Synthesis
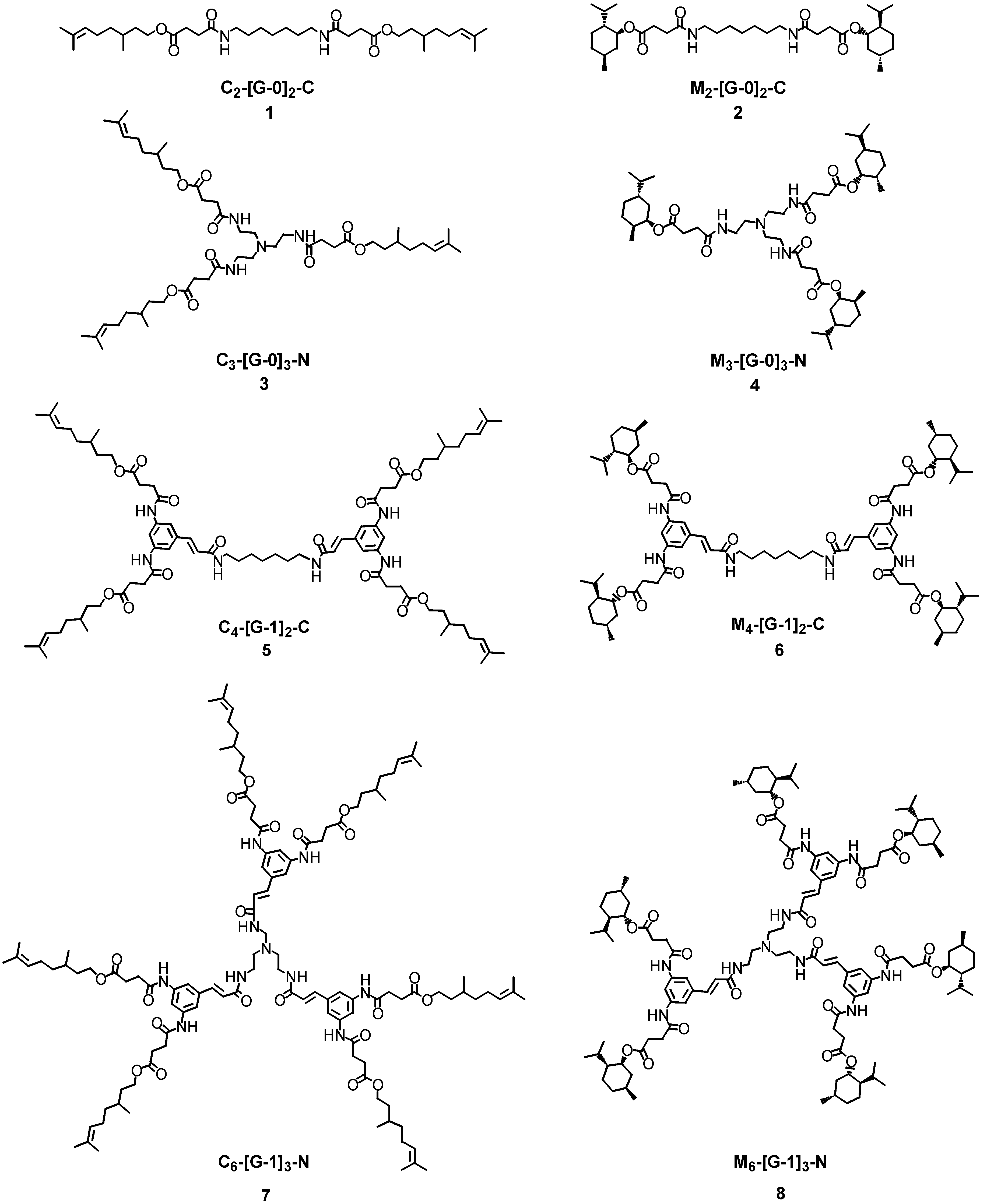
The linear and branched polyamides
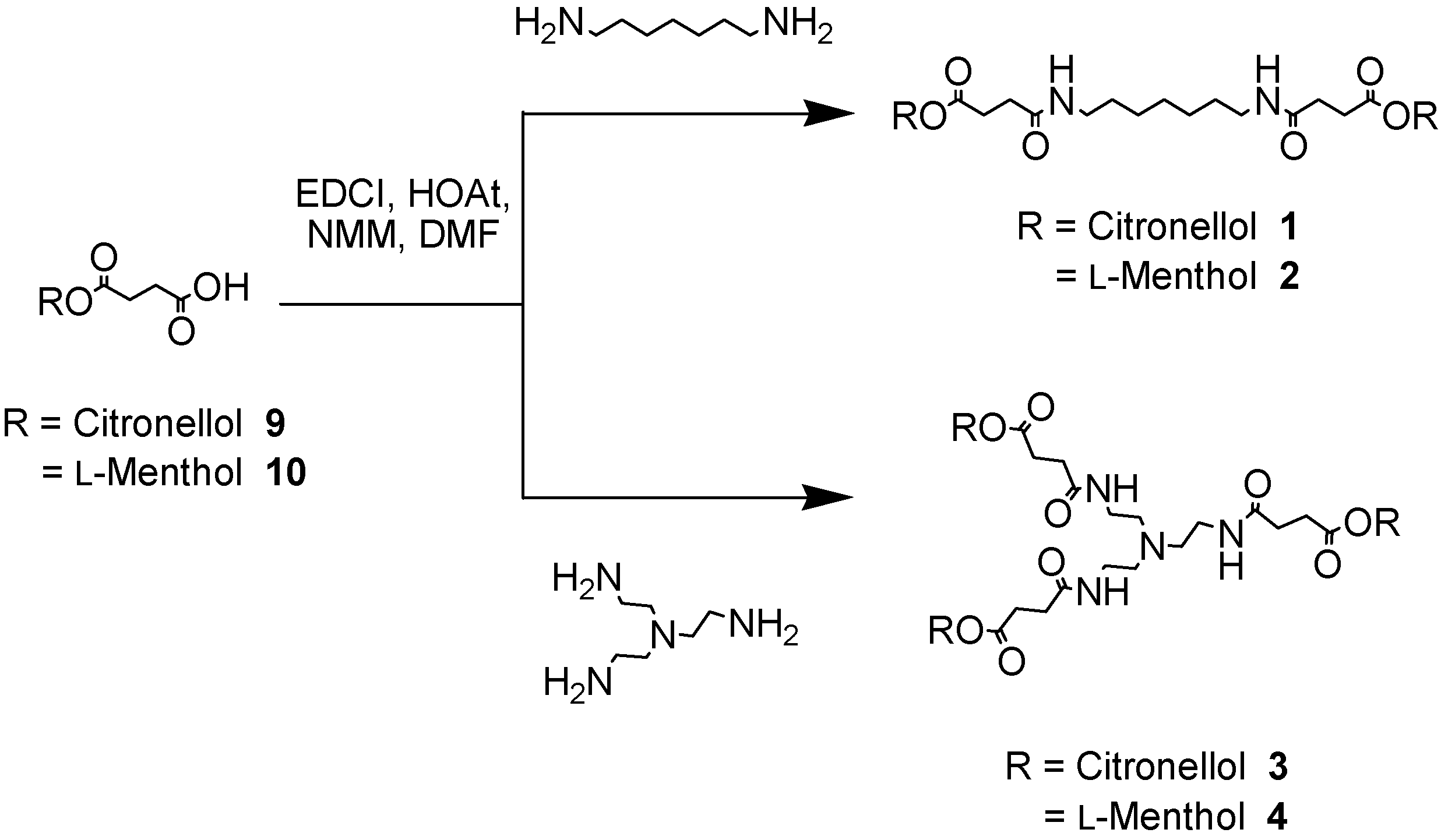
1-
8 bearing the ester functionalities on the external surfaces were synthesised in a convergent [
11] fashion starting from the corresponding primary or secondary alcohols, respectively [
12]. Succinic anhydride was ring opened under basic conditions [
13] using citronellol and
l-menthol, leading to the production of the carboxyester derivatives
9 as a white solid (in 95% yield) and
10 as a transparent oil (in 90% yield), respectively. The ester-carboxylic acid derivatives
9 and
10 were then coupled to the ‘core’ amine units such as 1,7-diaminoheptane or
tris-(2-ethylamino)amine to afford the desired polyamides
1-
4 featuring an increasing number of ester moieties (
Scheme 1).
Scheme 1.
Synthesis of simple and branched polyamides (1-4)
Scheme 1.
Synthesis of simple and branched polyamides (1-4)
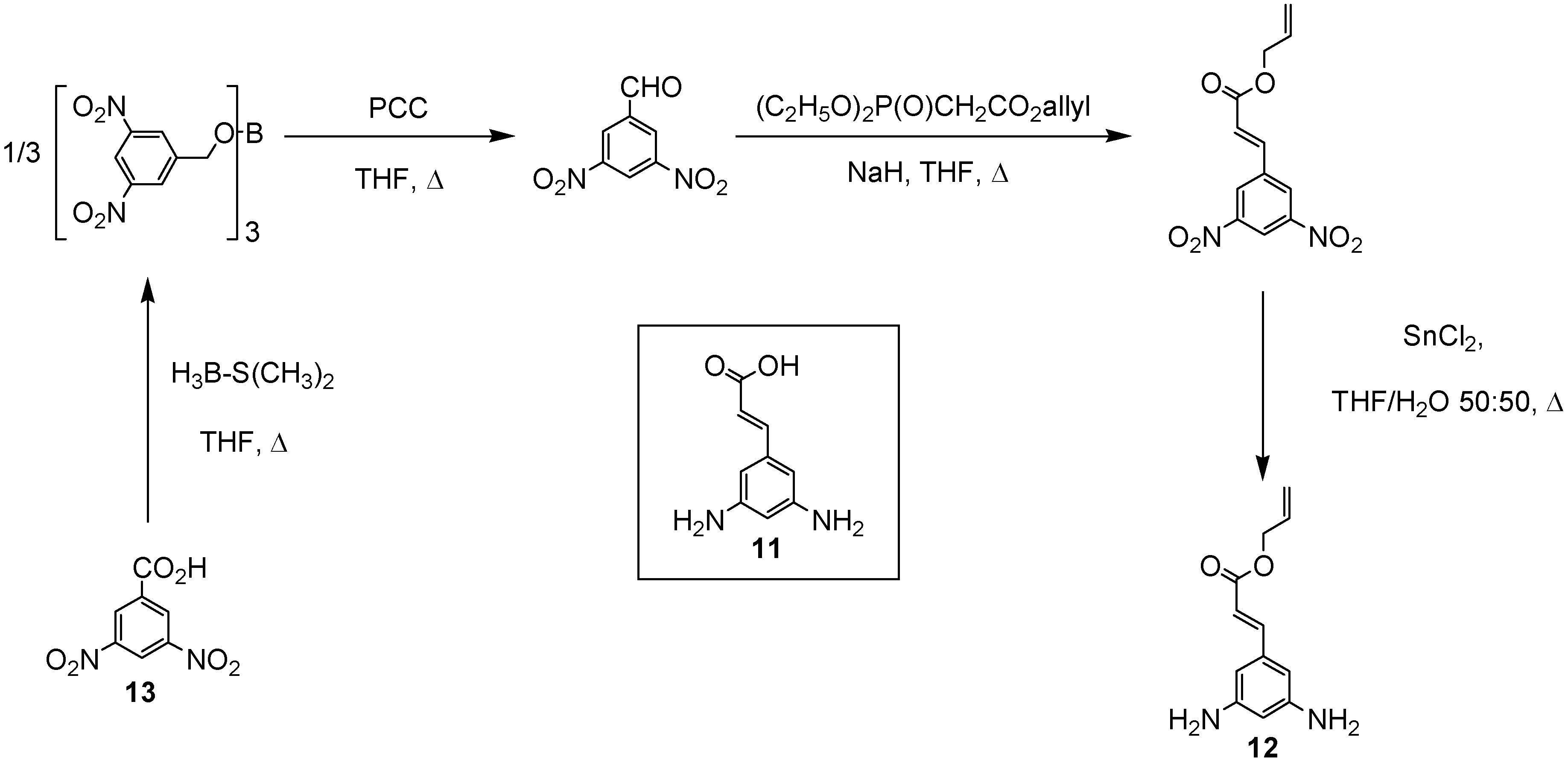
In order to increase the number of ester groups on the core units described above, the cinnamic acid derivative 3,5-diaminocinnamic acid
11 [
12] was used as a branching moiety in the construction of the branched polyamides. The selection of the convergent approach [
11] to the desired branched polyamides via selective coupling between the orthogonal amino moieties of 3,5-diaminocinnamic acid
11 and the carboxylic acid functionalities of the
l-menthol and citronellol esters
9 and
10 necessitated the use of protecting group chemistries. Therefore, the allyl derivative of 3,5-diamino-cinnamic acid
12 was synthesised in four steps from the commercially available 3,5-dinitrobenzoic acid
13 (
Scheme 2) in good yield.
Scheme 2.
Synthesis of 3,5-diaminocinnamic acid allyl ester (12)
Scheme 2.
Synthesis of 3,5-diaminocinnamic acid allyl ester (12)
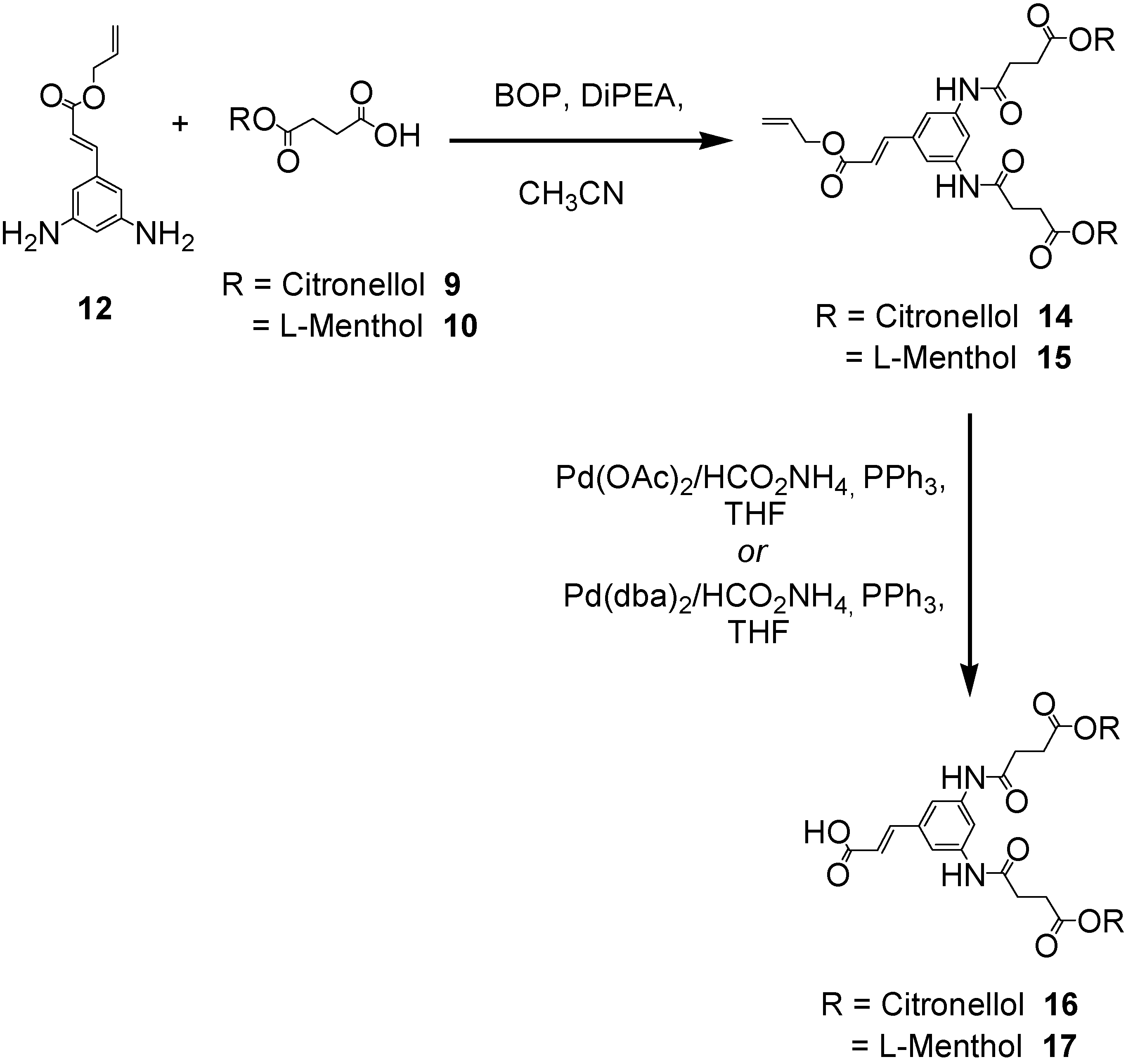
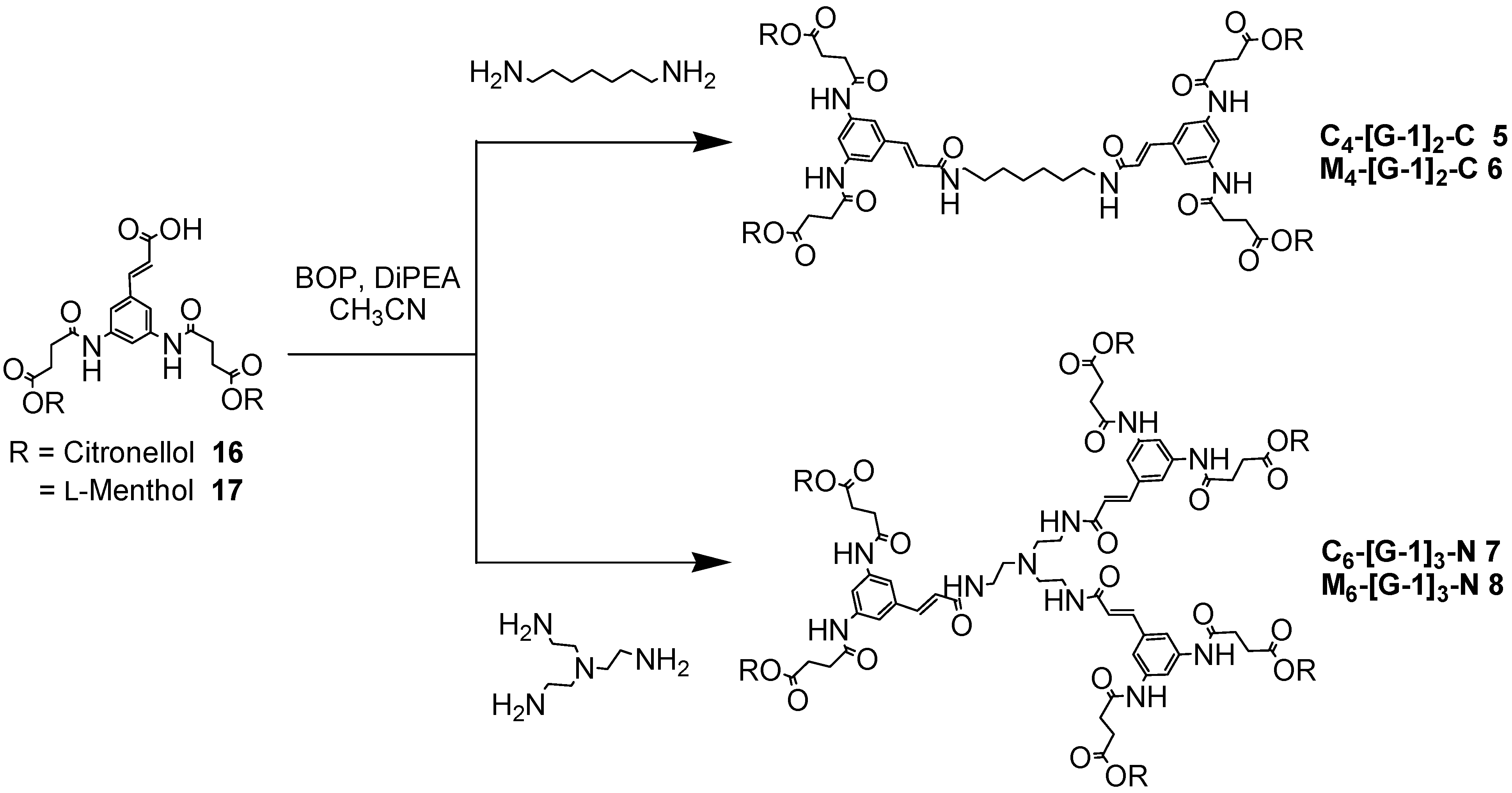
The first generation polyamide dendrons C
2-[G-1]-CO
2allyl (
14) and M
2-[G-1]-CO
2allyl (
15) were constructed in excellent yields (90-98%) using either 1-ethyl-3-(3’-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) and 1–hydroxy-7-azabenzotriazole (HOAt) or benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) coupling reagents (
Scheme 3) [
14]. Cleavage of the allyl ester at the dendrons’ focal point required very mild deprotection conditions (either Pd(OAc)
2, Pd(dba)
2, Pd(PPh
3)
4 in conjunction with ammonium formate) in order to prevent detrimental decomposition of the terpene ester linkages and to afford acceptable yields (64-74%) of the desired carboxylic acid derivatives
16 and
17.
Scheme 3.
Synthesis of the first generation polyamide dendrons featuring either citronellol or l-menthol at the peripheral surface.
Scheme 3.
Synthesis of the first generation polyamide dendrons featuring either citronellol or l-menthol at the peripheral surface.
The dendrimers were constructed from [G-0] to [G-2] by coupling each dendron to either bifunctional or trifunctional central core units. Synthesis of the first generation dendrimers was carried out initially using EDCI/HOAt as the coupling reagent system, but the yields were very poor. Use of BOP afforded M
4-[G-1]
2-C (
6) as a white solid in excellent yield (96%) and M
6-[G-1]
3-N (
8) as pale yellow solids in moderate yield (48%) over a prolonged reaction time (48 hours). The BOP reagent was also used in the synthesis of C
4-[G-1]
2-C (
5) and C
6-[G-1]
3-N (
7). These dendrimers were obtained after purification by column chromatography as pale yellow solids in acceptable yields (98% and 28%, respectively), in agreement with the results obtained for the series of
l-menthol first generation dendrimers (
Scheme 4).
Scheme 4.
Synthesis of the branched polyamides 5-8.
Scheme 4.
Synthesis of the branched polyamides 5-8.
Hydrolysis Studies
Preliminary studies of alcohol release under enzymatic hydrolysis were conducted on the ester groups situated on the peripheral layer of the linear and branched polyamides
1-
8 with lipase from
Candida cylindracea in an aqueous solution buffered to pH 7.2 [
15]. The sampling was carried out over variable times (between 40 and 96 hours) and the alcohol released from the peripheral surface of the polyamides was extracted from the aqueous phases with an organic solvent (CHCl
3) and its concentration monitored by RP-HPLC or GC analyses. The extracts were also analysed by MALDI-TOF mass spectrometry, from which it was possible to identify several of the major hydrolytic products. For example, when C
2-[G-0]
2-C (
1) was subjected to the hydrolysis in the presence of the lipase an increase in the concentration of citronellol (~15%) was exhibited. However, a similar trend was also observed during the hydrolysis of
1 carried out without the lipase. This result may suggest that a slow hydrolytic process occurred as a consequence of the slightly basic conditions of the solution, and the enzyme did not participate in the catalysis of the process. However, the MALDI-TOF mass spectra of the samples extracted from the reaction mixtures did not reveal the presence of species that could be attributable to products of the hydrolysis. Therefore, it was not possible to confirm the occurrence of the hydrolytic process in this case.
In contrast, unambiguous results were obtained from the hydrolysis of M2-[G-0]2-C (2), where cleavage of the ester linkages was not observed. This result was not unexpected since compound 2 features esters that are derived from a secondary alcohol, and hence more difficult to cleave either under basic conditions or by enzymatic hydrolysis.
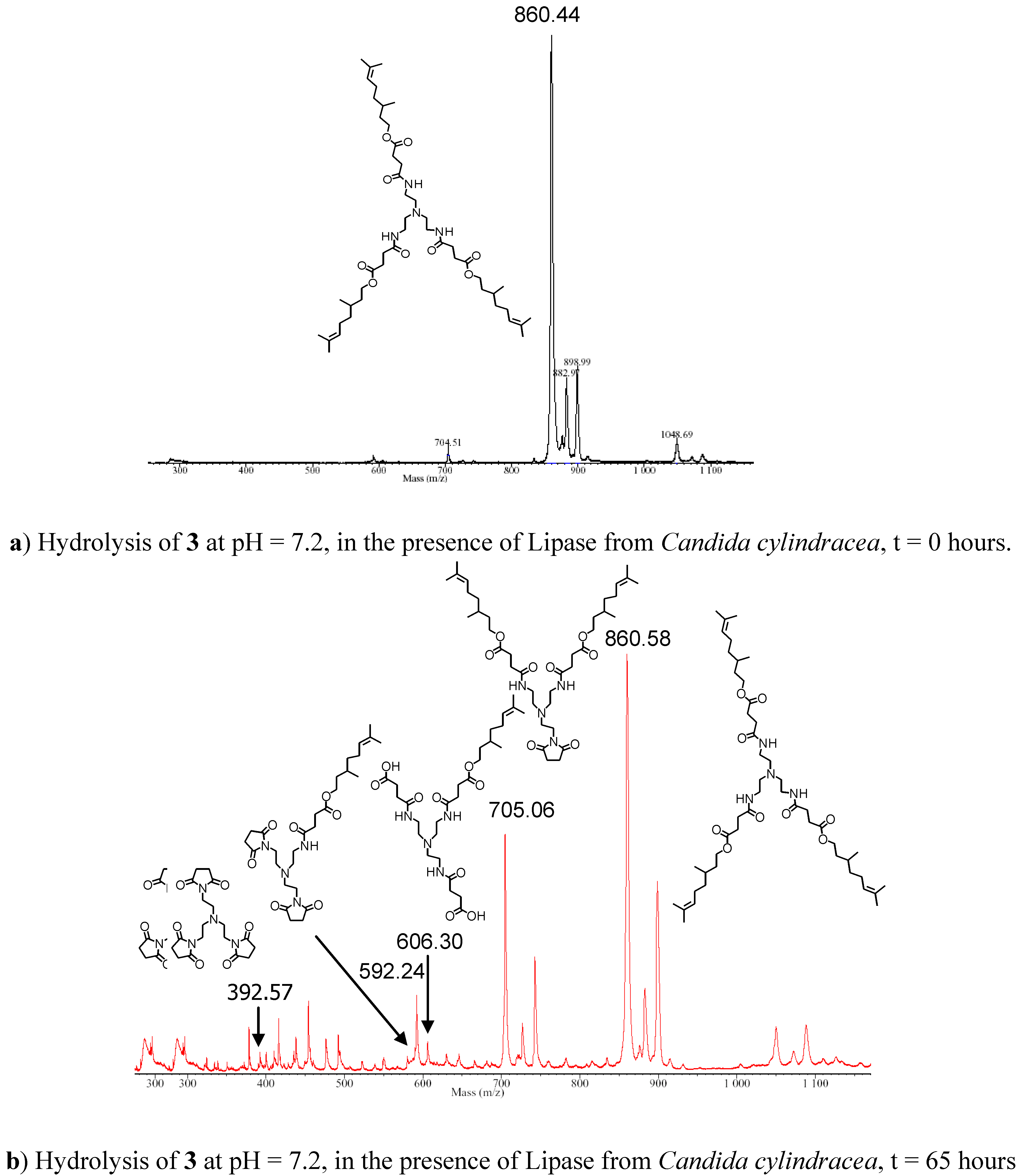
More interesting results were obtained from the hydrolysis studies of C
3-[G-0]
3-N (
3). This compound was characterised by the presence of three citronellol moieties bound to the aliphatic polyamide structure
via a succinate spacer. Furthermore, a tertiary amine functionality was present in the core unit of the molecule. When
3 was subjected to the hydrolytic conditions in the presence of lipase from
Candida cylindracea progressive increase in the concentration of citronellol (~50%) was revealed by the RP-HPLC analysis. Although a certain degree of ester cleavage was also observed in the absence of enzyme (
ca. 25 %), in this case a significant difference between the enzymatic hydrolysis and that performed without the enzyme was evident. MALDI-TOF mass spectrometric analysis of the samples during the hydrolysis study revealed important information about the hydrolytic process. In both reactions (the hydrolysis in the presence and absence of the enzyme) the appearance of two peaks corresponding to the loss of one molecule of citronellol (MH
+=704 Da) and of two citronellol units (MH
+=548 Da, or as the MK
+=589 Da), was observed, which suggested formation of cyclic imides (
Figure 2). The peak at 704 Da increased throughout the reaction, while the peak corresponding to the loss of two molecules of citronellol (592 Da) appeared at the initial stage of the hydrolytic process, but its relative intensity did not increase considerably as the hydrolysis proceeded. However, from the mass spectrometric analysis, it was not possible to obtain any correlation between peak intensities and concentration of the species present, therefore, it was only possible to suggest that the main hydrolytic pathway involved the release of a single molecule of citronellol per molecule of starting material C
3-[G-0]
3-N (
3), and formation of a cyclic succinimide followed by subsequent release from the monoimide species. The other peaks detected in the spectra were attributed to the matrix used (4-hydroxy-α-cyanocinnamic acid), its aggregates and fragments, as confirmed by the spectrum obtained by the analysis of the matrix on its own recorded under identical analytical conditions. Formation of succinimides from succinates under neutral and basic conditions are well-documented in the literature [
16]. For example, Matsumoto
et al. [
17] have reported recently the use of succinyl ester derivatives as an easily hydrolysable spacer for the conjugation of a new class of prodrug form of an HIV protease inhibitor. The esters of succinamic acids are known to undergo rapid hydrolysis
via succinimide formation under mild alkaline conditions [
18].
Figure 2.
MALDI-TOF Mass Spectra of the hydrolysis of C3-[G-0]3-N (3) recorded at (a) time = 0 and (b) after 65 Hours.
Figure 2.
MALDI-TOF Mass Spectra of the hydrolysis of C3-[G-0]3-N (3) recorded at (a) time = 0 and (b) after 65 Hours.
Recently, the use of lipase from
Candida cylindracea to generate amide bonds in organic solvents from the corresponding esters has been reported by Gotor and co-workers [
19]. Furthermore, the lipase from
Pseudomonas cepacia has been employed in the enzymatic hydrazinolysis of diesters and the synthesis of
N-aminosuccinimide derivatives in organic solvents [
20]. The principle of ring closure with consequent elimination of an alcohol as a leaving group has also been embraced for the development of controlled release of fragrance alcohols from poly(propyleneimine) dendrimers [
21]. In this case, fragrance release was based upon proton abstraction from a carbamoyl moiety by a basic buffer system. The intermediate nucleophile generated then underwent an intramolecular ring closure on the neighbouring ester moiety to release the desired fragrance and generate a cyclic imide by-product. The cyclisation occurred rapidly in slightly alkaline conditions and represented the driving force for the efficient release of even tertiary alcohols. In the last example, the efficiency of the cyclisation was promoted by the presence of a rigid benzene ring that held the two reactive components in close proximity with the conformation most appropriate to afford efficient imide ring closure.
In the current study, the linker used to conjugate the alcohol unit to the dendritic surface was a flexible succinate chain. Therefore, it is possible to assume that the pH-promoted cyclisation was not favoured as in the study reported by Hermann and co-workers [
21], and occurred at a slower rate. This explanation does not contradict the different hydrolytic profiles exhibited by C
3-[G-0]
3-N (
3) in the presence and absence of the lipase. It is proposed that in this case (hydrolysis of
3 in the buffered system) the enzyme simply accelerated a process that was already occurring. It is also proposed that the presence of the tertiary amine at the core unit of the molecule participated in the de-protonation of the amide, hence promoting the cyclisation even in the absence of the enzyme. It was noticeable that the ring closing process did not occur in the case of C
2-[G-0]
2-C (
1), which was characterised by the same succinyl ester moieties, no central tertiary amine and a longer alkyl chain as the core unit.
When the hydrolysis of M3-[G-0]3-N (4) was investigated, release of the secondary alcohol was not observed either in the presence or absence of the enzyme. This result revealed: – i) the lack of interactions between the enzyme and the substrate, which may be a consequence of the rigidity of the structure of the alcohol moieties, and the presence of esters of a secondary alcohol and ii) the low tendency of these esters to undergo ring closing under neutral conditions, as a consequence of the poor ability of secondary alcohols to behave as leaving group under mildly basic conditions.
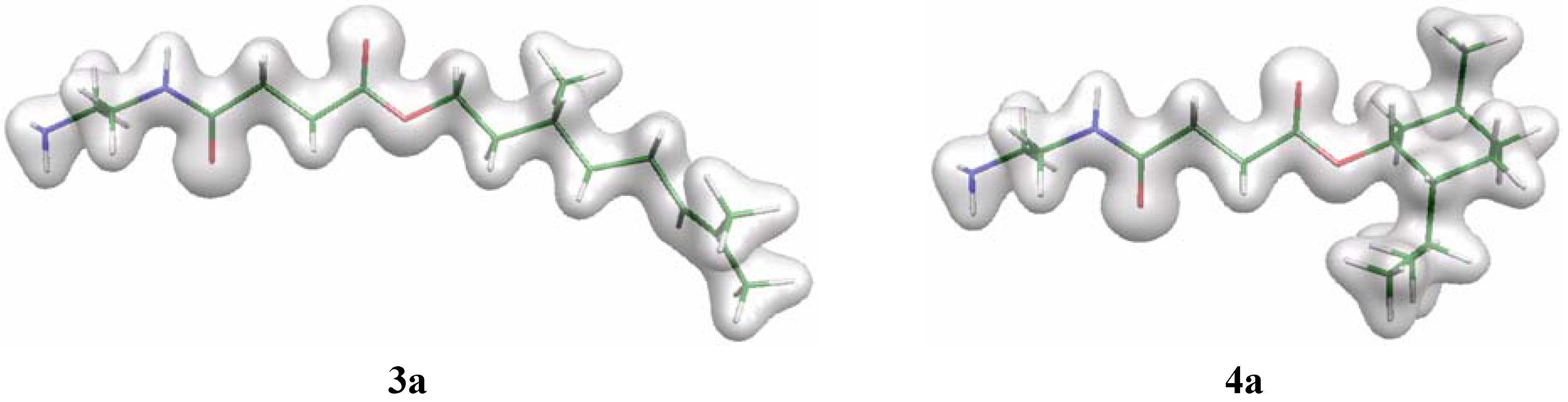
In order to examine the hydrolysis characteristics of molecules C
3-[G-0]
3-N (
3) and M
3-[G-0]
3-N (
4) further, preliminary computer modelling studies were performed on single ‘arms’ of the polyamides, (
3a and
4a, respectively – see
Figure 3). Structures were built within the Cerius2 software [
22] and geometry optimised at the HF/6-31G(d) level using Gaussian98 [
23]. For each system two geometries were studied with different values of the O=C–O–R torsion angle φ. The lowest-energy conformation was found with φ = 0° (denoted
cis) while the high-energy conformer has φ = 180° (denoted
trans). Firstly, it was noted that the energy differences between the
cis and
trans conformers for
3a and
4a were identical (8.52 kcal mol
–1). In contrast, the corresponding hydrolysis product in which either the citronellol or
l-menthol components have been removed to afford the corresponding carboxylic acid, the energy difference between conformers was 6.24 kcal mol
–1. The rotational energy profile of φ is similar for all three structures – the alcohol leaving group does not significantly affect the energy of the different conformers of a given molecule, thus steric repulsions between the alcohols and the remainder of the molecule are small. Plots of the electron density for the
cis conformers of
3a and
4a (
Figure 3) reveal that there is minimal difference in the local density around the carboxy group. While the isopropyl substituent of the cyclohexane ring in
4a-
cis would appear to occlude the reactive carbonyl centre, the approach angle onto the C=O group will be perpendicular to the C=O vector and thus not close to the bulky ester group. Based upon this preliminary evidence, it appears that the observed lack of hydrolysis of
4 cannot not attributed to a predominant steric effect, and is most likely related to the poor leaving ability of the alcohol functionality. Indeed, one calculated difference between
3a and
4a are the partial atomic charges of the carbon atom of the COOR group, with values of –0.38 and –0.34 e, respectively. While this difference is only a small distinction, it is likely that the cause is the increased positive inductive effect offered by the leaving group in
4a cf. that of
3a, which promotes an increased negative (more positive) charge on the adjacent atoms.
Figure 3.
Plots of the total electron density of the
cis conformers of
3a and
4a, drawn using the Molekel [
24] software.
Figure 3.
Plots of the total electron density of the
cis conformers of
3a and
4a, drawn using the Molekel [
24] software.
A study of the ester cleavage from the first generation polyamide dendrimers
5-
8 was conducted by GC analysis. Although sample preparation was carried out under similar conditions used for the branched molecules
1-
4, the chloroform solution resultant from the extraction of the aqueous mother liquor was injected directly into the GC column. This procedure eliminated one step in the sample preparation and decreased any potential error associated with this process. Furthermore, the analyses employed an internal standard to minimise the errors related with the sampling and injection processes. However, under the same hydrolytic conditions used in the other cases examined, release of the citronellol from C
6-[G-1]
3-N (
7) was not observed. This result was rationalised as a consequence of two major factors: increased bulkiness of the dendritic structure which may have inhibited the access of the molecule into the enzyme active site, and also increased rigidity of the system introduced by the presence of the cinnamate moieties. Furthermore, it was speculated that the increased rigidity might also render the deprotonation process of the amide unit more difficult to achieve and consequently reduce the ability of intramolecular nucleophilic addition/elimination that was observed in the case of C
3-[G-0]
3-N (
3) under mild basic conditions. Since the lipase from
Candida cylindracia was found to catalyse the cleavage of the ester moieties only from C
3-[G-0]
3-N
3, it was decided to investigate another enzyme which belonged to the class of hydrolytic enzymes referred to as cutinases. The enzyme used was a solid-supported cutinase from
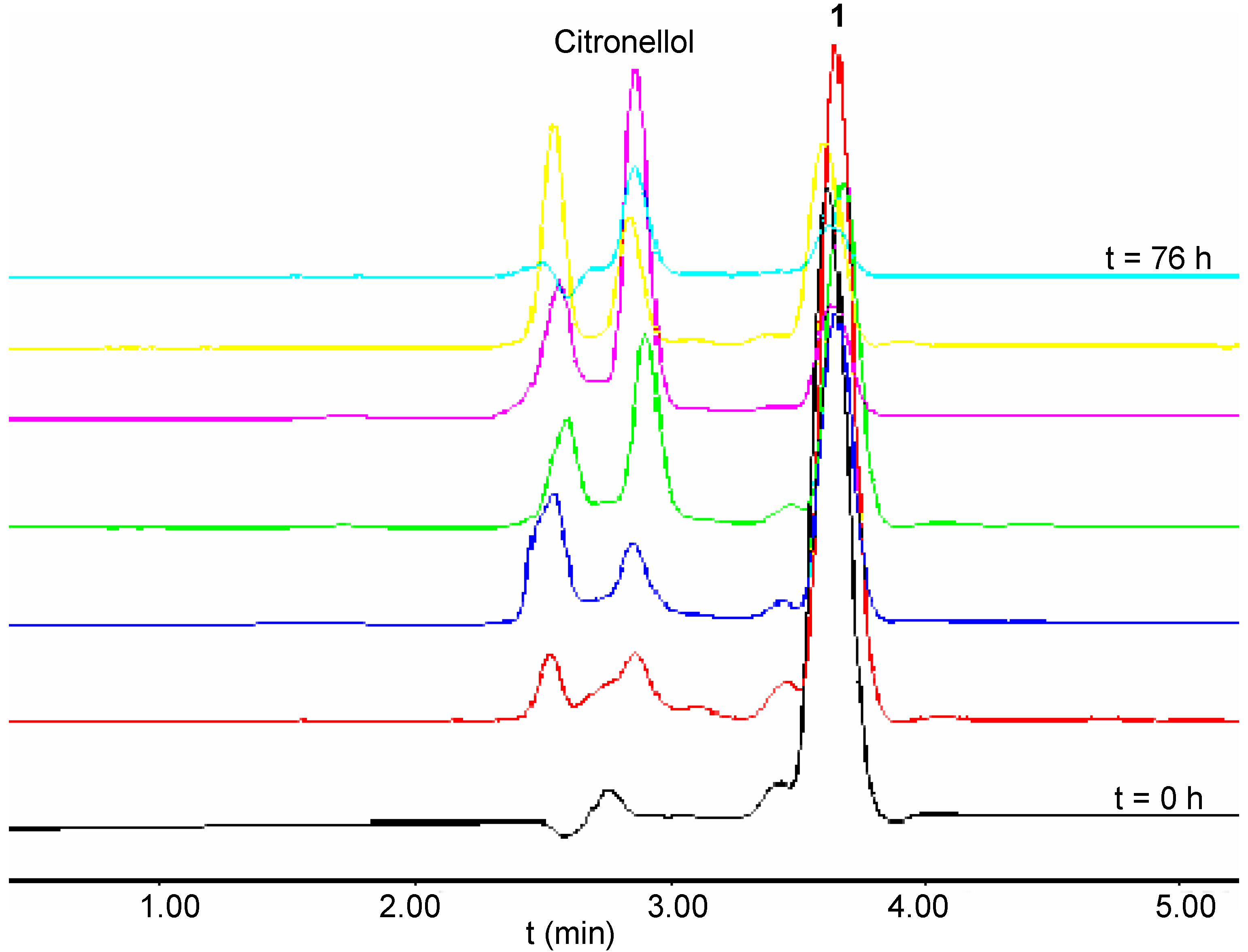
Fusarium solani pisii, used commonly in detergent products. The release profiles for the series of citronellol-based dendrimers obtained under the optimum conditions for this enzyme, were very surprising. For example, C
2-[G-0]
2-C
1 underwent cleavage of the ester moieties (~80%) within the first 40 hours of hydrolysis. However, a decrease in concentration of citronellol was observed after this period of time, thereby indicating the occurrence of possible side reactions involving the alcohol (
Figure 4).
Figure 4.
HPLC Traces of the Hydrolysis of 1 in the presence of a Cutinase, recorded at Different Times During the Reactions.
Figure 4.
HPLC Traces of the Hydrolysis of 1 in the presence of a Cutinase, recorded at Different Times During the Reactions.
In contrast, when the hydrolysis was carried out in the absence of the enzyme, significant release of alcohol was not detected. MALDI-TOF mass spectrometry did not reveal significant information regarding the hydrolytic pathway, although a decrease in intensity of the peaks corresponding to the starting material was observed with the proceeding of the reaction. Unfortunately, citronellol was not detectable under the conditions used in the mass spectrometric analysis, and also was not observed in the spectra of standard samples. Furthermore, analysis of negative ions via MALDI-TOF MS was not conducted, therefore, possible negatively charged species resulted from the hydrolysis were not observed. From the results obtained for the hydrolysis of 1, it was concluded that the hydrolysis of the ester moieties was enzyme-catalysed although it occurred without formation of the cyclic imide. The presence of the enzyme also appeared to influence much more significantly the hydrolytic process in comparison to the other enzyme tested (lipase) thus indicating more favourable interactions between the substrate and the enzyme active site. Similar behaviour was also observed in the case of the citronellol derived first generation polyamide dendrimers 5 and 7.
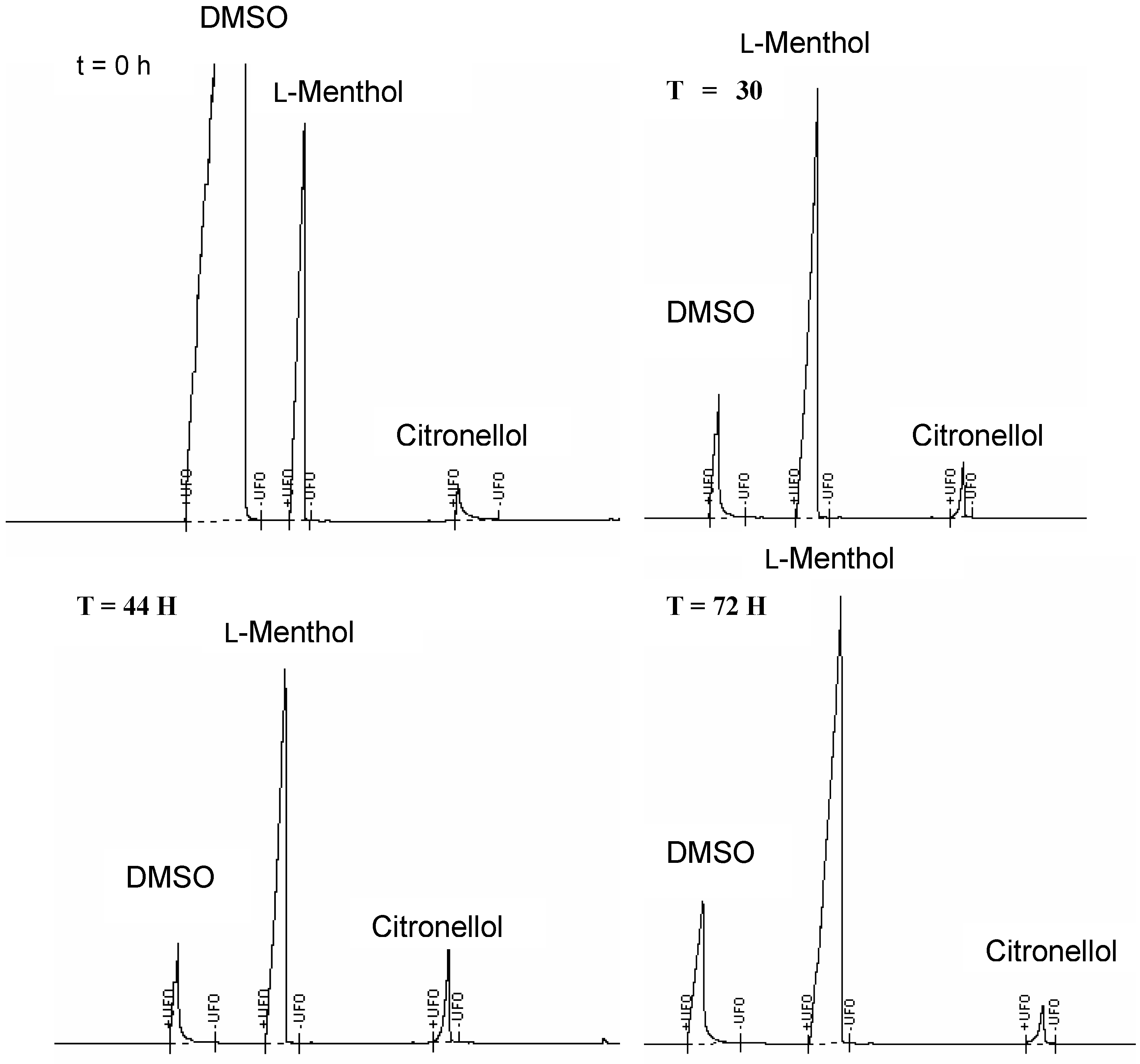
Figure 5.
GC Chromatograms of the Hydrolysis of C4-[G-1]2-C (5) in the Presence of Cutinase, Recorded after 0, 30, 44 and 72 Hours.
Figure 5.
GC Chromatograms of the Hydrolysis of C4-[G-1]2-C (5) in the Presence of Cutinase, Recorded after 0, 30, 44 and 72 Hours.
The analysis of the hydrolytic products was carried out using GC, in which each sample was spiked with
l-menthol as the internal standard. When the hydrolysis was performed in the presence of a lipase, cleavage of the ester groups was not detected. However, improved results were obtained in the presence of the cutinase, whereas a small percentage of citronellol was released from the dendritic structures (~15-25%). The dendrimer C
4-[G-1]
2-C (
5) exhibited release of citronellol over a period of 40 hours after which decrease in the concentration of citronellol was observed in a similar fashion as described above (
Figure 5). Furthermore, the hydrolysis carried out in the absence of enzyme did not exhibit relevant release of citronellol, thus indicating that the ester cleavage was promoted by the enzyme rather than dictated by the reaction conditions. MALDI-TOF mass spectrometric analysis in this case also did not prove to be informative: – the almost complete absence of the peak corresponding to the dendritic starting material was observed from the beginning of the hydrolysis. This result was attributed to the experimental procedure required for the preparation of the analytical samples. Since the enzyme was solid supported, each sample was subjected to filtration and extraction with an organic solvent prior to analysis. However, the lack of the signal corresponding to the dendritic species in the MALDI-TOF mass spectrometric analysis led to the hypothesis that the dendrimers were absorbed onto the solid support and not eluted in the organic solvent used in the extraction step.
The polyamide dendrimer C6-[G-1]3-N (7) also exhibited similar behaviour in the presence of the cutinase, although the increase in concentration of citronellol was very moderate (~15%). This moderate hydrolytic process – as in the case of the hydrolysis carried out in the presence of a lipase – may be attributable to the increased rigidity of the structure C6-[G-1]3-N (7) when compared with C4-[G-1]2-C (5). The dendrimer C6-[G-1]3-N (7) was characterised by a tetrafunctional core with three arms possessing only two carbon chains. In contrast, C4-[G-1]2-C (5) possessed a bifunctional core with longer alkyl chains (seven methylenic residues), which imparted a higher degree of flexibility to the structure. Furthermore, 7 was characterised by the presence of three rigid polyamide dendrons around the core unit, with considerable increase of the bulkiness of the molecules.
The series of dendrimers bearing l-menthol esters at the periphery were also subjected to the hydrolytic conditions in the presence of the cutinase. However, as in the case of the hydrolysis in the presence of lipase, these molecules did not exhibit any evidence of alcohol release, confirming the selectivity of these classes of hydrolases towards esters of primary alcohols.