Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle
Abstract
:1. Introduction
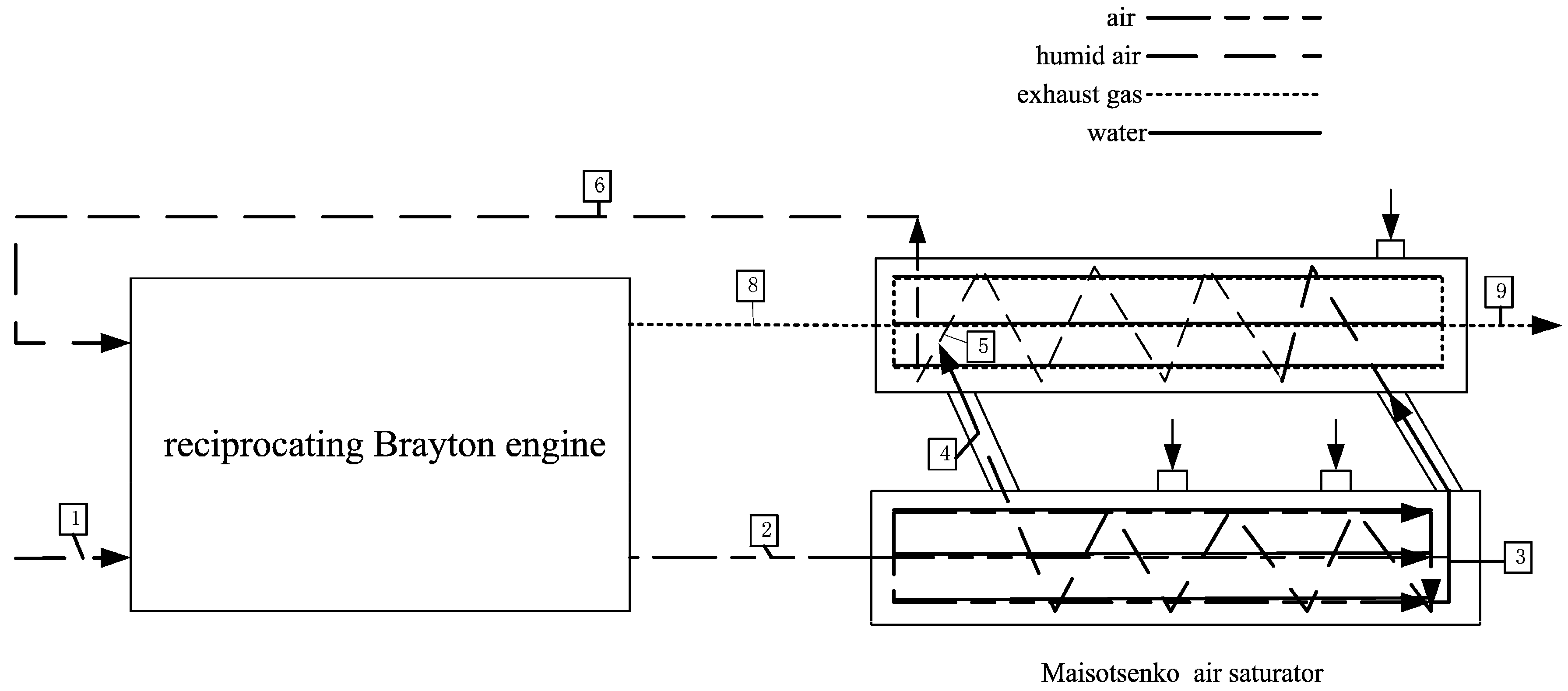
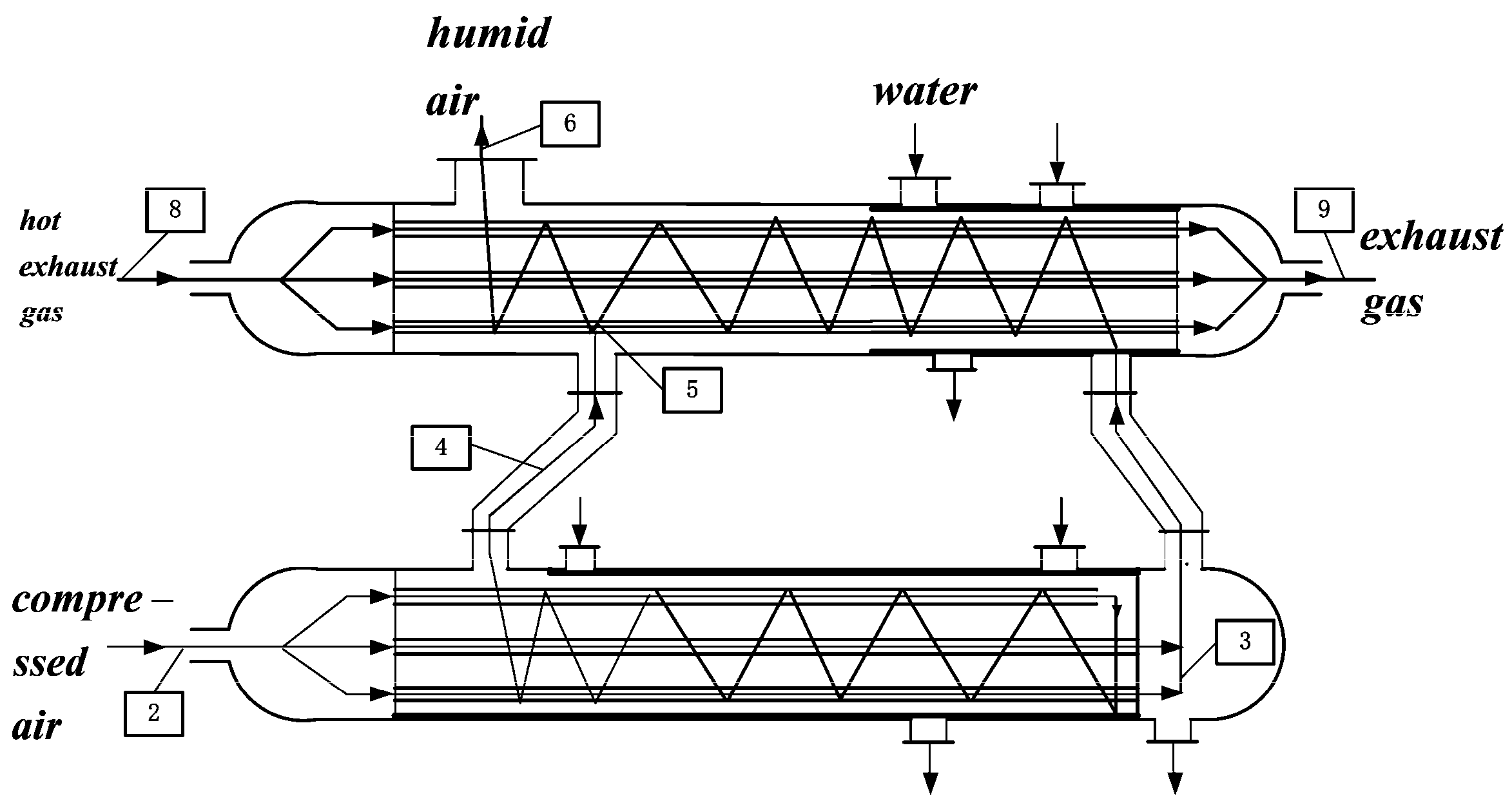
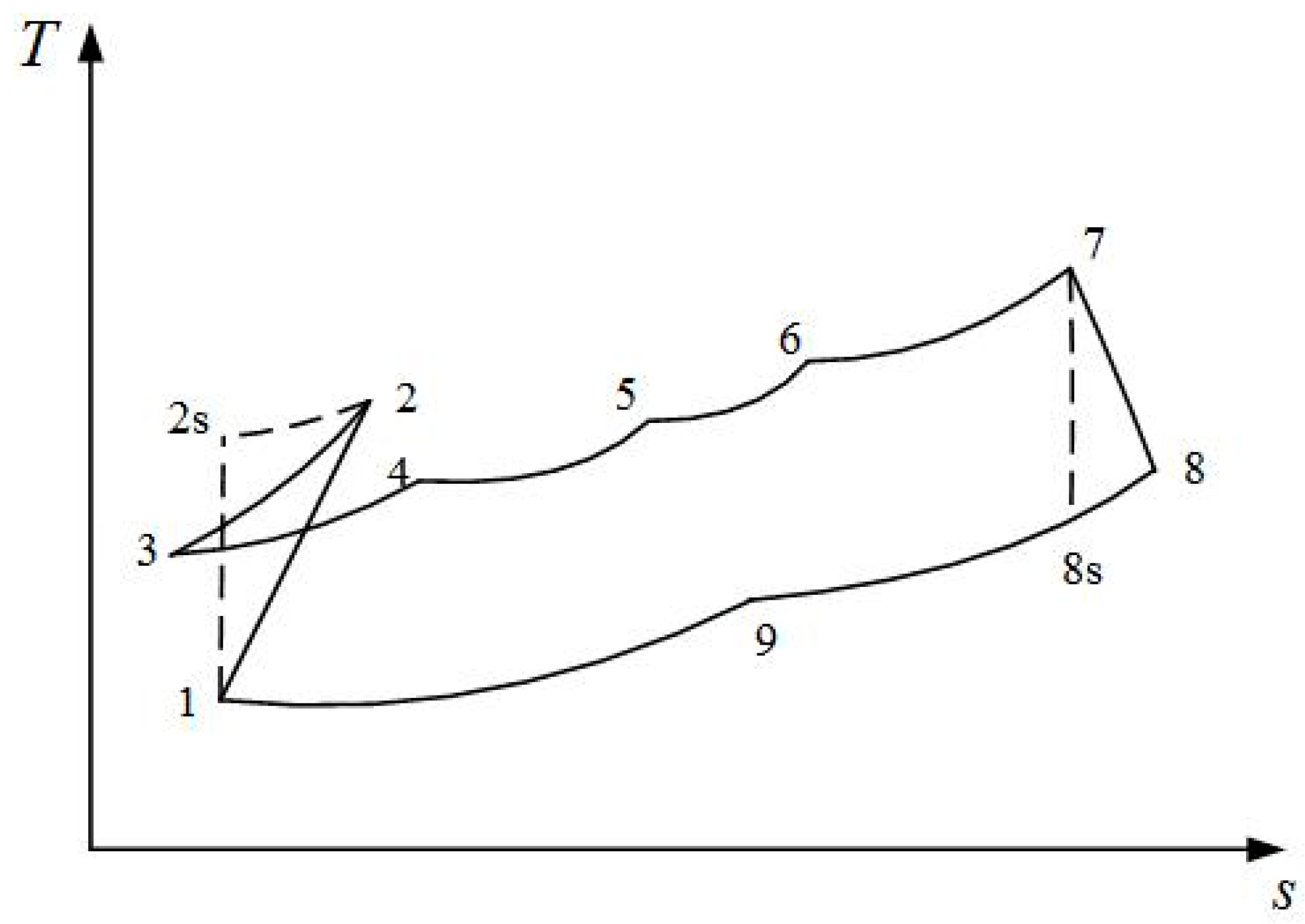
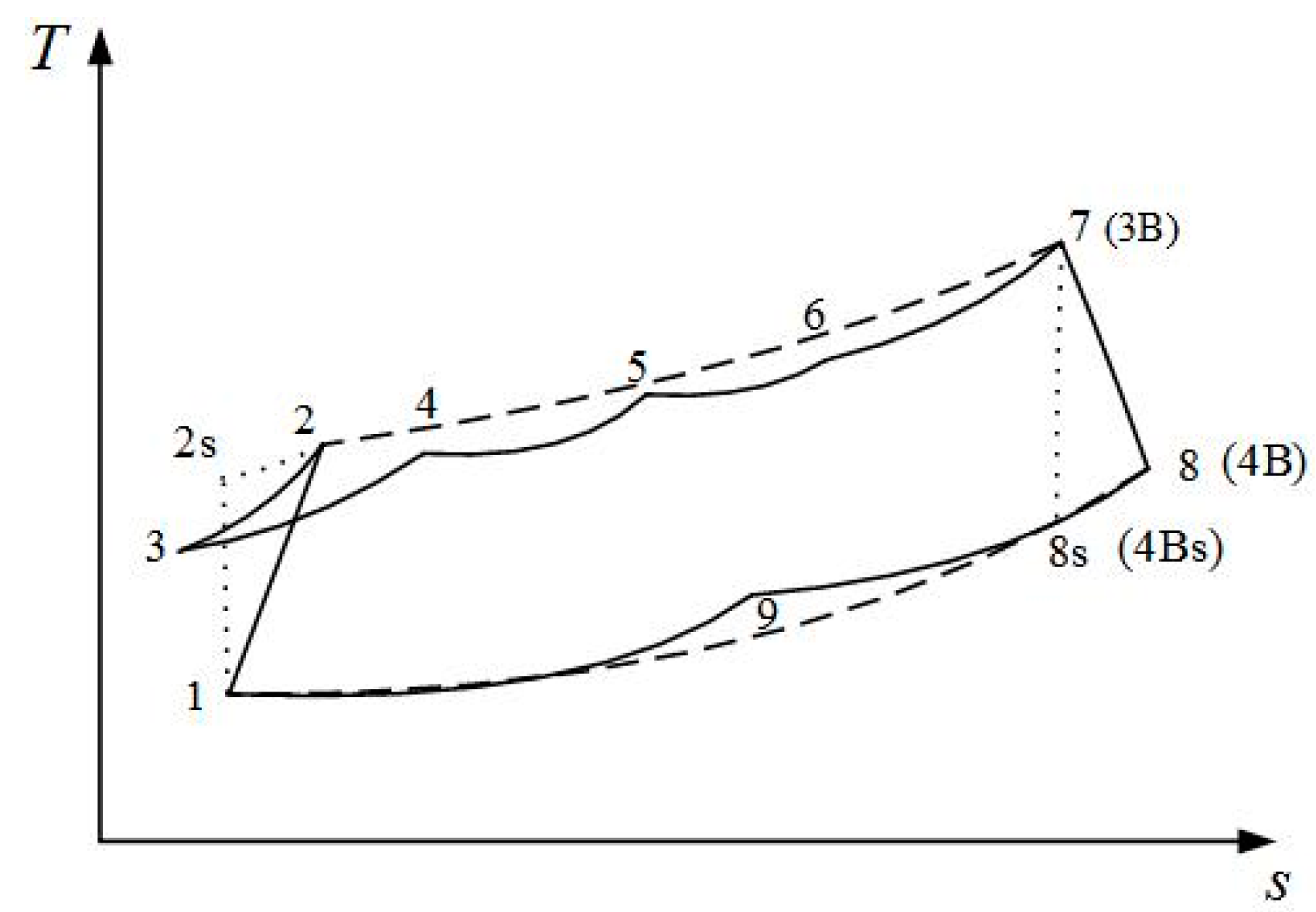
2. Cycle Model and Performance Analyses of Irreversible Maisotsenko Reciprocating Brayton Cycle
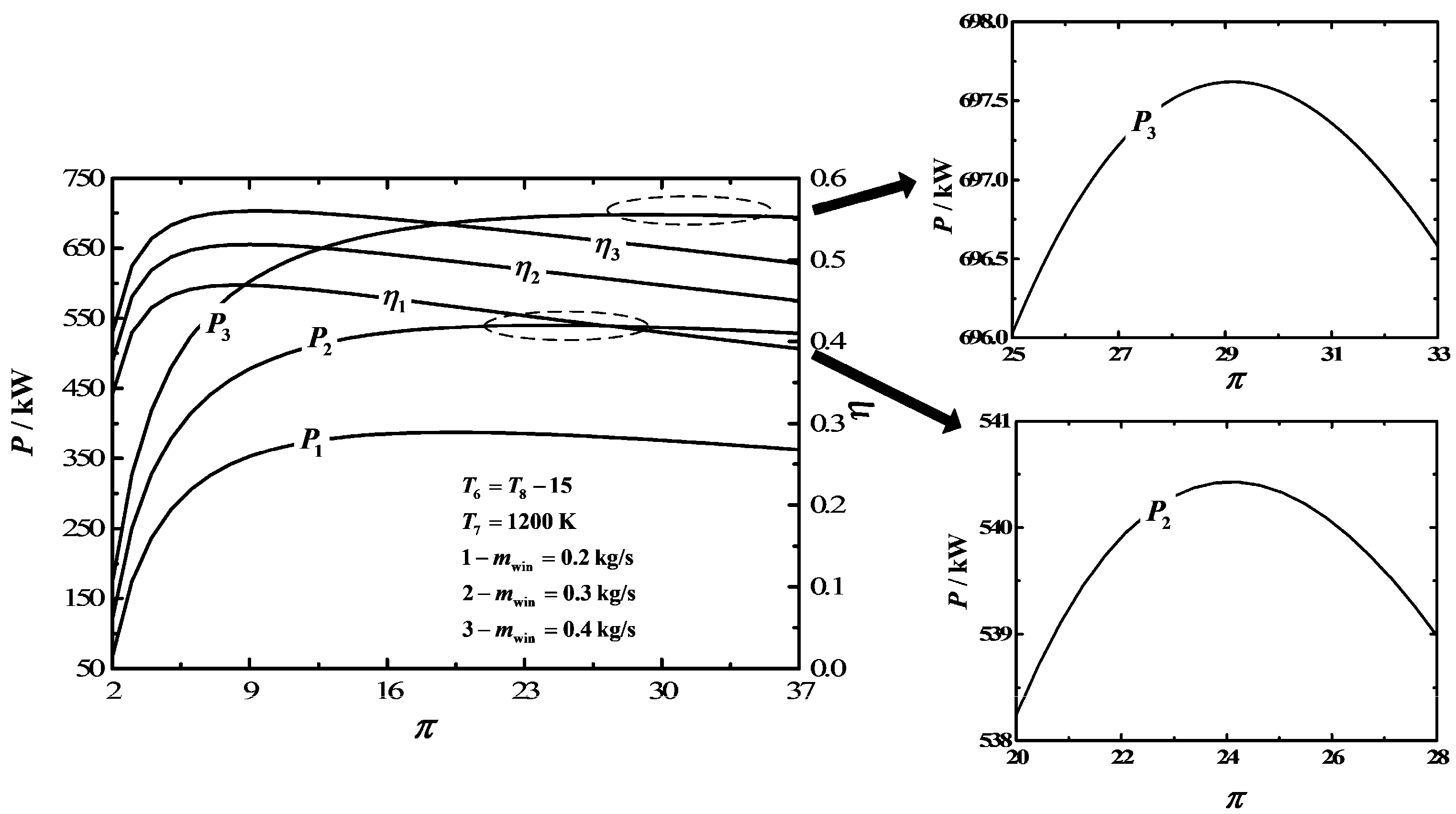
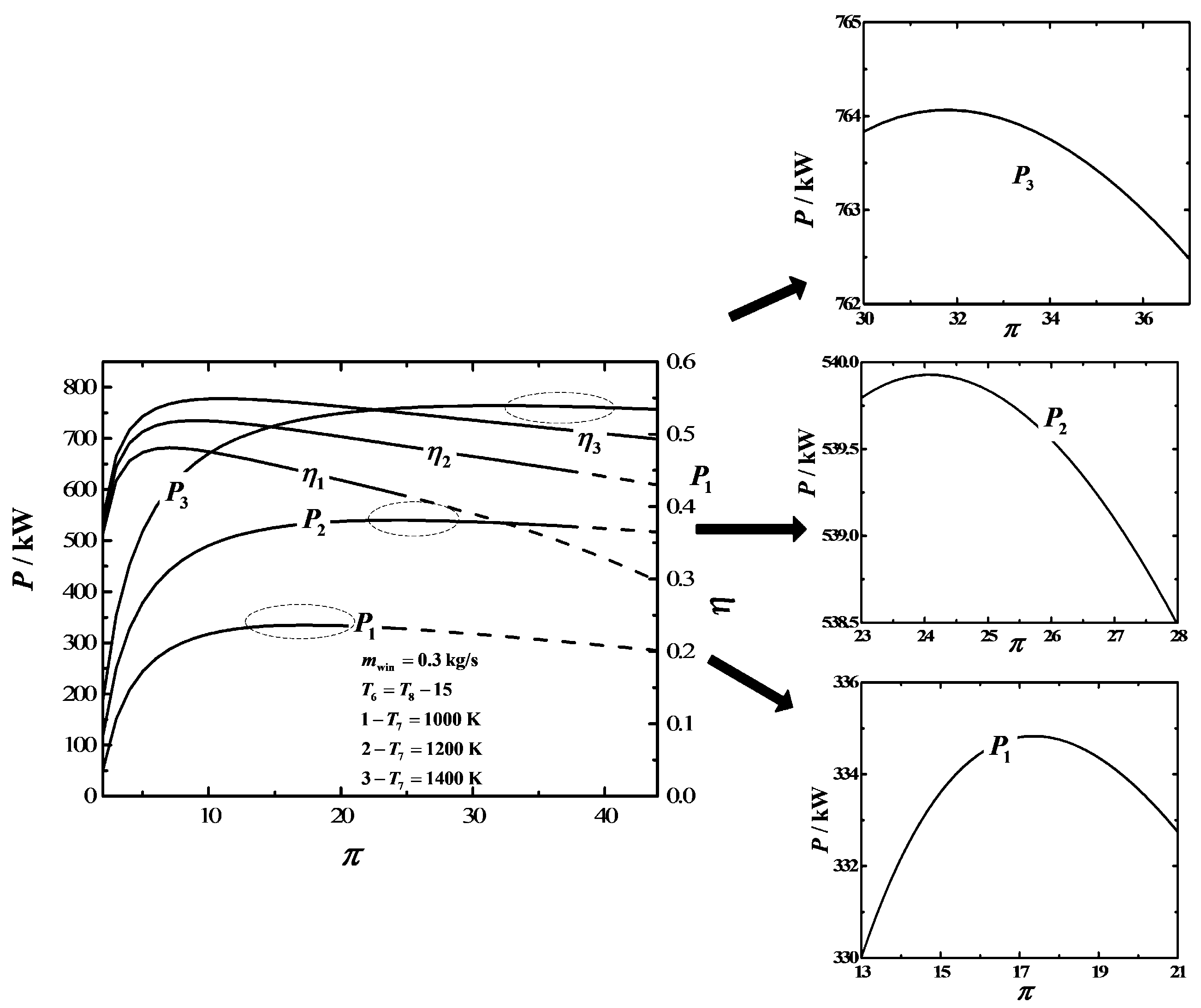
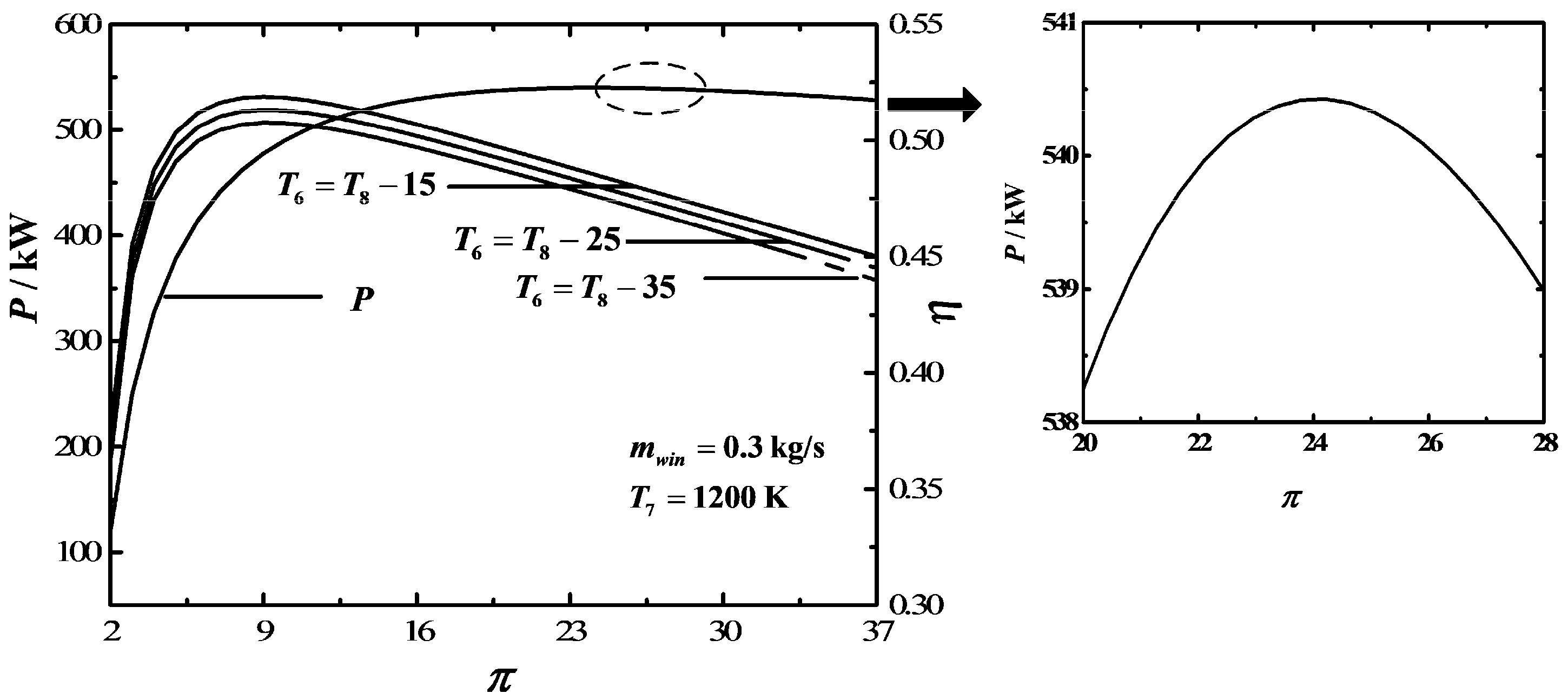
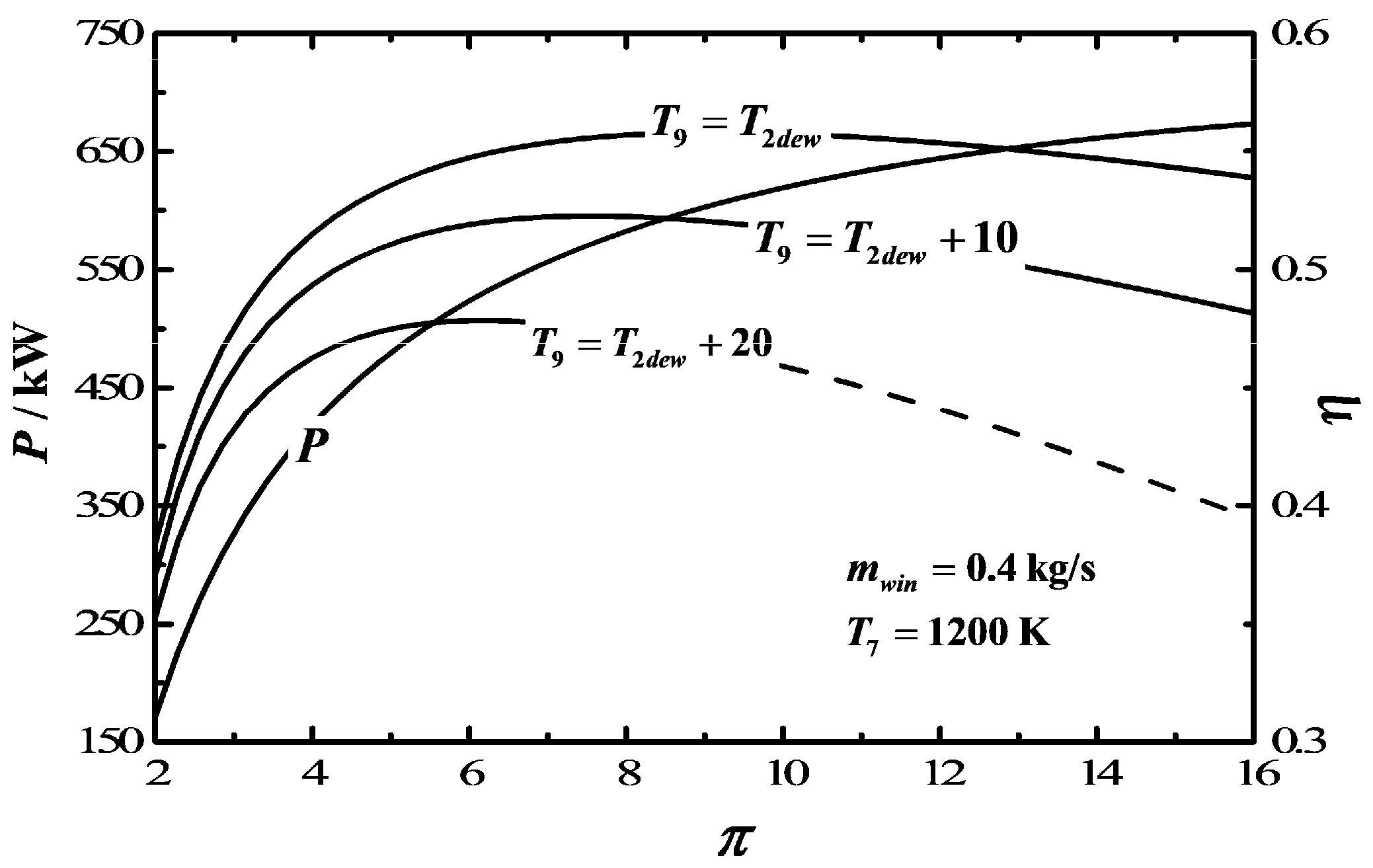
3. Numerical Examples and Discussion
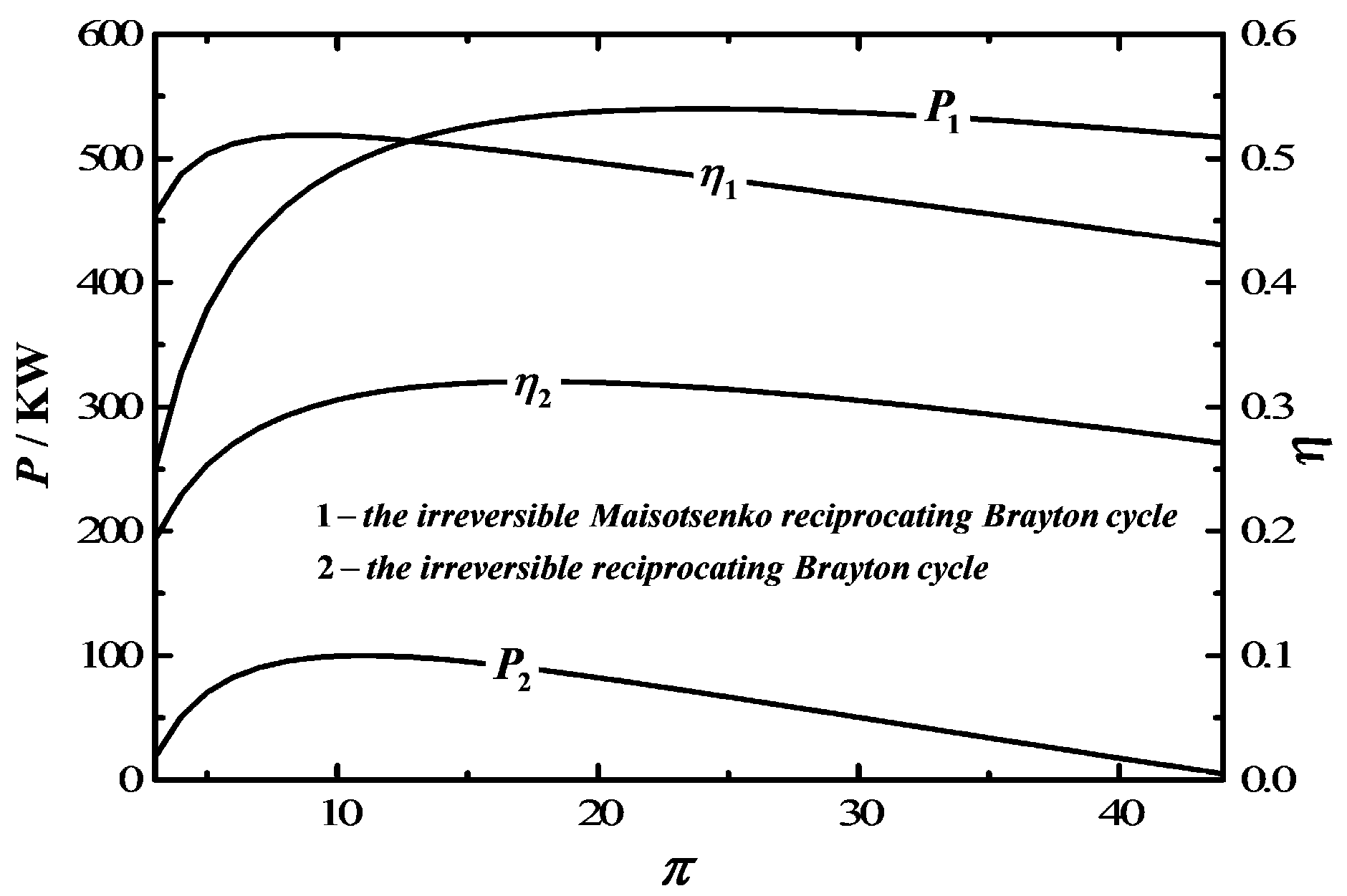
4. Comparison with the Traditional Irreversible Reciprocating Brayton Cycle
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| heat released rate by combustion | |
| heat leakage coefficient of combustion charmer | |
| specific heat at constant pressure | |
| friction force | |
| enthalpy | |
| air adiabatic exponent | |
| L | the distance of the piston travels per cycle |
| molecular weight | |
| mass flow rate | |
| cycle index per second | |
| mole number | |
| power output | |
| pressure | |
| heat rate of addition or rejection | |
| Rg | ideal gas constant |
| entropy generation | |
| temperature | |
| time | |
| V | volume |
| piston speed | |
| work | |
| displacement of piston | |
| dryness of steam | |
| compressor isentropic temperature rate |
| mole fraction of steam in humid air | |
| efficiencies of irreversible compression | |
| efficiency of irreversible expansion | |
| friction coefficient | |
| compression ratio |
| air | |
| reciprocating Brayton cycle | |
| dew | dew point temperature |
| heat addition | |
| leak | leakage |
| maximum | |
| heat rejection | |
| maximum power out | |
| steam | |
| water | |
| air saturator water inlet | |
| above air saturator water inlet | |
| below air saturator water inlet | |
| maximum efficiency | |
| effect of friction | |
| cycle state points |
Abbreviations
| AS | air saturator |
| FTT | finite time thermodynamic |
| HTL | heat transfer loss |
| IIL | internal irreversible loss |
| MFR | mass flow rate |
| MGTC | Maisotsenko gas turbine cycle |
| MRBC | Maisotsenko reciprocating Brayton cycle |
| PLF | piston friction loss |
| RBC | reciprocating Brayton cycle |
References
- Maisotsenko, V.; Gillan, L.E.; Heaton, T.L.; Gillan, A.D. Method and Plate Apparatus for Dew Point Evaporative Cooler Using a trough Wetting System. U.S. Patent No 6705096, 16 March 2004. [Google Scholar]
- Maisotsenko, V.; Treyger, I. Way to energy abundance can be found through the Maisotsenko cycle. Int. J. Energy A Clean Environ. 2011, 12, 319–326. [Google Scholar] [CrossRef]
- Maisotsenko, V.S.; Gillan, L.E.; Heaton, T.L.; Gillan, A.D. Power System and Method. U.S. Patent No 7007453, 7 March 2006. [Google Scholar]
- Gillan, L.E.; Maisotsenko, V. Maisotsenko open cycle used for gas turbine power generation. In Proceedings of the International Joint Power Generation Conference, Georgia, GA, USA, 16–19 June 2003; ASME: New York, NY, USA, 2003; Volume 3, pp. 75–84. [Google Scholar]
- Mahmood, M.H.; Sultan, M.; Miyazaki, T.; Koyama, S.; Maisotsenko, V.S. Overview of Maisotsenko cycle—A way towards dew point evaporative cooling. Renew. Sustain. Energy Rev. 2016, 66, 537–555. [Google Scholar] [CrossRef]
- Reyzin, I. Evaluation of Maisotsenko power cycle thermodynamic efficiency. Int. J. Energy A Clean Environ. 2011, 12, 129–139. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gadalla, M. Analysis of Maisotsenko open gas turbine power cycle with a detailed air saturator model. Appl. Energy 2015, 149, 338–353. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gadalla, M. Analysis of Maisotsenko open gas turbine bottoming cycle. Appl. Therm. Eng. 2015, 82, 351–359. [Google Scholar] [CrossRef]
- Khalatov, A.A.; Severin, S.D.; Brodetskiy, PI.; Maisotsenko, V.S. Sub-atmospheric Reverse Brayton Cycle with Waste Heat Regeneration According to the Maisotsenko Cycle. Rep. Natl. Acad. Sci. Ukraine 2015, 1. [Google Scholar]
- Saghafifar, M.; Gadalla, M. Thermo-economic optimization of hybrid solar Maisotsenko bottoming cycles using heliostat field collector: Comparative analysis. Applied Energy 2017, 190, 686–702. [Google Scholar] [CrossRef]
- Andresen, B.; Salamon, P.; Barry, R.S. Thermodynamics in finite time. Phys. Today 1984, 37, 62–70. [Google Scholar] [CrossRef]
- Bejan, A. Entropy generation minimization: The new thermodynamics of finite-size devices and finite-time process. J. Appl. Phys. 1996, 79, 1191–1218. [Google Scholar] [CrossRef]
- Chen, L.G.; Sun, F.R. Advances in Finite Time Thermodynamics: Analysis and Optimization; Nova Science Publishers: New York, NY, USA, 2004. [Google Scholar]
- Feidt, M. Optimal thermodynamics-New upperbounds. Entropy 2009, 11, 529–547. [Google Scholar] [CrossRef]
- Gonca, G.; Sahin, B.; Ust, Y.; Parlak, A. Determination of the optimum temperatures and mass ratios of steam injected into turbocharged internal combustion engines. J. Renew. Sustain. Energy 2013, 5, 023119. [Google Scholar] [CrossRef]
- Kosloff, R. Quantum thermodynamics: A dynamical viewpoint. Entropy 2013, 15, 2100–2128. [Google Scholar] [CrossRef]
- Gonca, G.; Sahin, B. The influences of the engine design and operating parameters on the performance of a turbocharged and steam injected diesel engine running with the Miller cycle. Appl. Math. Model. 2016, 40, 3764–3782. [Google Scholar] [CrossRef]
- Ge, Y.L.; Chen, L.G.; Sun, F.R. Progress in finite time thermodynamic studies for internal combustion enginecycles. Entropy 2016, 18, 139. [Google Scholar] [CrossRef]
- Liu, S.N.; Ou, C.J. Maximum power output of quantum heat engine with energy bath. Entropy 2016, 18, 205. [Google Scholar] [CrossRef]
- Feidt, M.; Costea, M.; Petrescu, S.; Stanciu, C. Nonlinear thermodynamic analysis and optimization of a Carnot engine cycle. Entropy 2016, 18, 243. [Google Scholar] [CrossRef]
- Chen, L.G.; Xia, S.J. Generalized Thermodynamic Dynamic-Optimization of Irreversible Processes; Science Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Chen, L.G.; Xia, S.J. Generalized Thermodynamic Dynamic-Optimization of Irreversible Cycles—Thermodynamic and Chemical Theoretical Cycles; Science Press: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Gonzalez-Ayala, J.; Roco, J.M.M.; Medina, A.; Calvo-Hernandez, A. Carnot-like heat engines versus low-dissipation models. Entropy 2017, 19, 182. [Google Scholar] [CrossRef]
- Bejan, A. Theory of heat transfer-irreversible power plants. Int. J. Heat Mass Transf. 1988, 31, 1211–1219. [Google Scholar] [CrossRef]
- Açıkkalp, E.; Yamik, H. Modeling and optimization of maximum available work for irreversible gas power cycles with temperature dependent specific heat. J. Non-Equilibrium Thermodyn. 2015, 40, 25–39. [Google Scholar] [CrossRef]
- Açıkkalp, E.; Caner, N. Performance assessment of an irreversible nano Brayton cycle operating with Maxwell-Boltzmann gas. Eur. Phys. J. Plus 2015, 130, 93. [Google Scholar] [CrossRef]
- Açıkkalp, E.; Caner, N. Application of exergetic sustainability index to a nano-scale irreversible Brayton cycle operating with ideal Bose and Fermi gasses. Phys. Lett. A 2015, 379, 1990–1997. [Google Scholar] [CrossRef]
- Gonca, G.; Sahin, B. Thermo-ecological performance analysis of a Joule-Brayton cycle (JBC) turbine with considerations of heat transfer losses and temperature-dependent specific heats. Energy Convers. Manag. 2017, 138, 97–105. [Google Scholar] [CrossRef]
- Açıkkalp, E. Performance analysis of irreversible solid oxide fuel cell—Brayton heat engine with ecological based thermo-environmental criterion. Energy Convers. Manag. 2017, 148, 279–286. [Google Scholar] [CrossRef]
- Kumar, R.; Kaushik, S.C.; Kumar, R. Performance analysis of an irreversible regenerative Brayton cycle based on ecological optimization criterion. Int. J. Therm. Environ. Eng. 2015, 9, 25–32. [Google Scholar]
- Naserian, M.M.; Farahat, S.; Sarhaddi, F. Finite time exergy analysis and multi-objective ecological optimization of a regenerative Brayton cycle considering the impact of flow rate variations. Energy Convers. Manag. 2015, 103, 790–800. [Google Scholar] [CrossRef]
- Kumar, R.; Kaushik, S.C.; Kumar, R.; Hans, R. Multi-objective thermodynamic optimization of an irreversible regenerative Brayton cycle using evolutionary algorithm and decision making. Ain Shams Eng. J. 2016, 7, 741–753. [Google Scholar] [CrossRef]
- Kaushik, S.C.; Kumar, R.; Arora, R. Thermo-economic optimization and parametric study of an irreversible regenerative Brayton cycle. J. Therm. Eng. 2016, 2, 861–870. [Google Scholar] [CrossRef]
- Malali, P.D.; Chaturvedi, S.K.; Abdel-Salam, T. Performance optimization of a regenerative Brayton heat engine coupled with a parabolic dish solar collector. Energy Convers. Manag. 2017, 143, 85–95. [Google Scholar] [CrossRef]
- Chen, L.G.; Wang, W.H.; Sun, F.R. Ecological performance optimization for an open-cycle ICR gas turbine power plant. Part 1 thermodynamic modeling. J. Energy Inst. 2010, 83, 235–241. [Google Scholar] [CrossRef]
- Wang, W.H.; Chen, L.G.; Sun, F.R. Ecological performance optimization for an open-cycle ICR gas turbine power plant. Part 2 Optimization. J. Energy Inst. 2010, 83, 242–248. [Google Scholar] [CrossRef]
- Zhang, W.L.; Chen, L.G.; Sun, F.R. Power and efficiency optimization for combined Brayton and inverse Brayton cycles. Appl. Therm. Eng. 2009, 29, 2885–2894. [Google Scholar] [CrossRef]
- Gonca, G. Exergetic and ecological performance analyses of a gas turbine system with two intercoolers and two re-heaters. Energy 2017, 124, 579–588. [Google Scholar] [CrossRef]
- Qin, X.Y.; Chen, L.G.; Sun, F.R. The universal power and efficiency characteristics for irreversible reciprocating heat engine cycles. Eur. J. Phys. 2003, 24, 359–366. [Google Scholar] [CrossRef]
- Ge, Y.L.; Chen, L.G.; Sun, F.R.; Wu, C. Performance of reciprocating Brayton cycle with heat transfer, friction and variable specific heats of working fluid. Int. J. Ambient Energy 2008, 29, 65–75. [Google Scholar] [CrossRef]
- Shen, W.D.; Tong, J.G. Engineering Thermodynamics; High Education Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Zhang, J.M. Optimal Design and Working Condition Analysis of Marine Steam Turbine; Naval University of Engineering Press: Wuhan, China, 1987. (In Chinese) [Google Scholar]
- Parlak, A. Comparative performance analysis of irreversible Dual and Diesel cycles under maximum power conditions. Energy Convers. Manag. 2005, 46, 351–359. [Google Scholar] [CrossRef]
- Klein, S.A. An explanation for observed compression ratios in internal combustion engines. J. Eng. Gas Turbine Power 1991, 113, 511–513. [Google Scholar] [CrossRef]
- Mozurkewich, M.; Berry, R.S. Finite-time thermodynamics: Engine performance improved by optimized piston motion. Proc. Natl. Acad. Sci. USA 1981, 78, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- Mozurkewich, M.; Berry, R.S. Optimal paths for thermodynamic systems: The ideal Otto cycle. J. Appl. Phys. 1982, 53, 34–42. [Google Scholar] [CrossRef]
- Chen, L.G.; Ge, Y.L.; Sun, F.R.; Wu, C. Effects of heat transfer, friction and variable specific-heats of a working fluid on performance of an irreversible Dual cycle. Energy Convers. Manag. 2006, 47, 3224–3234. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Chen, L.; Wang, W. Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle. Entropy 2018, 20, 167. https://doi.org/10.3390/e20030167
Zhu F, Chen L, Wang W. Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle. Entropy. 2018; 20(3):167. https://doi.org/10.3390/e20030167
Chicago/Turabian StyleZhu, Fuli, Lingen Chen, and Wenhua Wang. 2018. "Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle" Entropy 20, no. 3: 167. https://doi.org/10.3390/e20030167



