Real-Time ECG-Based Detection of Fatigue Driving Using Sample Entropy
Abstract
:1. Introduction
2. Materials and Methods
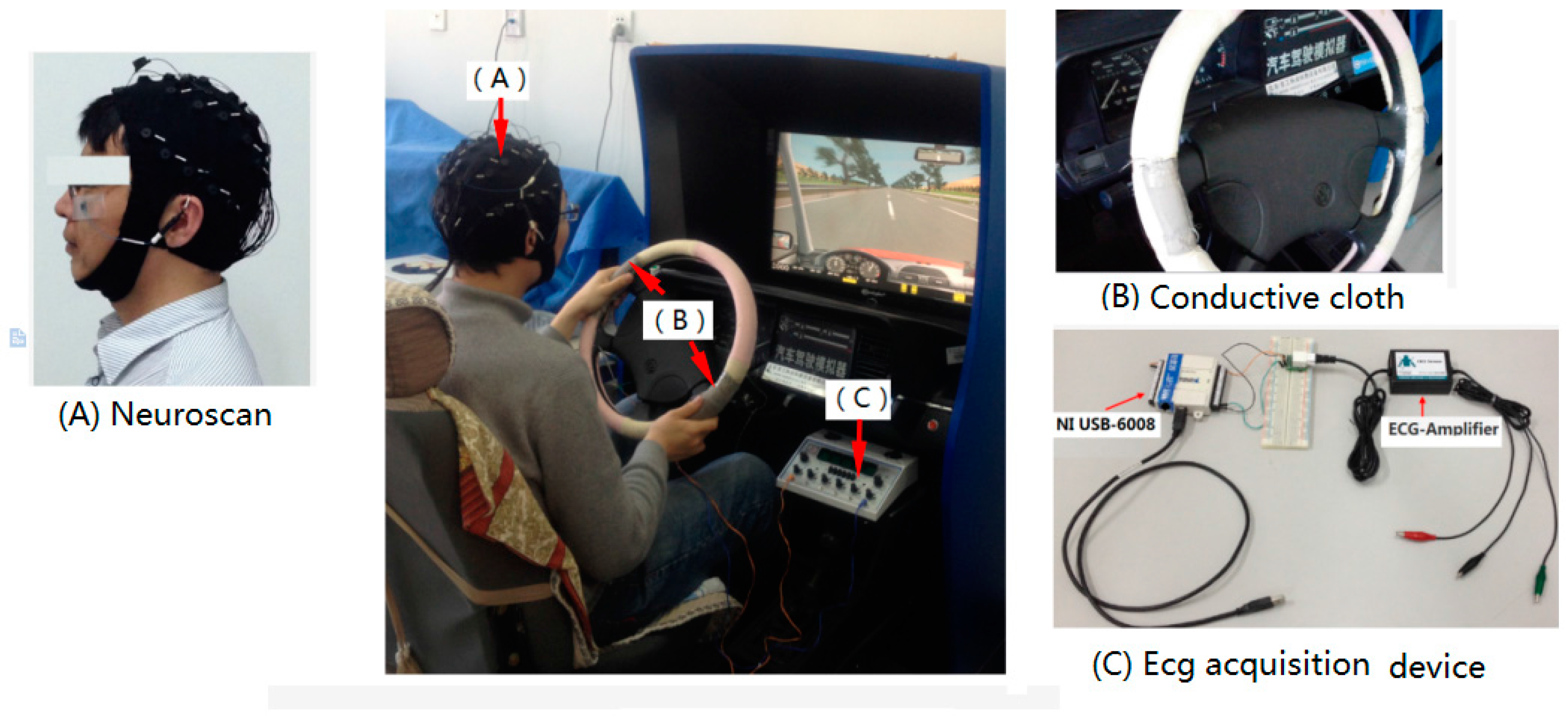
2.1. Experiment
2.1.1. Subjects
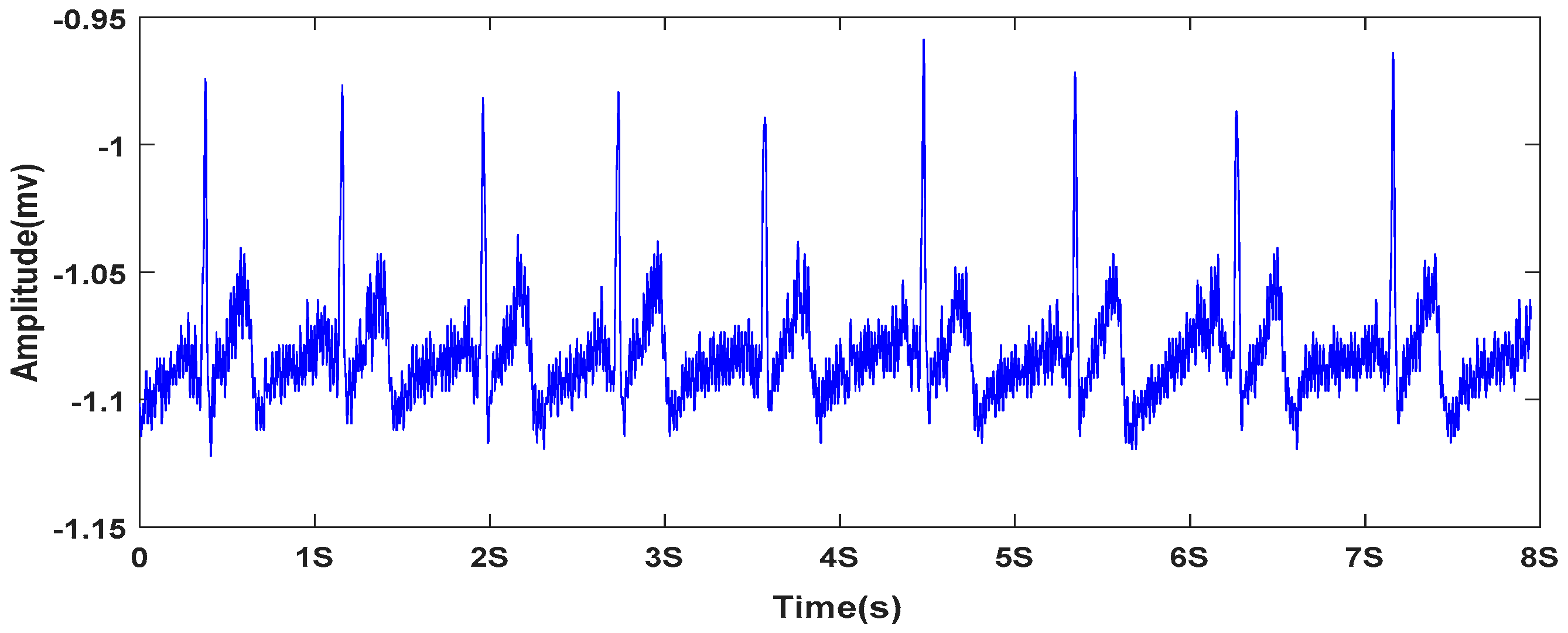
2.1.2. ECG
2.1.3. EEG
2.2. Methods
2.2.1. Sample Entropy
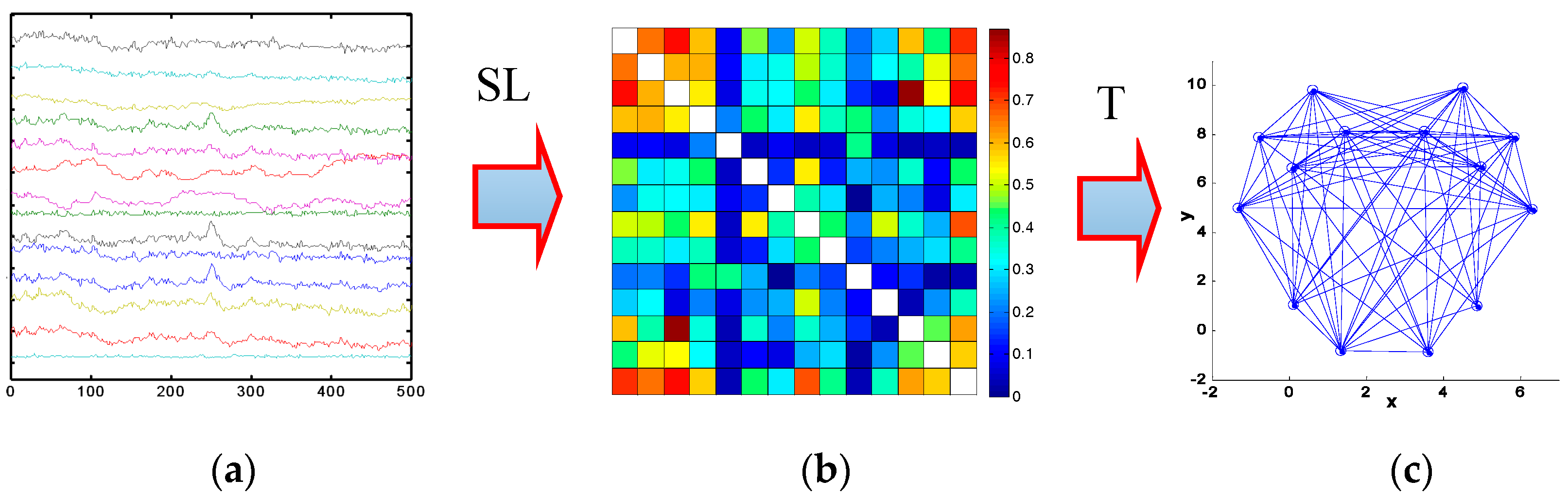
2.2.2. Brain Network
- Preprocessing and artifact removal using WPD
- Formation of a brain network
- Degree of connectivity (Ki)
2.2.3. The Relative Power Spectrum
2.2.4. Statistical Analysis Algorithm
3. Results
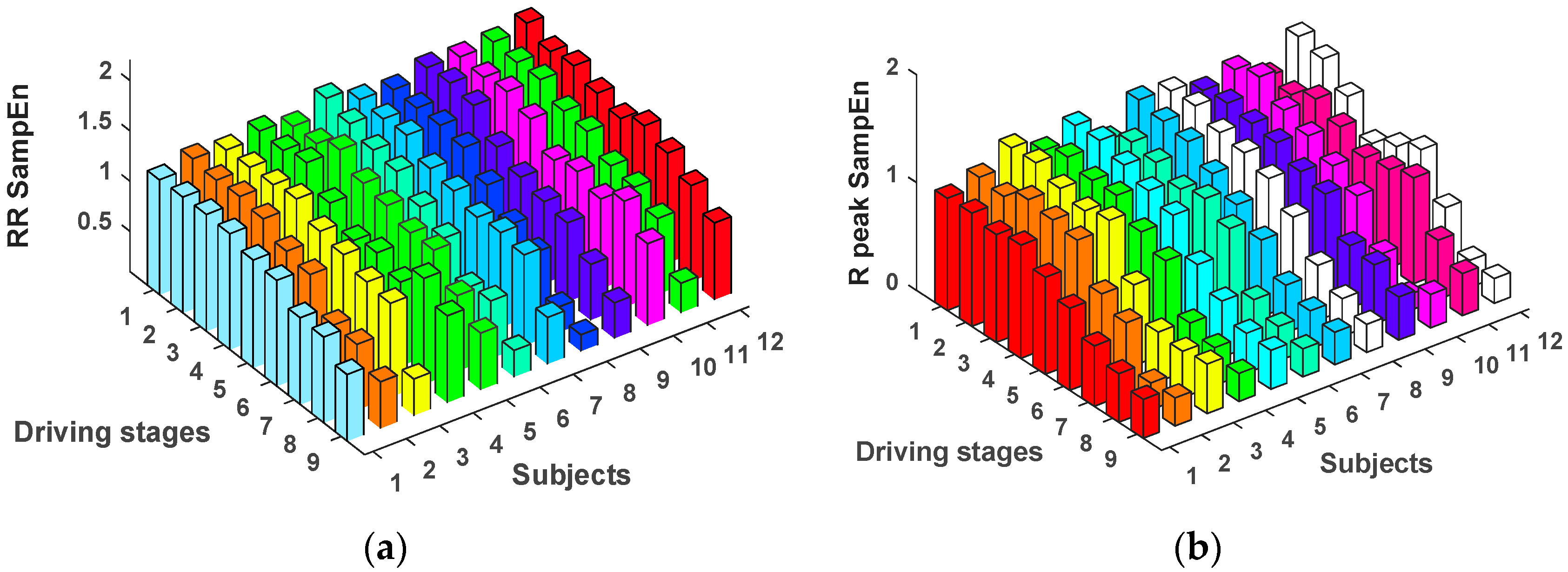
3.1. HRV Characteristics
3.2. Brain Network
3.2.1. Choice Threshold T
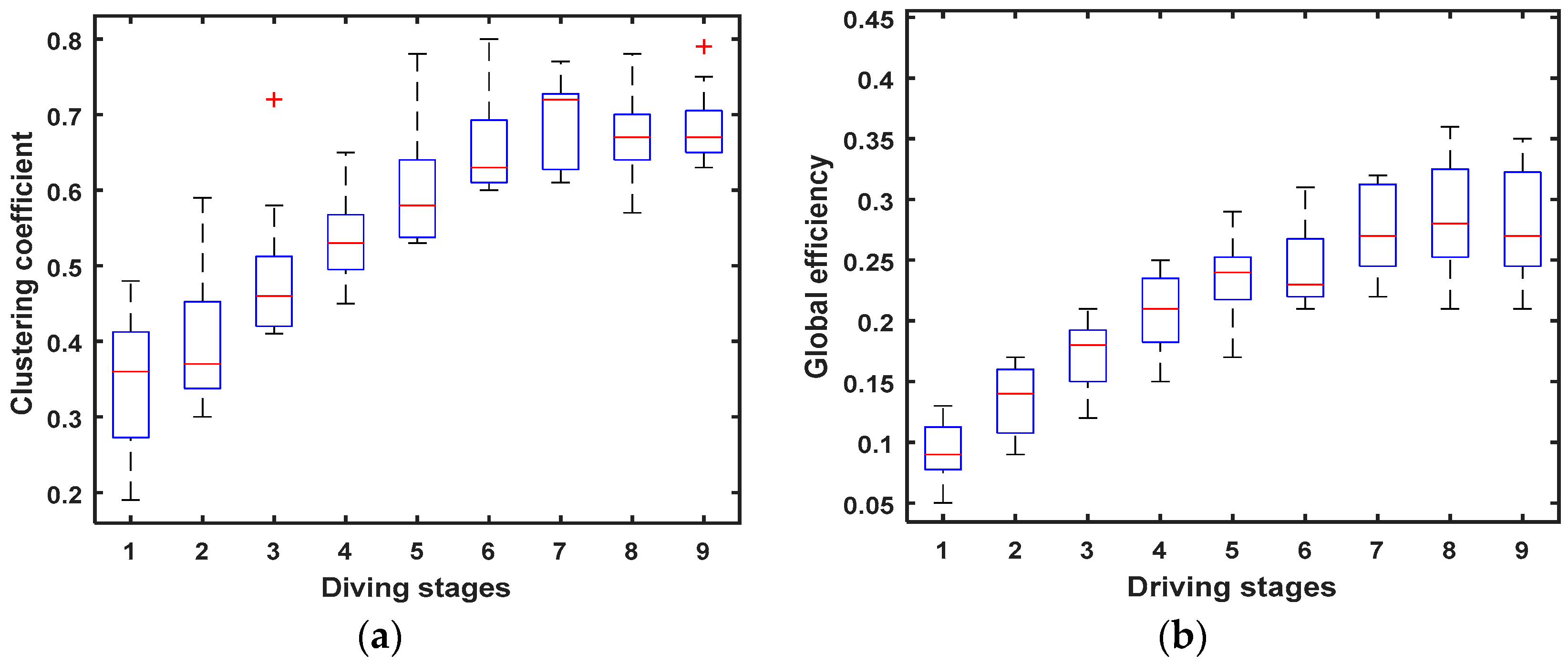
3.2.2. Cluster Coefficient C and Global Efficiency G
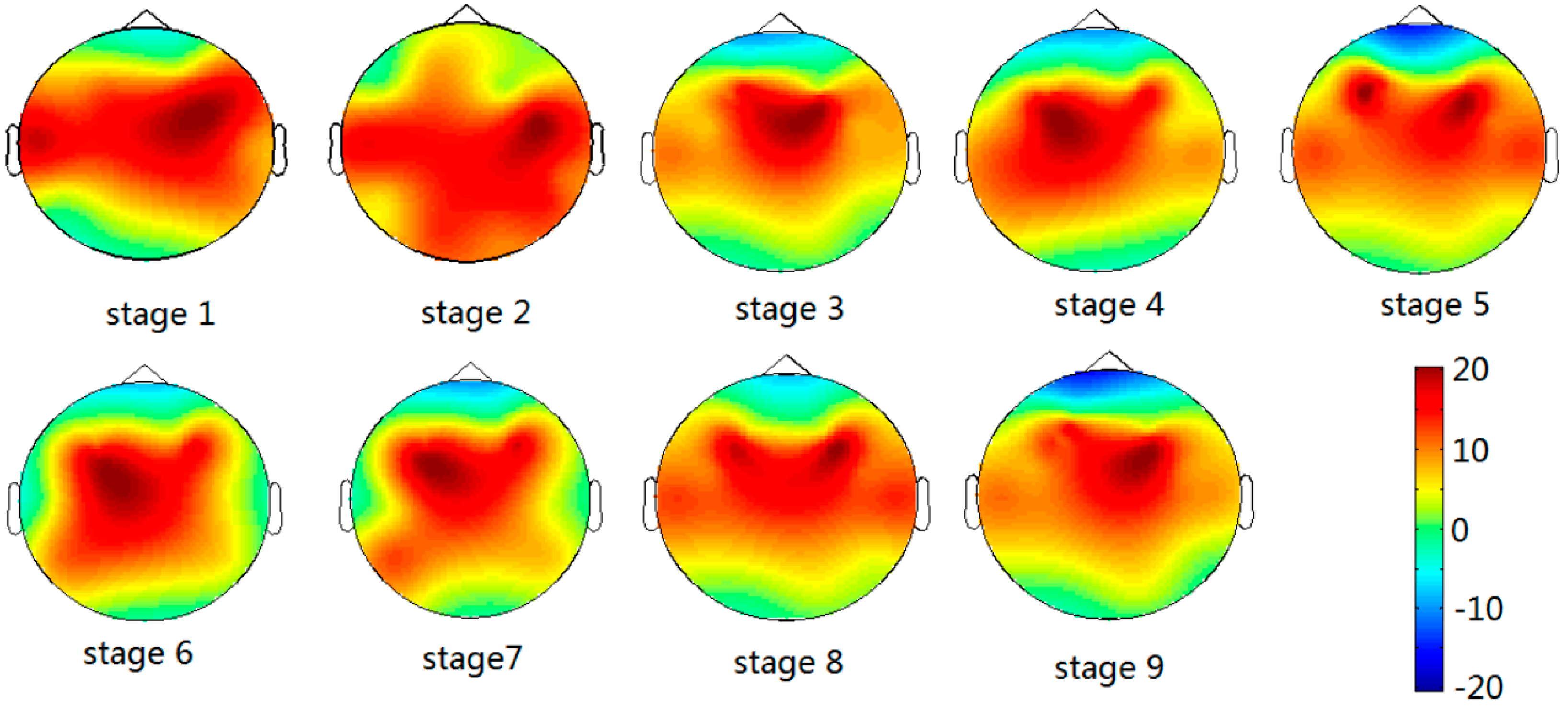
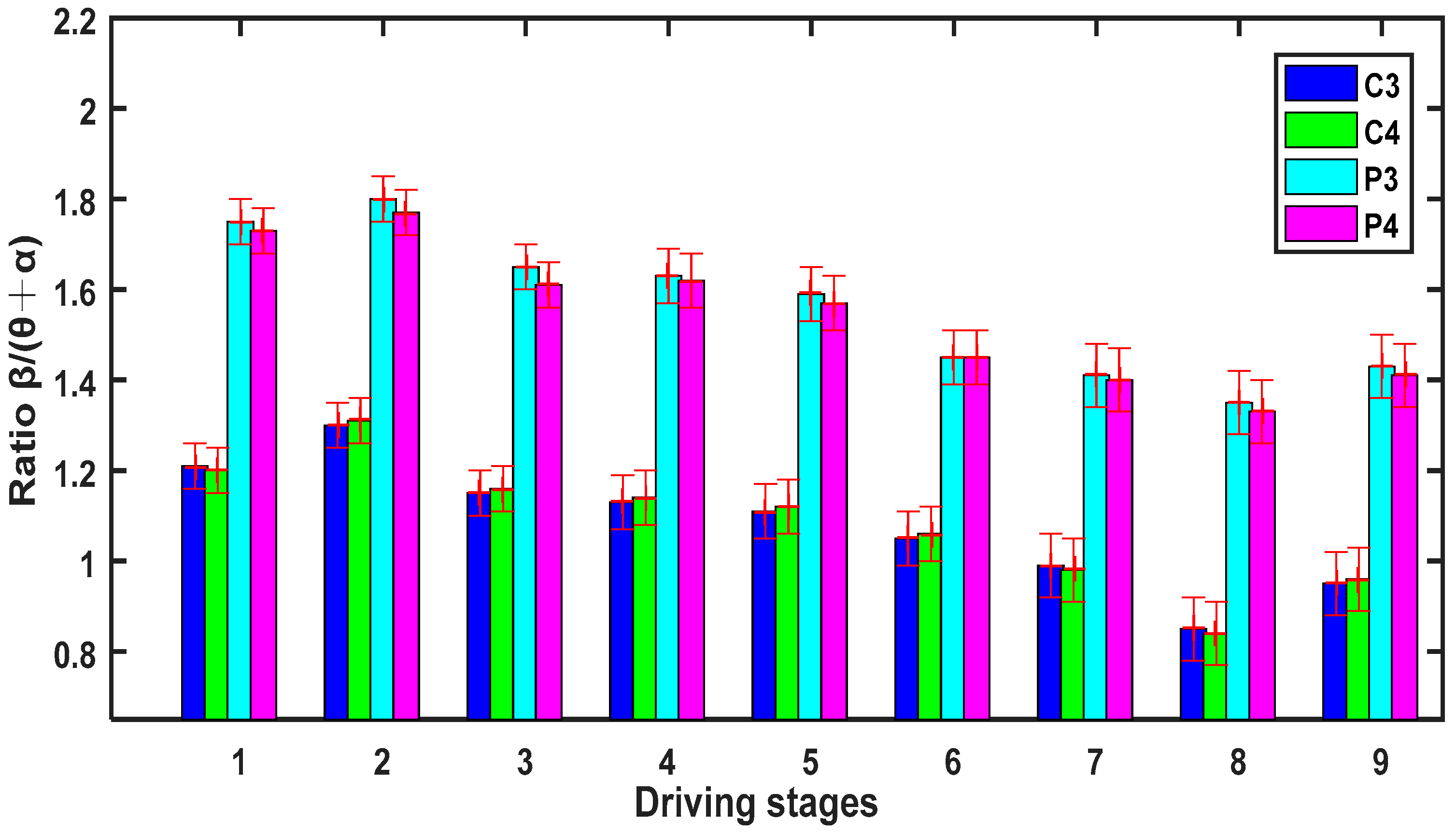
3.3. The Relative Power Spectrum
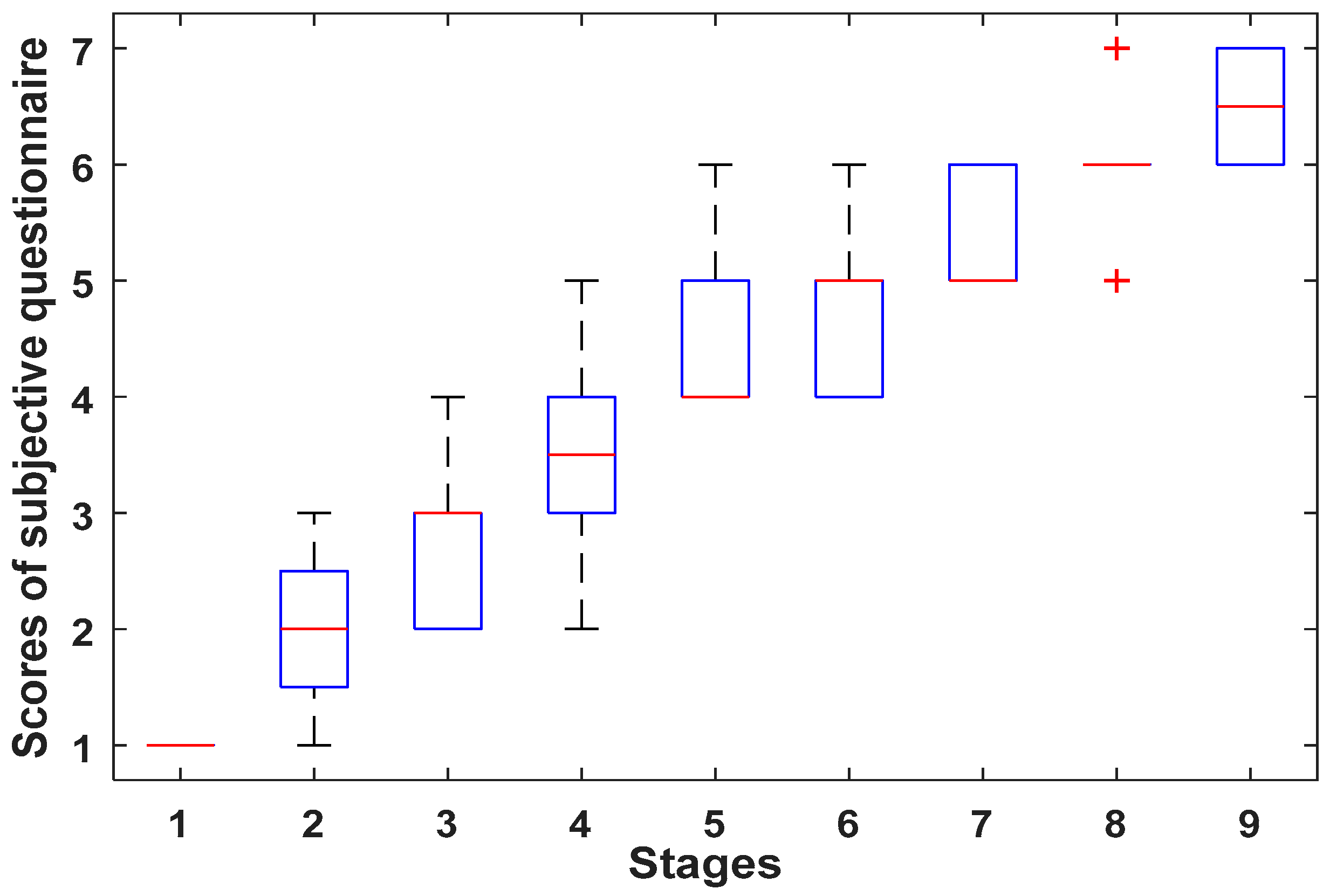
3.4. Subjective Questionnaire
3.5. Comparative Analysis
3.5.1. Correlation Analysis
3.5.2. HRV Characteristics and Subjective Questionnaire
3.5.3. Methods Comparison
4. Discussion
4.1. Previous Studies
4.2. Novel Findings of This Study
4.3. Limitations and Future Research Lines
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williamson, A.; Friswell, R. The effect of external non-driving factors, payment type and waiting and queuing on fatigue in long distance trucking. Accid. Anal. Prev. 2013, 58, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, H.; Zhao, W. Dynamic driver fatigue detection using hidden Markov model in real driving condition. Expert Syst. Appl. 2016, 63, 397–411. [Google Scholar] [CrossRef]
- Lal, S.K.L.; Craig, A.; Boord, P.; Kirkup, L.; Nguyen, H. Development of an algorithm for an EEG-based driver fatigue countermeasure. J. Saf. Res. 2003, 34, 321–328. [Google Scholar] [CrossRef]
- Desmond, P.A.; Matthews, G. Individual differences in stress and fatigue in two field studies of driving. Transp. Res. Part F Traffic Psychol. Behav. 2009, 12, 265–276. [Google Scholar] [CrossRef]
- Schmidt, E.A.; Schrauf, M.; Simon, M.; Buchner, A.; Kincses, W.E. The short-term effect of verbally assessing drivers’ state on vigilance indices during monotonous daytime driving. Transp. Res. Part F Traffic Psychol. Behav. 2011, 14, 251–260. [Google Scholar] [CrossRef]
- Morales, J.M.; Díazpiedra, C.; Rieiro, H.; Roca-González, J.; Romero, S.; Catena, A.; Fuentes, L.J.; di Stasi, L.L. Monitoring driver fatigue using a single-channel electroencephalographic device: A validation study by gaze-based, driving performance, and subjective data. Accid. Anal. Prev. 2017, 109, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Vakulin, A.; D’Rozario, A.; Kim, J.W.; Watson, B.; Cross, N.; Wang, D.; Coeytaux, A.; Bartlett, D.; Wong, K.; Grunstein, R. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Ling, S.H.; San, P.P.; Naik, G.R.; Nguyen, T.N.; Tran, Y.; Craig, A.; Nguyen, H.T. Improving EEG-Based Driver Fatigue Classification Using Sparse-Deep Belief Networks. Front. Neurosci. 2017, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Naik, G.R.; Nguyen, T.N.; Ling, S.H.; Tran, Y.; Craig, A.; Nguyen, H.T. Driver Fatigue Classification with Independent Component by Entropy Rate Bound Minimization Analysis in an EEG-Based System. IEEE J. Biomed. Health Inform. 2017, 21, 715–724. [Google Scholar] [CrossRef]
- Wei, C.S.; Wang, Y.T.; Lin, C.T.; Jung, T.P. Toward Drowsiness Detection Using Non-Hair-Bearing EEG-Based Brain-Computer Interfaces. IEEE Trans. Syst. Rehabilit. Eng. 2018, 26, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Khushaba, R.N.; Kodagoda, S.; Liu, D.; Dissanayake, G. Muscle computer interfaces for driver distraction reduction. Comput. Methods Progr. Biomed. 2013, 110, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, M.; Liu, J.; Zheng, C. Electroencephalogram and electrocardiograph assessment of mental fatigue in a driving simulator. Accid. Anal. Prev. 2012, 45, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, Z.; Szypulska, M. Classification of falling asleep states using HRV analysis. Biocybern. Biomed. Eng. 2017, 37, 290–301. [Google Scholar] [CrossRef]
- Benedetto, S.; Pedrotti, M.; Minin, L.; Baccino, T.; Re, A.; Montanari, R. Driver workload and eye blink duration. Transp. Res. Part F Traffic Psychol. Behav. 2011, 14, 199–208. [Google Scholar] [CrossRef]
- Mandal, B.; Li, L.; Wang, G.S.; Lin, J. Towards Detection of Bus Driver Fatigue Based on Robust Visual Analysis of Eye State. IEEE Trans. Intell. Transp. Syst. 2017, 18, 545–557. [Google Scholar] [CrossRef]
- Fan, X.; Sun, Y.; Yin, B.; Guo, X. Gabor-based dynamic representation for human fatigue monitoring in facial image sequences. Pattern Recognit. Lett. 2010, 31, 234–243. [Google Scholar] [CrossRef]
- Verwey, W.B.; Zaidel, D.M. Preventing drowsiness accidents by an alertness maintenance device. Accid. Anal. Prev. 1999, 31, 199–211. [Google Scholar] [CrossRef]
- Wu, Q. An ECG-Based Approach to Driving Fatigue Detection; Zhejiang University: Zhejiang, China, 2008. [Google Scholar]
- Itoh, Y.; Hayashi, Y.; Tsukui, I.; Saito, S. Heart rate variability and subjective mental workload in flight task validity of mental workload measurement using HRV method. In Work with Computers: Organizational, Management, Stress and Health Aspects, Proceedings of the Third International Conference on Human-Computer Interaction, Boston, MA, USA, 18–22 September 1989; Elsevier Science Inc.: Amsterdam, The Netherlands, 1989; Volume 1, pp. 209–216. [Google Scholar]
- O’Hanlon, J.F. Heart Rate Variability: A New Index of Driver Alertness/Fatigue; SAE Technical Paper; SAE: Warrendale, PA, USA, 1972. [Google Scholar]
- Kramer, A.F. Physiological Metrics of Mental Workload: A Review of Recent Progress; Illinois University at Urbana-Chamapaign: Chamapaign, IL, USA, 1990. [Google Scholar]
- Kalsbeek, J.W.H.; Ettema, J.H. Scored regularity of the heart rate pattern and the measurement of perceptual or mental load. Ergonomics 1963, 6, 306–307. [Google Scholar]
- Kalsbeek, J.W.H.; Sykes, R.N. Objective measurement of mental load. Acta Psychol. 1967, 27, 253–261. [Google Scholar] [CrossRef]
- Mulder, G.; Mulder-Hajonides van der Meulen, W.R.E.H. Mental load and the measurement of heart rate variability. Ergonomics 1973, 16, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Mann, K.; Röschke, J.; Gopinathan, M.S. Nonlinear analysis of continuous ECG during sleep II. Dynamical measures. Biol. Cybern. 2000, 82, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Ouyang, G. Adaptive neuro-fuzzy inference system for classification of background EEG signals from ESES patients and controls. Sci. World J. 2014, 2014, 140863. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Y.; Ren, Y.; Li, D.; Voss, L.; Sleigh, J.; Li, X. Detection of burst suppression patterns in EEG using recurrence rate. Sci. World J. 2014, 2014, 295070. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.K.L.; Craig, A. A critical review of the psychophysiology of driver fatigue. Biol. Psychol. 2001, 55, 173–194. [Google Scholar] [CrossRef]
- Artaud, P.; Planque, S.; Lavergne, C.; Cara, H.; de Lepine, P.; Tarriere, C.; Gueguen, B. An on-board system for detecting lapses of alertness in car driving. In Proceedings of the 14th International Technical Conference on Experimental Safety Vehicles, Munich, Germany, 23–26 May 1994; pp. 350–359. [Google Scholar]
- Stam, C.J.; Reijneveld, J.C. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed. Phys. 2007, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Routray, A.; Nayak, B.P. Functional network changes associated with sleep deprivation and fatigue during simulated driving: Validation using blood biomarkers. Clin. Neurophysiol. 2011, 122, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Jap, B.T.; Lal, S.; Fischer, P.; Bekiaris, E. Using EEG spectral components to assess algorithms for detecting fatigue. Expert Syst. Appl. 2009, 36, 2352–2359. [Google Scholar] [CrossRef]
- Siemionow, V.; Fang, Y.; Calabrese, L.; Sahgal, V.; Yue, G.H. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin. Neurophysiol. 2004, 115, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Papadelis, C.; Kourtidou-Papadeli, C.; Bamidis, P.D.; Chouvarda, I.; Koufogiannis, D.; Bekiaris, E.; Maglaveras, N. Indicators of sleepiness in an ambulatory EEG study of night driving. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6201–6204. [Google Scholar]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.S.; Yi, S.H. Effects of Detrending for Analysis of Heart Rate Variability and Applications to the Estimation of Depth of Anesthesia. J. Korean Phys. Soc. 2004, 44, 561. [Google Scholar]
- Pham, T.D. Time-Shift Multiscale Entropy Analysis of Physiological Signals. Entropy 2017, 19, 257. [Google Scholar] [CrossRef]
- Costa, M.; Peng, C.K.; Goldberger, A.L.; Hausdorff, J.M. Multiscale entropy analysis of human gait dynamics. Phys. A Stat. Mech. Appl. 2003, 330, 53–60. [Google Scholar] [CrossRef]
- Pincus, D.S.M.; Gladstone, I.M.; Ehrenkranz, R.A. A regularity statistic for medical data analysis. J. Clin. Monit. 1991, 7, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Chialvo, D.R.; Kaiser, M.; Hilgetag, C.C. Organization, development and function of complex brain networks. Trends Cogn. Sci. 2004, 8, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Wang, M.S.; Liu, H. Effects of sleep deprivation on gamma oscillation of waking human EEG. Prog. Nat. Sci. 2009, 18, 1533–1538. [Google Scholar] [CrossRef]
- Makeig, S.; Jung, T.P. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Cogn. Brain Res. 1996, 4, 15–25. [Google Scholar] [CrossRef]
- Bullmore, E.T.; Bassett, D.S. Brain graphs: Graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011, 7, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Kranczioch, C.; Zich, C.; Schierholz, I.; Sterr, A. Mobile EEG and its potential to promote the theory and application of imagery-based motor rehabilitation. Int. J. Psychophysiol. 2014, 91, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Whitham, E.M.; Pope, K.J.; Fitzgibbon, S.P.; Lewis, T.; Clark, C.R.; Loveless, S.; Broberg, M.; Wallace, A.; DeLosAngeles, D.; Lillie, P.; et al. Scalp electrical recording during paralysis: Quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin. Neurophysiol. 2007, 118, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R. Skin conductance, alpha activity, and vigilance. Am. J. Psychol. 1965, 78, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Messé, A.; Marrelec, G.; Bellec, P.; Perlbarg, V.; Doyon, J.; Pélégrini-Issac, M.; Benali, H. Comparing structural and functional graph theory features in the human brain using multimodal MRI. IRBM 2012, 33, 244–253. [Google Scholar] [CrossRef]
- Breckel, T.P.K.; Thiel, C.M.; Giessing, C. The efficiency of functional brain networks does not differ between smokers and non-smokers. Psychiatry Res. 2013, 214, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Eoh, H.J.; Chung, M.K.; Kim, S.-H. Electroencephalographic study of drowsiness in simulated driving with sleep deprivation. Int. J. Ind. Ergon. 2005, 35, 307–320. [Google Scholar] [CrossRef]
- Jap, B.T.; Lal, S.; Fischer, P. Comparing combinations of EEG activity in train drivers during monotonous driving. Expert Syst. Appl. 2011, 38, 996–1003. [Google Scholar] [CrossRef]
- Van Wijk, B.C.M.; Stam, C.J.; Daffertshofer, A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 2010, 5, e13701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.L.G.; Jiménez, B.M.; Hernández, E.G.; López, A.L. Spanish version of the Swedish Occupational Fatigue Inventory (SOFI): Factorial replication, reliability and validity. Int. J. Ind. Ergon. 2005, 35, 737–746. [Google Scholar] [CrossRef]
- Samn, S.W.; Perelli, L.P. Estimating Aircrew Fatigue: A Technique with Implications to Airlift Operations; Technical Report No. SAM-TR-82-21; USAF School of Aerospace Medicine: San Antonio, TX, USA, 1982. [Google Scholar]
- Ponten, S.C.; Bartolomei, F.; Stam, C.J. Small-world networks and epilepsy: Graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin. Neurophysiol. 2007, 118, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, M.; Yang, Y.; Gao, J.; Rao, N.; Lin, P. The Reorganization of Human Brain Networks Modulated by Driving Mental Fatigue. IEEE J. Biomed. Health Inform. 2017, 21, 743–755. [Google Scholar] [CrossRef] [PubMed]
- The Ratio of Male to Female Drivers in Jiangsu is 7:3, and Female Drivers Tend to Make Wonderful Mistakes. Available online: http://jiangsu.china.com.cn/html/jsnews/news/1185549_2.html (accessed on 13 March 2018).

| SampEn (RR) | SampEn (R Peaks) | C | G | P3β/θ+α | P4β/θ+α | C3β/θ+α | C4β/θ+α | SQ | |
|---|---|---|---|---|---|---|---|---|---|
| SampEn (RR) | 1 | 0.8578 | −0.6637 | −0.7133 | 0.8035 | 0.8167 | 0.7651 | 0.7411 | −0.9531 |
| SampEn (R peaks) | 0.8578 | 1 | −0.6781 | −0.7103 | 0.7792 | 0.7619 | 0.7366 | 0.7098 | −0.8909 |
| C | −0.6637 | −0.6781 | 1 | 0.9576 | −0.8356 | −0.7960 | −0.6513 | −0.6845 | 0.7764 |
| G | −0.7133 | −0.7103 | 0.9576 | 1 | −0.8501 | −0.8278 | −0.6812 | −0.6988 | 0.7452 |
| P3β/θ+α | 0.8035 | 0.7792 | 0.8356 | −0.8501 | 1 | 0.9822 | 0.8834 | 0.8602 | −0.8055 |
| P4β/θ+α | 0.8167 | 0.7619 | 0.7960 | −0.8278 | 0.9822 | 1 | 0.8577 | 0.8425 | −0.8134 |
| C3β/θ+α | 0.7651 | 0.7366 | −0.6513 | −0.6812 | 0.8834 | 0.8577 | 1 | 0.8919 | −0.7531 |
| C4β/θ+α | 0.7411 | 0.7098 | −0.6845 | −0.6988 | 0.8602 | 0.8425 | 0.8919 | 1 | −0.7319 |
| SQ | −0.9531 | −0.8909 | 0.7764 | 0.7452 | −0.8055 | −0.8134 | −0.7531 | −0.7319 | 1 |
| Subjects | Probability Value | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 | Stage 7 | Stage 8 | Stage 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | PRR Sampan (Fatigue Scale) | 2.6686 × 10−4 (1) | 0.0018 (2) | 0.0023 (3) | 0.0396 (4) | 0.0396 (4) | 1 (5) | 1 (5) | 0.0137 (6) | 0.0043 (7) |
| PR-Peak SampEn (Fatigue Scale) | 2.9266 × 10−4 (1) | 0.0033 (2) | 0.0059 (3) | 0.0443 (4) | 0.0443 (4) | 1 (5) | 1 (5) | 0.0261 (6) | 0.0078 (7) | |
| Subject 2 | PRR SampEn (Fatigue Scale) | 2.3667 × 10−4 (1) | 2.3667 × 10−4 (1) | 0.0019 (2) | 0.0019 (2) | 0.0165 (4) | 1 (5) | 1 (5) | 0.0217 (6) | 0.0217 (6) |
| PR-Peak SampEn (Fatigue Scale) | 2.8263 × 10−4 (1) | 2.8263 × 10−4 (1) | 0.0023 (2) | 0.0023 (2) | 0.0122 (4) | 1 (5) | 1 (5) | 0.0115 (6) | 0.0115 (6) | |
| Subject 3 | PRR SampEn (Fatigue Scale) | 6.2561 × 10−5 (1) | 6.2561 × 10−5 (1) | 0.0013 (2) | 0.0185 (3) | 0.0449 (4) | 0.0449 (4) | 1 (5) | 0.0377 (6) | 0.0377 (6) |
| PR-Peak SampEn (Fatigue Scale) | 8.8713 × 10−5 (1) | 8.8713 × 10−5 (1) | 0.0077 (2) | 0.0238 (3) | 0.0316 (4) | 0.0316 (4) | 1 (5) | 0.0192 (6) | 0.0192 (6) | |
| Subject 4 | PRR SampEn (Fatigue Scale) | 2.5912 × 10−5 (1) | 0.0011 (2) | 0.0115 (3) | 0.0115 (3) | 0.0399 (4) | 0.0399 (4) | 1 (5) | 0.0296 (6) | 0.0296 (6) |
| PR-Peak SampEn (Fatigue Scale) | 3.1764 × 10−5 (1) | 0.0026 (2) | 0.0188 (3) | 0.0188 (3) | 0.0471 (4) | 0.0471 (4) | 1 (5) | 0.0366 (6) | 0.0366 (6) | |
| Subject 5 | PRR SampEn (Fatigue Scale) | 4.5612 × 10−4 (1) | 0.0025 (2) | 0.0246 (3) | 0.0483 (4) | 1 (5) | 1 (5) | 0.0419 (6) | 0.0419 (6) | 0.0419 (6) |
| PR-Peak SampEn (Fatigue Scale) | 3.9371 × 10−4 (1) | 0.0012 (2) | 0.0211 (3) | 0.0392 (4) | 1 (5) | 1 (5) | 0.0388 (6) | 0.0388 (6) | 0.0388 (6) | |
| Subject 6 | PRR SampEn (Fatigue Scale) | 6.2613 × 10−5 (1) | 0.0017 (2) | 0.0017 (2) | 0.0093 (3) | 0.0274 | 0.0274 | 1 (5) | 1 (5) | 0.0436 (6) |
| PR-Peak SampEn (Fatigue Scale) | 3.5732 × 10−5 (1) | 0.0012 (2) | 0.0012 (2) | 0.0037 (3) | 0.0127 | 0.0127 | 1 (5) | 1 (5) | 0.0335 (6) | |
| Subject 7 | PRR SampEn (Fatigue Scale) | 0.0044 (1) | 0.0199 (3) | 0.0478 (4) | 1 (5) | 0.0226 (6) | 0.0226 (6) | 0.0226 (6) | 0.0031 (7) | 0.0031 (7) |
| PR-Peak SampEn (Fatigue Scale) | 0.0013 (1) | 0.0175 (3) | 0.0417 (4) | 1 (5) | 0.0352 (6) | 0.0352 (6) | 0.0352 (6) | 0.0018 (7) | 0.0018 (7) | |
| Subject 8 | PRR SampEn (Fatigue Scale) | 0.0016 (1) | 0.0215 (2) | 0.0386 (4) | 0.0386 (4) | 1 (5) | 1 (5) | 0.0483 (6) | 0.0483 (6) | 0.0162 (7) |
| PR-Peak SampEn (Fatigue Scale) | 0.0011 | 0.0224 (2) | 0.0414 (4) | 0.0414 (4) | 1 (5) | 1 (5) | 0.0446 (6) | 0.0446 (6) | 0.0126 (7) | |
| Subject 9 | PRR SampEn (Fatigue Scale) | 0.0025 (1) | 0.0178 (3) | 0.0178 (3) | 0.0415 (4) | 0.0415 (4) | 1 (5) | 0.0361 (6) | 0.0361 (6) | 0.0021 (7) |
| PR-Peak SampEn (Fatigue Scale) | 0.0031 (1) | 0.0199 (3) | 0.0199 (3) | 0.0471 (4) | 0.0471 (4) | 1 (5) | 0.0427 (6) | 0.0427 (6) | 0.0036 (7) | |
| Subject 10 | PRR SampEn (Fatigue Scale) | 3.4407 × 10−4 (1) | 0.0037 (2) | 0.0131 (3) | 0.0435 (4) | 0.0435 (4) | 1 (5) | 0.0481 (6) | 0.0481 (6) | 0.0065 (7) |
| PR-Peak SampEn (Fatigue Scale) | 4.1711 × 10−4 (1) | 0.0017 (2) | 0.0061 (3) | 0.0355 (4) | 0.0355 (4) | 1 (5) | 0.0412 (6) | 0.0412 (6) | 0.0033 (7) | |
| Subject 11 | PRR SampEn (Fatigue Scale) | 5.6702 × 10−4 (1) | 5.6702 × 10−4 (1) | 0.0027 (2) | 0.0087 (3) | 0.0279 (4) | 0.0279 (4) | 1 (5) | 1 (5) | 0.0396 (6) |
| PR-Peak SampEn (Fatigue Scale) | 9.0146 × 10−4 (1) | 9.0146 × 10−4 (1) | 0.0046 (2) | 0.0112 (3) | 0.0318 (4) | 0.0318 (4) | 1 (5) | 1 (5) | 0.0413 (6) | |
| Subject 12 | PRR SampEn (Fatigue Scale) | 0.0016 (1) | 0.0078 (2) | 0.0078 (2) | 0.0466 (3) | 1 (5) | 1 (5) | 1 (5) | 0.0413 (6) | 0.0413 (6) |
| PR-Peak SampEn (Fatigue Scale) | 7.1642 × 10−4 (1) | 0.0054 (2) | 0.0054 (2) | 0.0419 (3) | 1 (5) | 1 (5) | 1 (5) | 0.0359 (6) | 0.0359 (6) |
| Fully Alert, Wide Awake | A Little Tired, Less Than Fresh | Moderately Tired, Let Down | Extremely Tired, Very Difficult to Concentrate | |
|---|---|---|---|---|
| RR SampEn | 1.3081 ± 0.0965 | 1.1575 ± 0.0615 | 0.8053 ± 0.0833 | 0.5419 ± 0.1059 |
| R-Peak SampEn | 1.1967 ± 0.0792 | 1.0813 ± 0.0922 | 0.7258 ± 0.0943 | 0.3255 ± 0.1127 |
| Methods | Fully Alert, Wide Awake | A Little Tired, Less Than Fresh | Moderately Tired, Let Down | Extremely Tired, Very Difficult to Concentrate | |
|---|---|---|---|---|---|
| Moderately tired, let down | SampEn (RR) | 0.0071 | 0.0437 | 1 | 0.0245 |
| SampEn (R peaks) | 0.0104 | 0.0453 | 1 | 0.0336 | |
| C | 0.0043 | 0.0153 | 1 | 0.0246 | |
| G | 0.0182 | 0.0287 | 1 | 0.0215 | |
| P3β/θ+α | 0.0082 | 0.0359 | 1 | 0.0288 | |
| P4β/θ+α | 0.0057 | 0.0361 | 1 | 0.0291 | |
| C3β/θ+α | 0.0106 | 0.0375 | 1 | 0.0372 | |
| C4β/θ+α | 0.0113 | 0.0419 | 1 | 0.0355 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Wang, H.; Fu, R. Real-Time ECG-Based Detection of Fatigue Driving Using Sample Entropy. Entropy 2018, 20, 196. https://doi.org/10.3390/e20030196
Wang F, Wang H, Fu R. Real-Time ECG-Based Detection of Fatigue Driving Using Sample Entropy. Entropy. 2018; 20(3):196. https://doi.org/10.3390/e20030196
Chicago/Turabian StyleWang, Fuwang, Hong Wang, and Rongrong Fu. 2018. "Real-Time ECG-Based Detection of Fatigue Driving Using Sample Entropy" Entropy 20, no. 3: 196. https://doi.org/10.3390/e20030196





