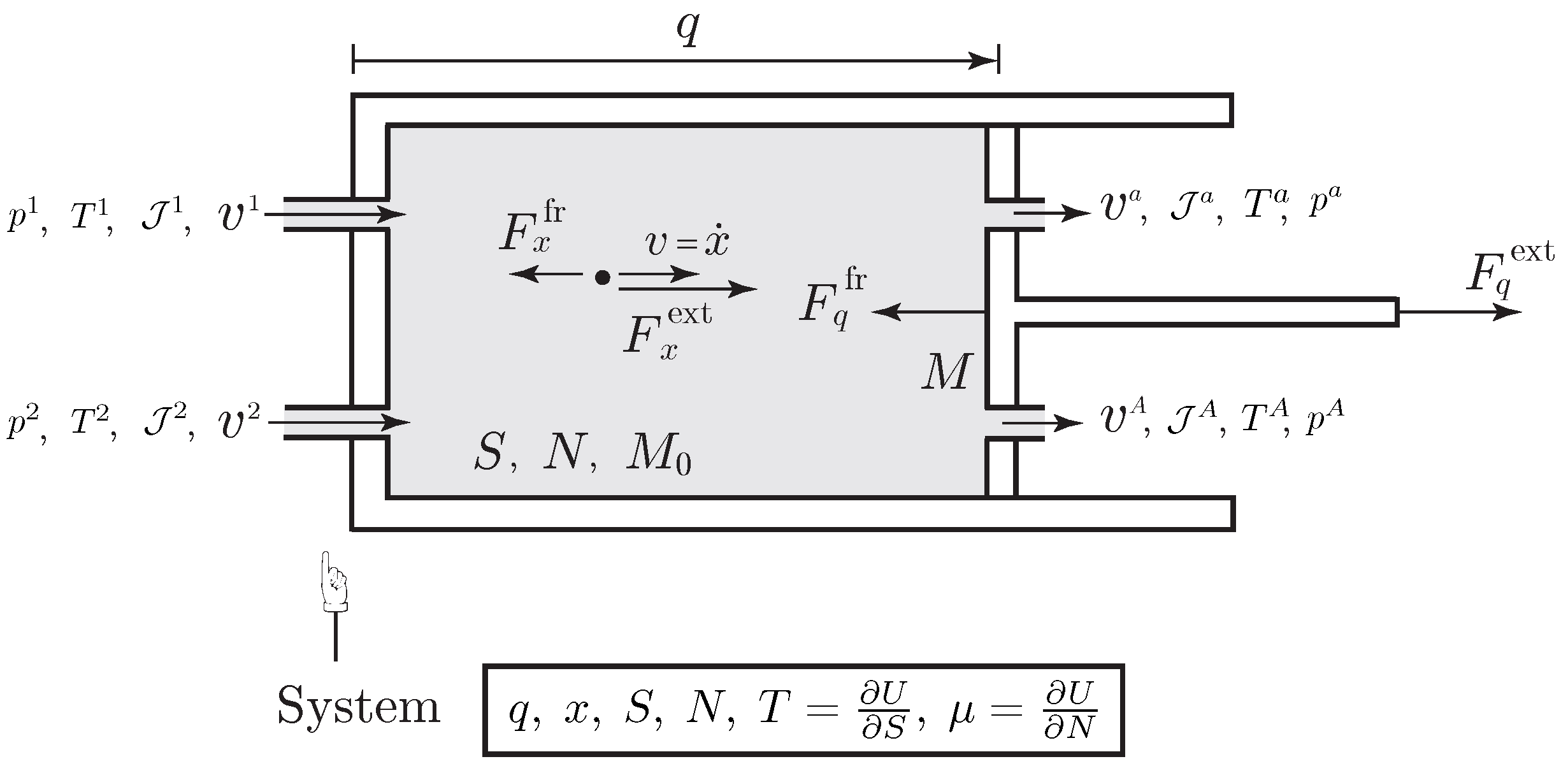
Example 2 (Flowing Fluid Carrying Kinetic Energy into a Piston Device).
We consider a piston with mass M moving in a cylinder containing a species with internal energy . We assume that the cylinder has A ports through which species is injected into or flows out of the cylinder with molar flow rates and velocities . The momentum and entropy flow rates accompanying these exchanges of species are given by where is the molecular weight of the species. We assume that the species flows through the a-th port and in the cylinder in a one dimensional direction, i.e., is parallel to , for each , as illustrated in Figure 1. The variable q characterizes the one-dimensional motion of the piston, such that the volume occupied by the species is , with the sectional area of the cylinder. The Lagrangian of the system is the sum of the kinetic energy of the piston and the species, to which is subtracted the internal energy of the species By a direct application of the variational formulation, the evolution equations are obtained as where is the pressure, together with the entropy Equation (20) which yields where we used the expression of the temperature and the expression of the chemical potential By translational invariance, the frictions forces verify , thus from this equation and the last equation in (19), we get the rate of entropy production within the system as where the first term represents the entropy production associated to the friction experiencing by the moving piston and the moving species, the second term is the entropy production associated to the mixing of gas at different velocities, and the last term is the entropy production associated to the mixing of gas at different pressure, and temperature. The second law requires that each of these terms is positive. Concerning the first term, the friction force must be dissipative and can be chosen as , where the phenomenological coefficient is determined experimentally, see [1]. The second term is positive as long as and it is zero if since in this case . Finally, the last term has been already analyzed in the previous example in the case of the ideal gas, see (15). The energy balance is deduced from (22) as where denotes the total (kinetic plus internal) molar energy of the species flowing at the a-th port. This balance of energy is consistent with the general expression of the first law in (1). Potential energies can be easily included in this example. External heating will be considered in Section 4. 3.4. Variational Formulation of an Open Simple System with Internal Matter Diffusion
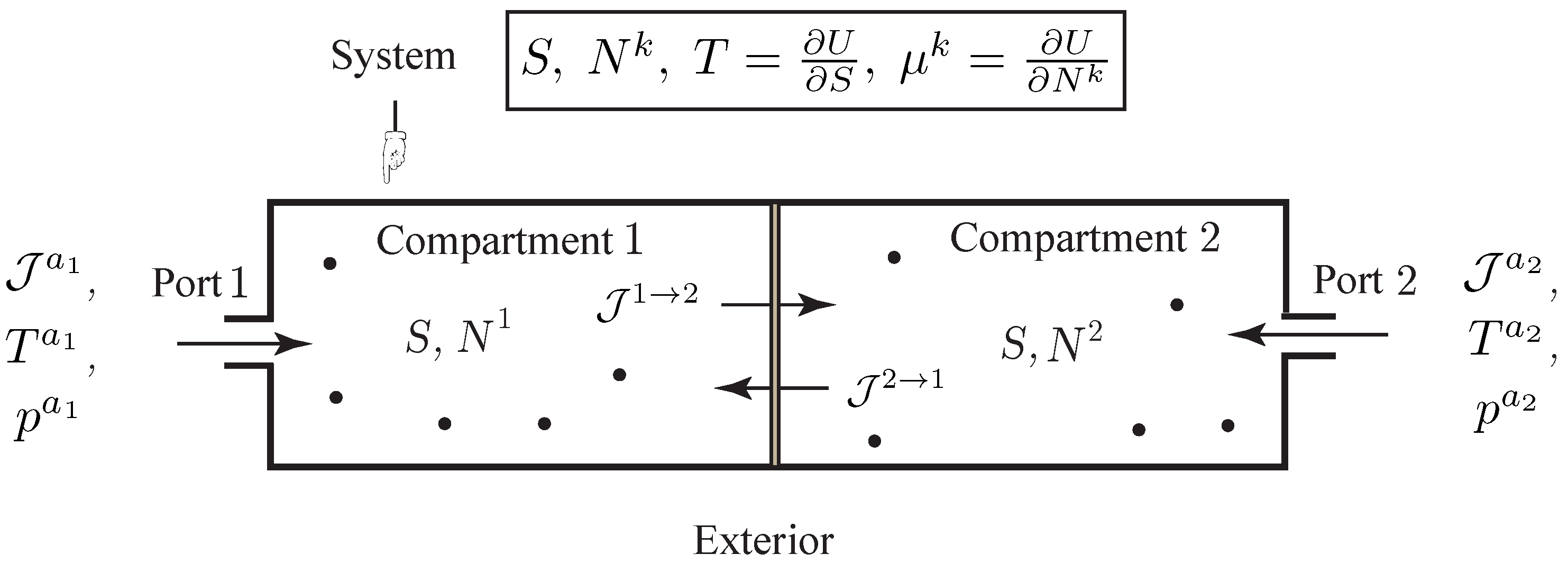
In this subsection, we extend the previous variational formulation to the case of open discrete systems experiencing internal diffusion processes. Diffusion is particularly important in biology where many processes depend on the transport of chemical species through bodies.
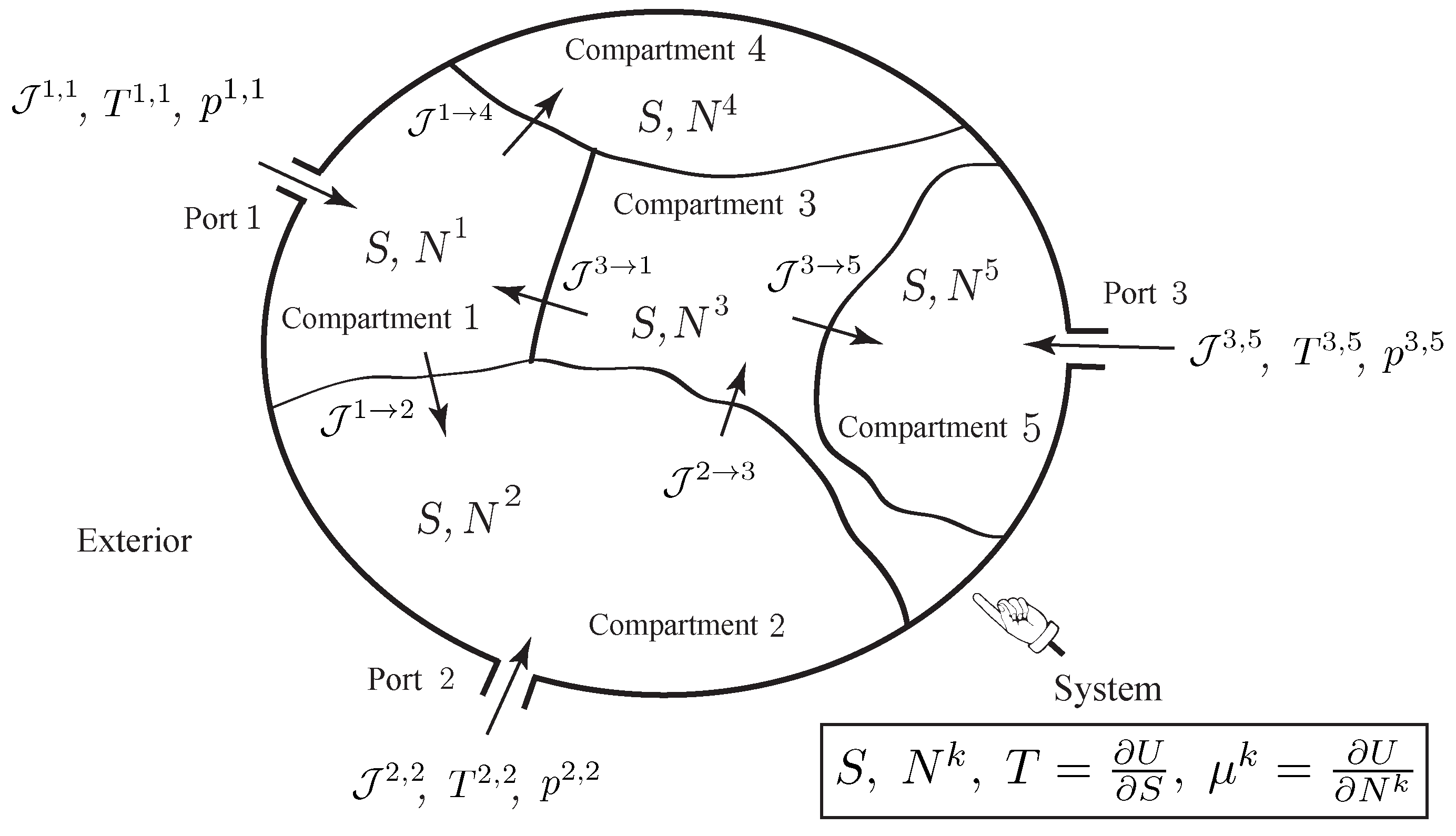
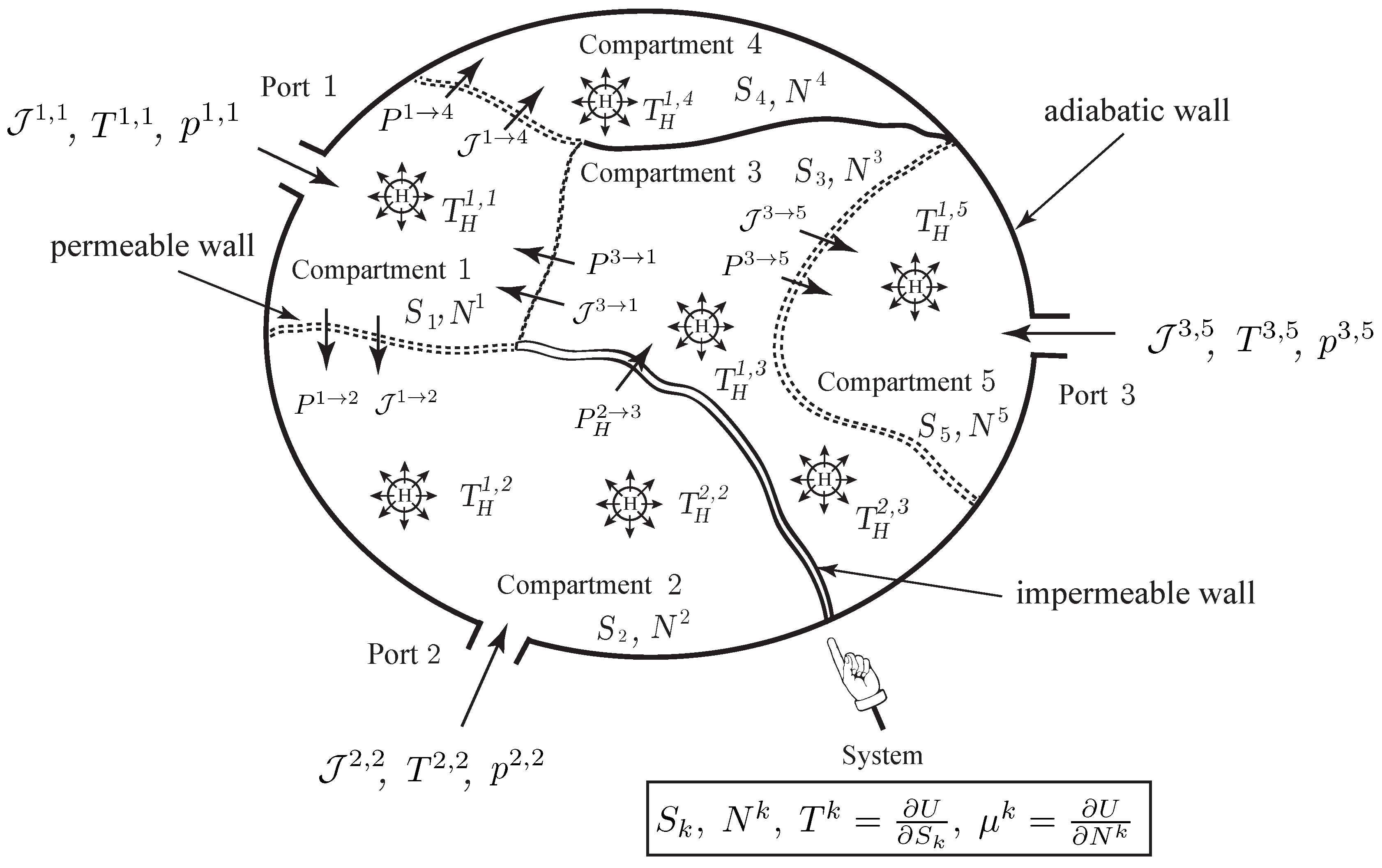
As illustrated in
Figure 2, we consider a thermodynamic system made of
K compartments that can exchange matter by diffusion across their common boundaries. Some compartments may have external ports, through which species can flow into or out of the compartment. We denote by
the number of ports of compartment
k, and by
, the molar flow rate flowing into or out of compartment
k through the
a-th port. We assume that the system has a single species and denote by
the number of moles of the species in the
k-th compartment,
. We assume that the thermodynamic system is simple; i.e., a uniform entropy
S, the entropy of the system, can be attributed to all the compartments. As earlier, the thermodynamic quantities known at the ports are usually the pressure and the temperature
,
, from which the other thermodynamic quantities, such as
or
, are deduced from the state equations of the gas.
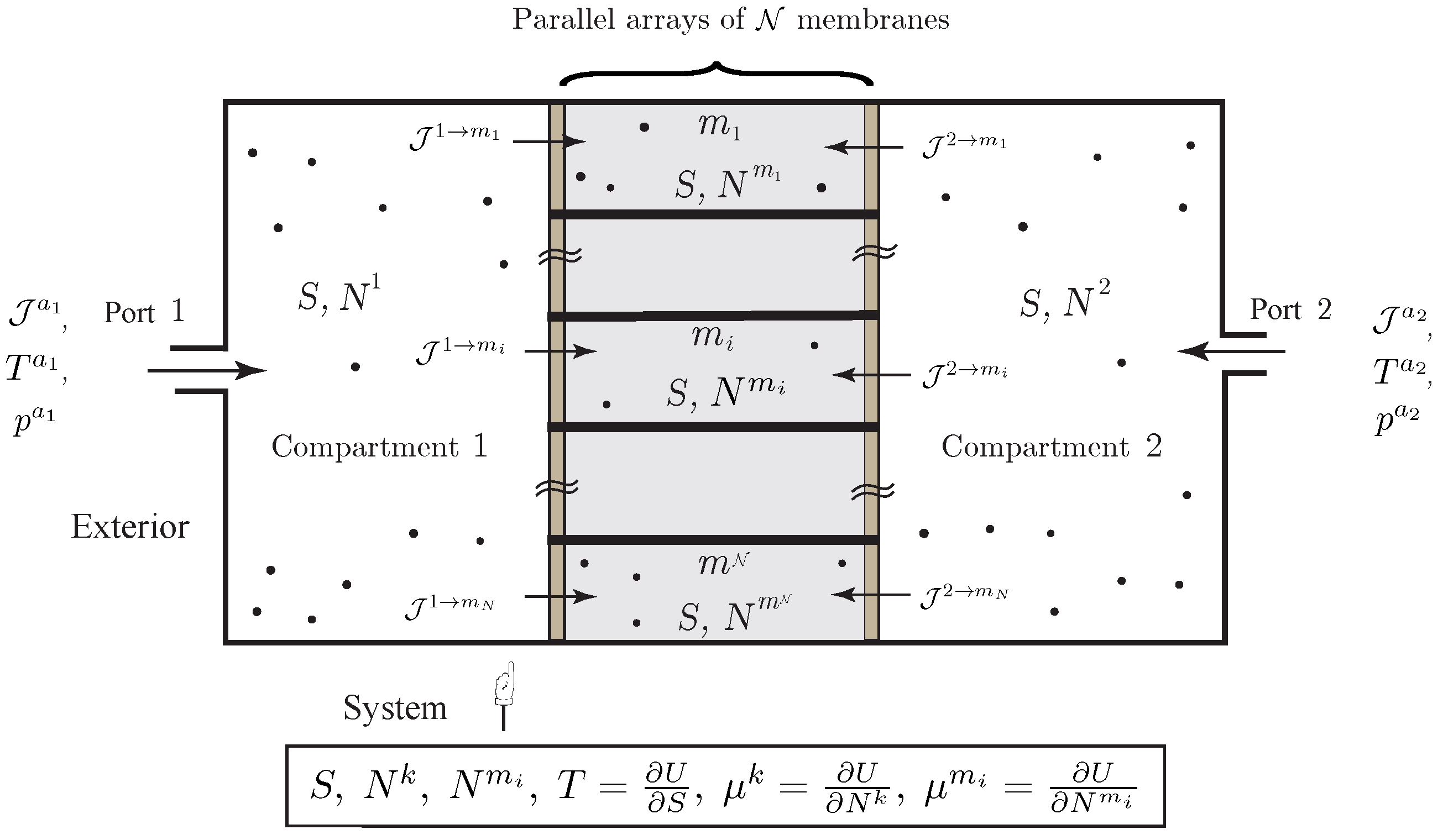
The setting that we develop is well appropriate for the description of diffusion across composite membranes, e.g., composed of different elements arranged in a series or parallel array, which occur frequently in living systems and have remarkable physical properties, see [
22,
23,
24,
25].
For each compartment
, the mole balance equation is
where
is the molar flow rate from compartment
l to compartment
k due to diffusion of the species, and
is the molar flow rate flowing into or out of compartment
k through the
a-th port.
Lagrangian variational formulation. By extending the variational formulation developed in
Section 3.2, we consider an open simple system with Lagrangian
where
are the mechanical variables of the system,
S the single entropy, and
the number of moles in the
k-th compartment,
. The additional mechanical interactions described in
Section 3.3, via the motion
x of the species, can be easily included in the present setting, too.
Given the external and friction forces
,
, the molar flow rates
,
, and the entropy flow rates
,
, the variational formulation is expressed as
where the curves satisfy the nonlinear nonholonomic constraint
and with respect to variations subject to the constraint
with
and
.
By taking the variation of the integral in (
26) and using the variational constraint (
28), we get the following conditions:
As before, we define the temperature and the chemical potential potentials of each compartment as
and
. By inserting the conditions (
29) into the phenomenological constraint (
27) and using
we get the following entropy equation
The rate of entropy production within the system is given by
where the three terms correspond to the entropy production associated to mechanical friction, diffusion, and mixing of gas flowing into the system through the ports.
By the second law, the entropy production must be always positive and hence suggests the phenomenological relations
where
and
are functions of the state variables, with
positive semi-definite and
, for all
. The third term has been already discussed earlier.
The total energy associated to the Lagrangian
is defined as
and satisfies the energy balance