Droplet Size Distribution in Sprays Based on Maximization of Entropy Generation
Abstract
:Introduction
2. Model Formulation
2.1 Entropy analysis


 , which is commonly referred to as the mass source term, and the corresponding specific entropy is denoted as ssource.
, which is commonly referred to as the mass source term, and the corresponding specific entropy is denoted as ssource.

 is the dimensionless source term.
is the dimensionless source term. . Since the control volume is around the liquid, ssource = s(l) ≅ s1(l) because the liquid is assumed isothermal. Eq. (3) can be rewritten as
. Since the control volume is around the liquid, ssource = s(l) ≅ s1(l) because the liquid is assumed isothermal. Eq. (3) can be rewritten as

 . The other part is due to the existence of the numerous individual droplets, represented as
. The other part is due to the existence of the numerous individual droplets, represented as  , which is directly associated with the particle nature of the droplets. Hence,
, which is directly associated with the particle nature of the droplets. Hence,


 is the probability of finding droplets with diameter Di in the spray. ṅi is the number of droplets produced per unit time and with diameter Di.
is the probability of finding droplets with diameter Di in the spray. ṅi is the number of droplets produced per unit time and with diameter Di.
 is the isothermal compressibility of the liquid, a thermodynamic property whose dependence on pressure is negligible for liquids.
is the isothermal compressibility of the liquid, a thermodynamic property whose dependence on pressure is negligible for liquids.






 . This equation may then be rewritten in the unit mass flow rate form as follows
. This equation may then be rewritten in the unit mass flow rate form as follows

 .
.2.2 Droplet size distribution model



 denotes the dimensionless mass source term.
denotes the dimensionless mass source term.


 and
and  , we have to evaluate
, we have to evaluate





 and
and  are the droplet diameters corresponding to the droplet volume
are the droplet diameters corresponding to the droplet volume  and
and  respectively; and f is the continuous droplet size probability density function (pdf). Thus,
respectively; and f is the continuous droplet size probability density function (pdf). Thus,





3. Results and Discussion

4. Conclusion
5. Acknowledgement
6. References
- Sellens, R.W.; Brzustowski, T.A. A Prediction of the Drop Size and Velocity Distribution in a Spray from First Principles. Atomization and Spray Technology 1985, 1, 195–201. [Google Scholar]
- Li, X.; Tankin, R.S. Droplet Size Distribution: A Derivation of a Nukiyama-Tanasawa Type Distribution Function. Combustion Science and Technology 1988, 55, 65–76. [Google Scholar]
- Ahmadi, M.; Sellens, R.W. A Simplified Maximum-Entropy-Based Drop Size Distribution. Atomization and Sprays 1993, 3, 291–310. [Google Scholar] [CrossRef]
- Cousin, J.; Yoon, S.J.; Dumouchel, C. Coupling of Classical Linear Theory and Maximum Entropy Formalism for Prediction of Drop Size Distribution in Sprays: Application to Pressure-Swirl Atomizers. Atomization and Sprays 1996, 6, 601–622. [Google Scholar] [CrossRef]
- Mitra, S.K.; Li, X. A Predictive Model for Droplet Size Distribution in Sprays. Atomization and Sprays 1999, 9, 29–50. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Fu, H. Modeling the Initial Droplet Size Distribution in Sprays Based on the Maximization of Entropy Generation. Atomization and Sprays. submitted. [CrossRef]
- Fu, F. Experimental Characterization of Sprays from an Air-blast Annular Research Nozzle. Master’s thesis, University of Waterloo, 2003. [Google Scholar]
- Kim, W.T.; Mitra, S.K.; Li, X.; Prociw, L.A.; Hu, T.C.J. A Predictive Model for the Initial Droplet Size and Velocity Distributions in Sprays and Comparison with Experiments. Part. Part. Syst. Charact. 2003, 20, 135–149. [Google Scholar] [CrossRef]

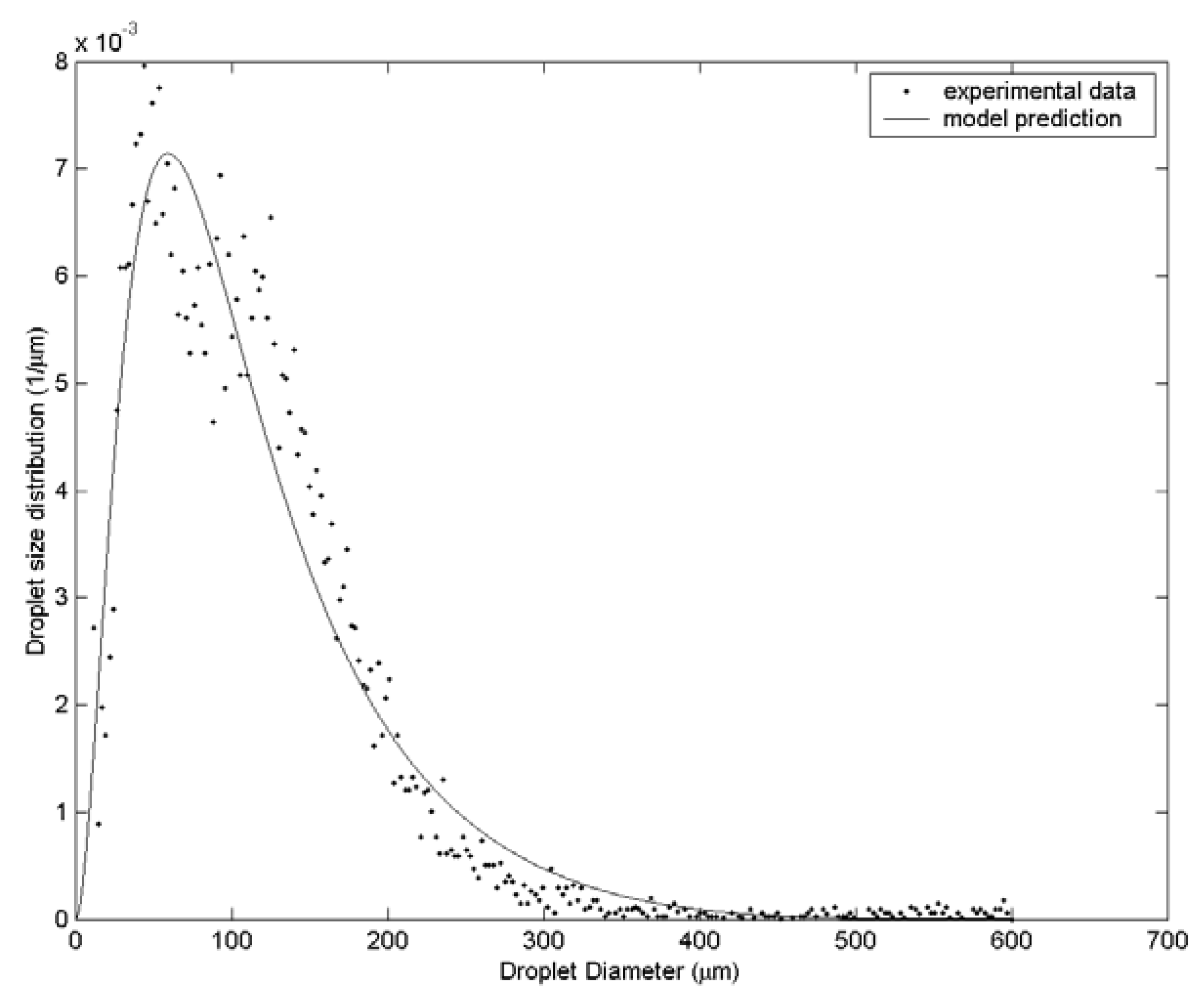

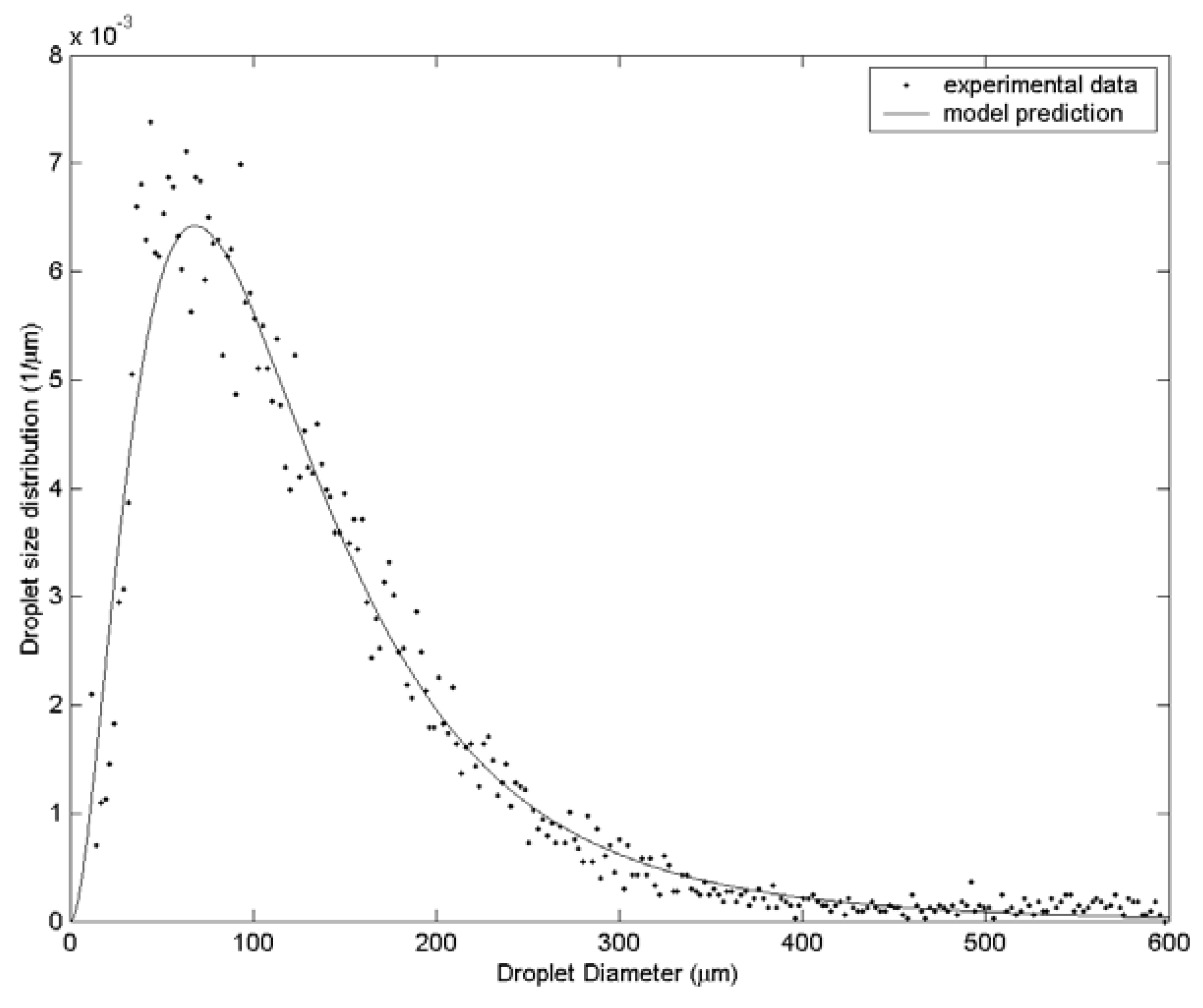
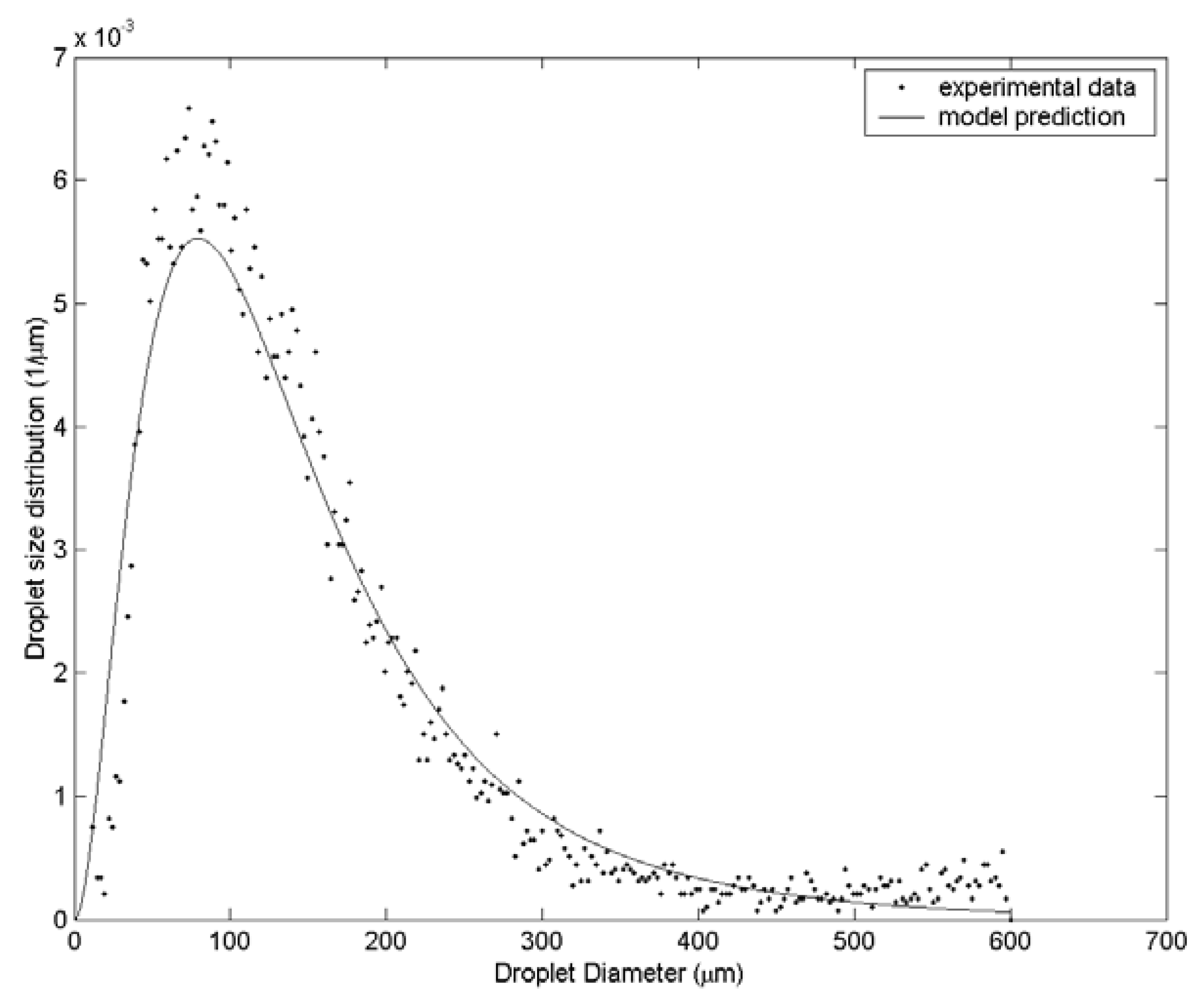
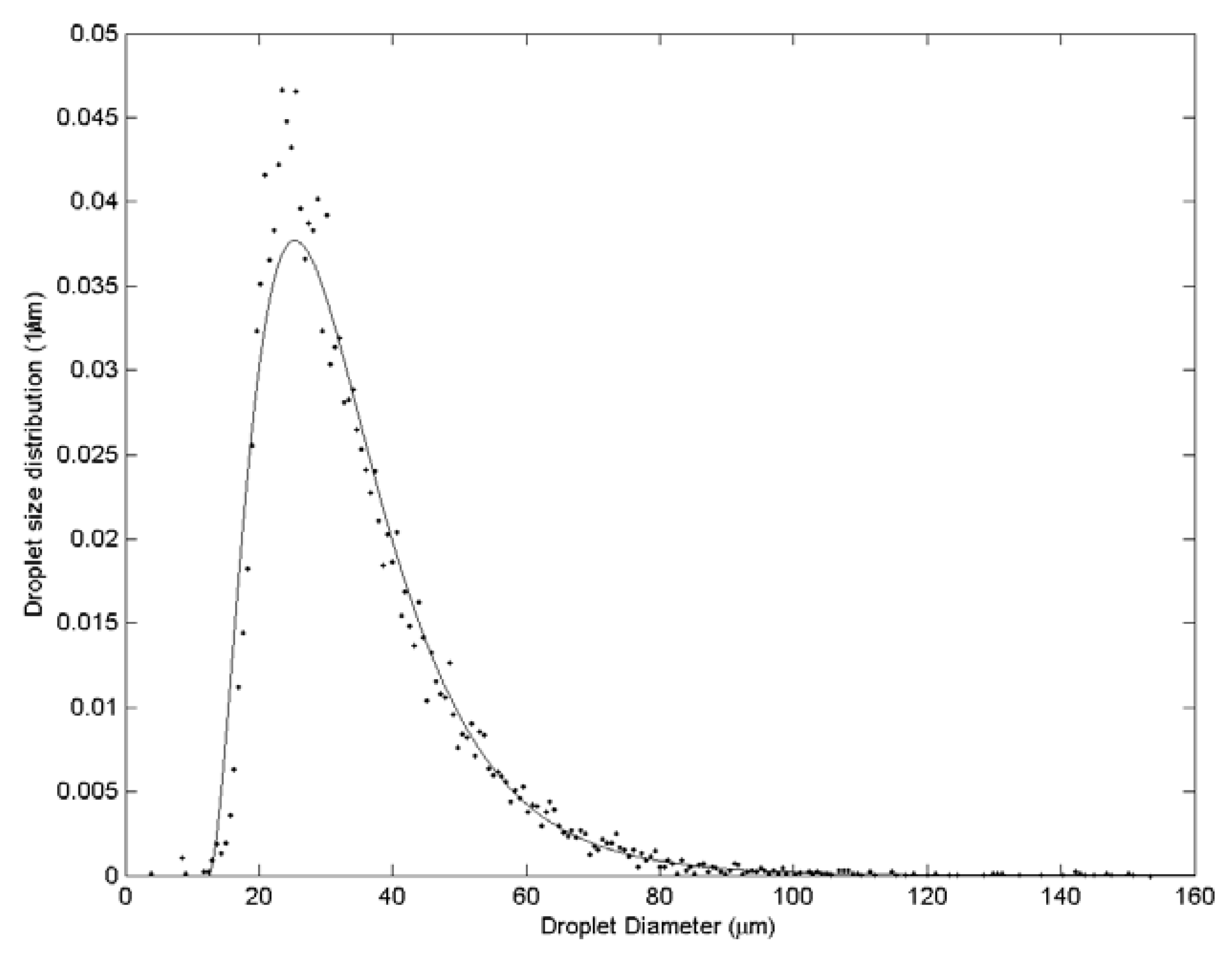
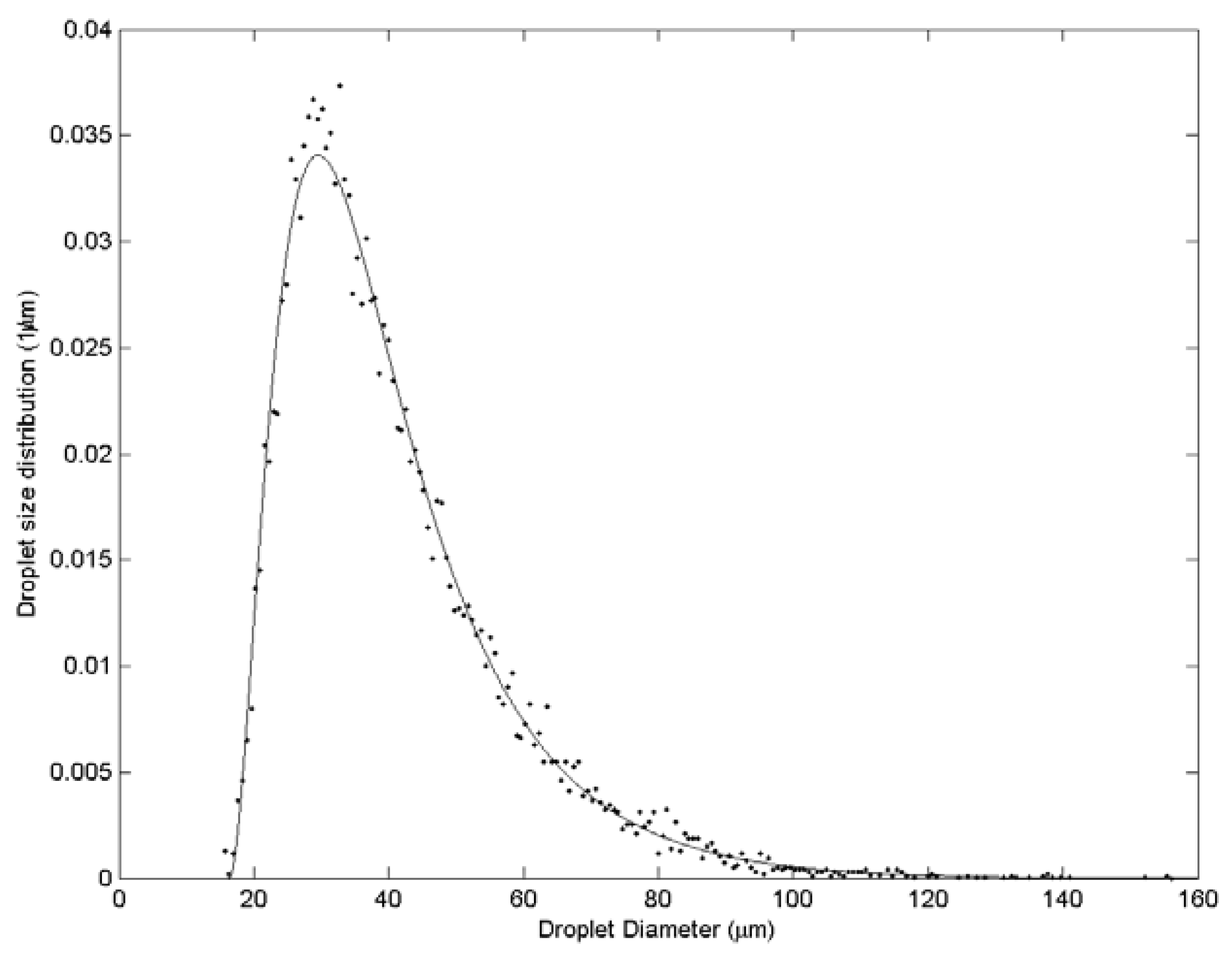
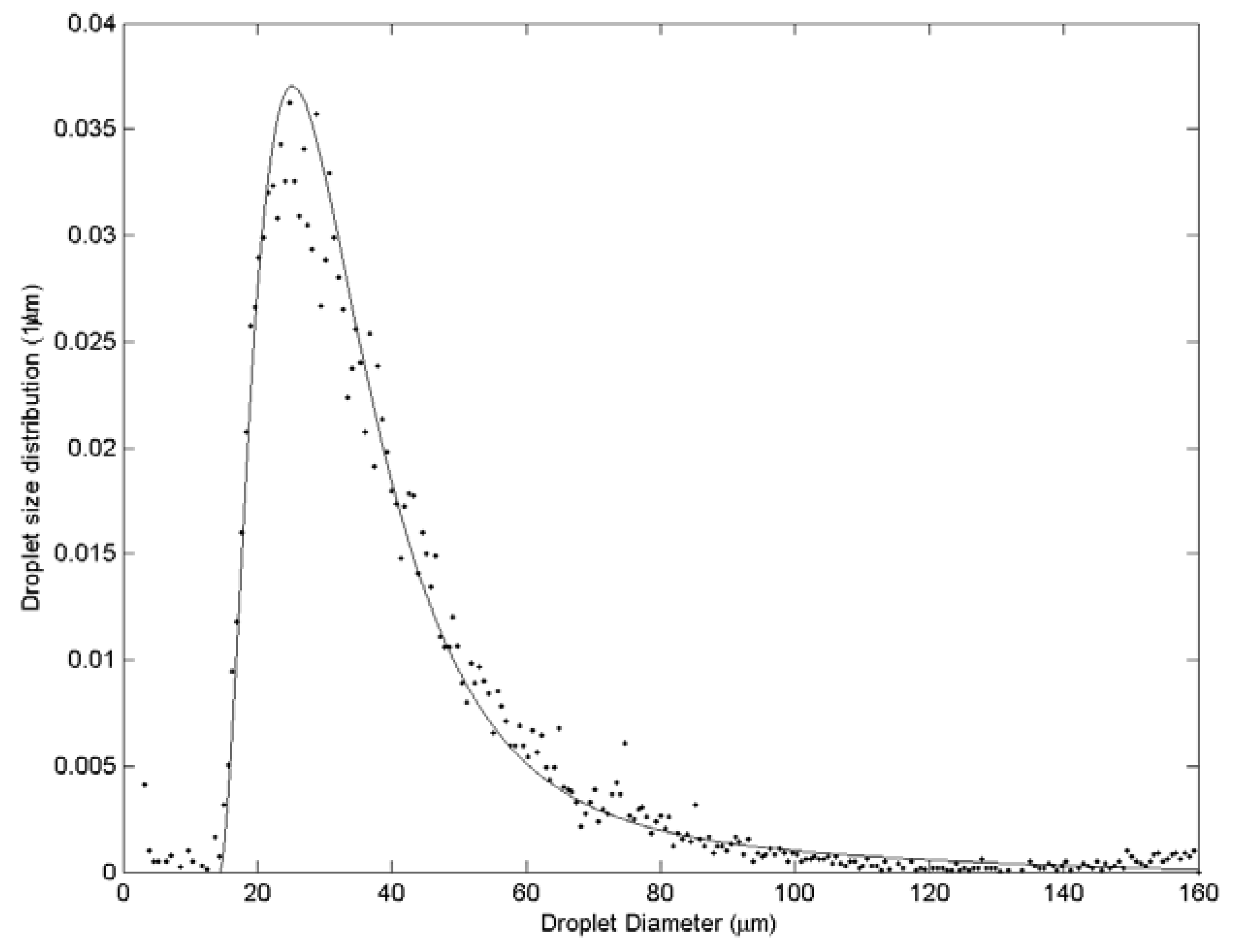
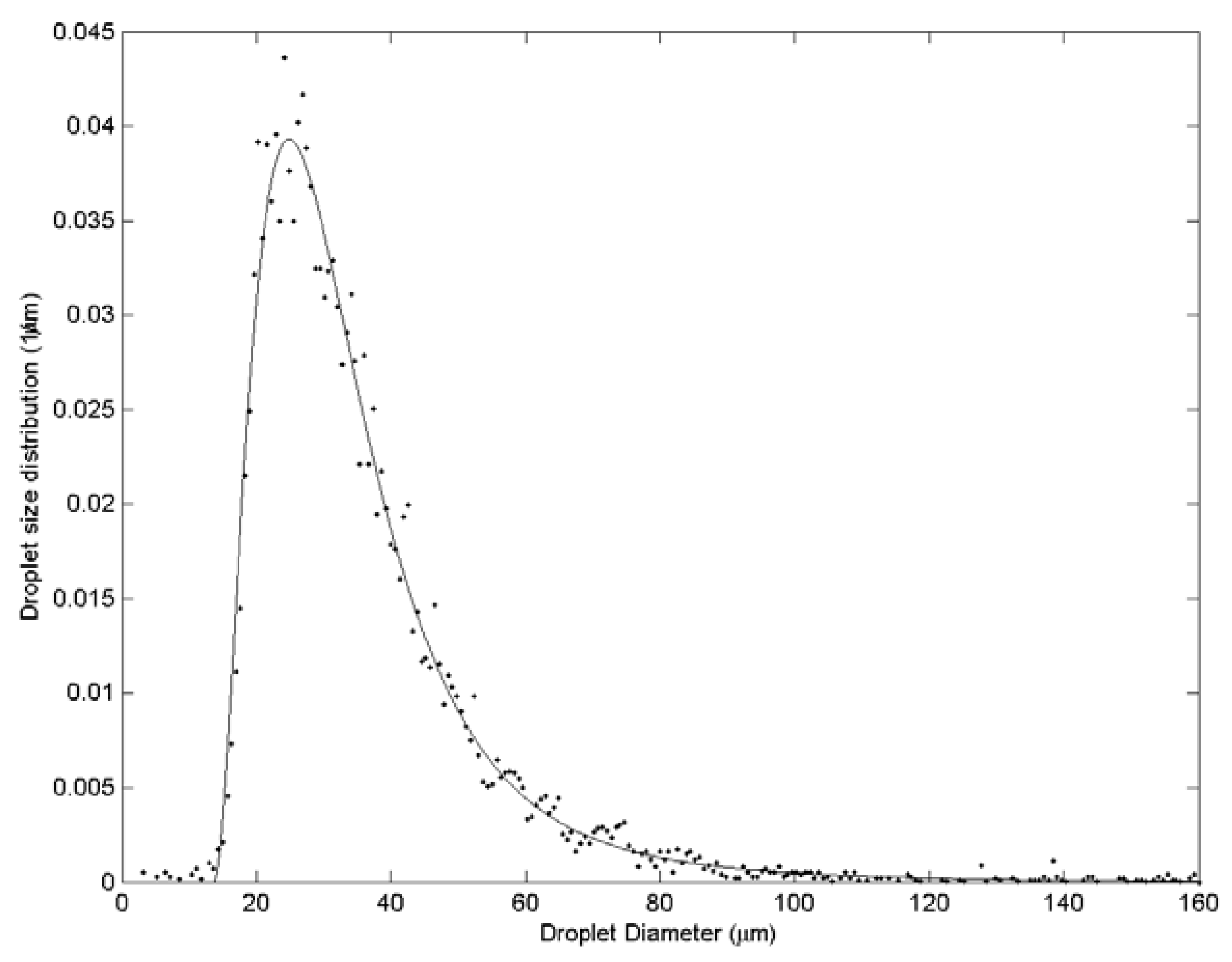
| Case | Water velocity (m/s) | Inner air velocity (m/s) | Outer air velocity (m/s) |
| 1 | 2.1 | 48 | 48 |
| 2 | 2.1 | 41 | 27 |
| 3 | 3.2 | 27 | 41 |
| 4 | 4.3 | 27 | 41 |
| Case | D10 (μm) | D20 (μm) | D30 (μm) | Validation Rate (%) |
| 1 | 112.8 | 135.4 | 161.3 | 61 |
| 2 | 108.1 | 142.3 | 182.0 | 48 |
| 3 | 131.6 | 163.3 | 197.5 | 61 |
| 4 | 152.5 | 188.6 | 227.1 | 54 |
| Case I | Case II | Case III | Case IV | |
| D0 (μm) | 12.385 | 16.321 | 14.350 | 13.697 |
© 2003 by MDPI (http://www.mdpi.org). Reproduction for noncommercial purposes permitted.
Share and Cite
Li, X.; Li, M. Droplet Size Distribution in Sprays Based on Maximization of Entropy Generation. Entropy 2003, 5, 417-431. https://doi.org/10.3390/e5050417
Li X, Li M. Droplet Size Distribution in Sprays Based on Maximization of Entropy Generation. Entropy. 2003; 5(5):417-431. https://doi.org/10.3390/e5050417
Chicago/Turabian StyleLi, Xianguo, and Meishen Li. 2003. "Droplet Size Distribution in Sprays Based on Maximization of Entropy Generation" Entropy 5, no. 5: 417-431. https://doi.org/10.3390/e5050417




