Pyrazolo[4,3-e][1,2,4]triazines: Purine Analogues with Electronic Absorption in the Visible Region
Abstract
:Introduction
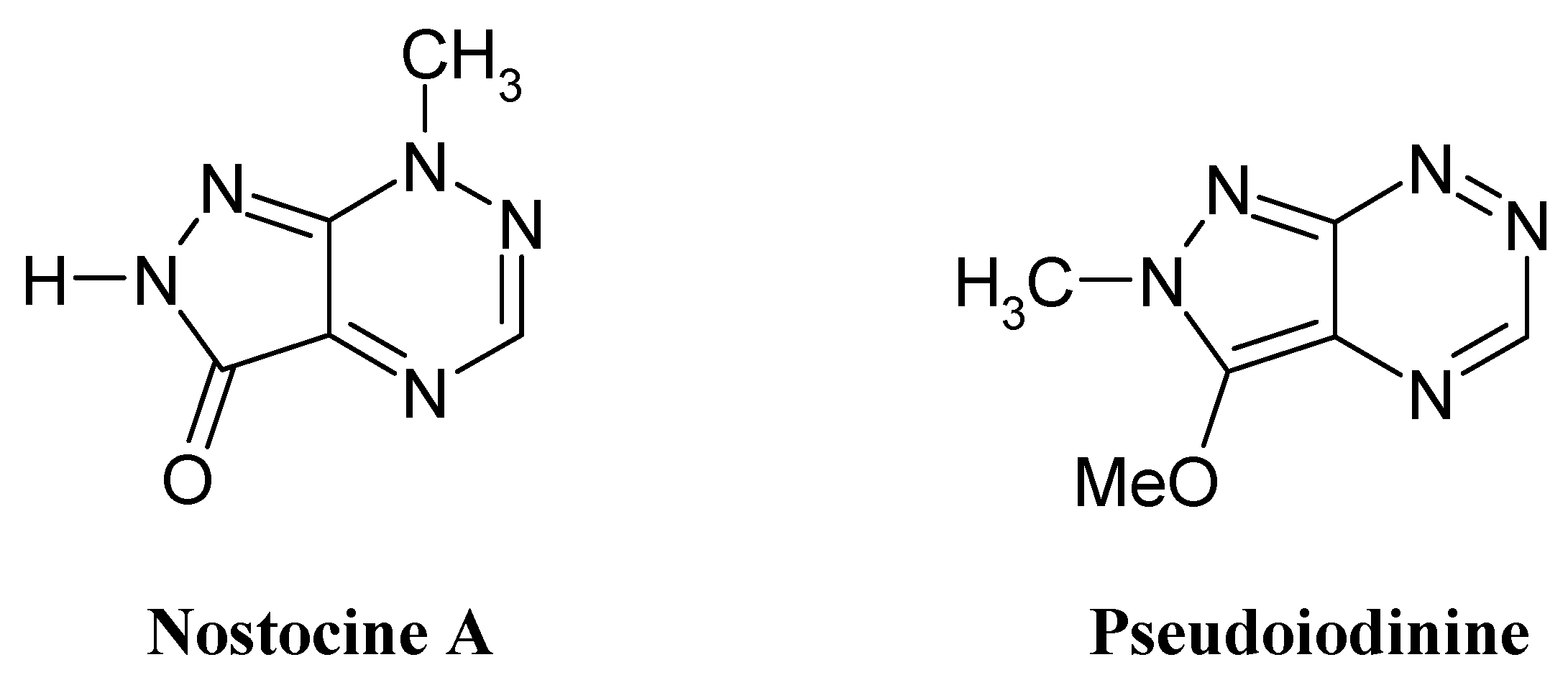
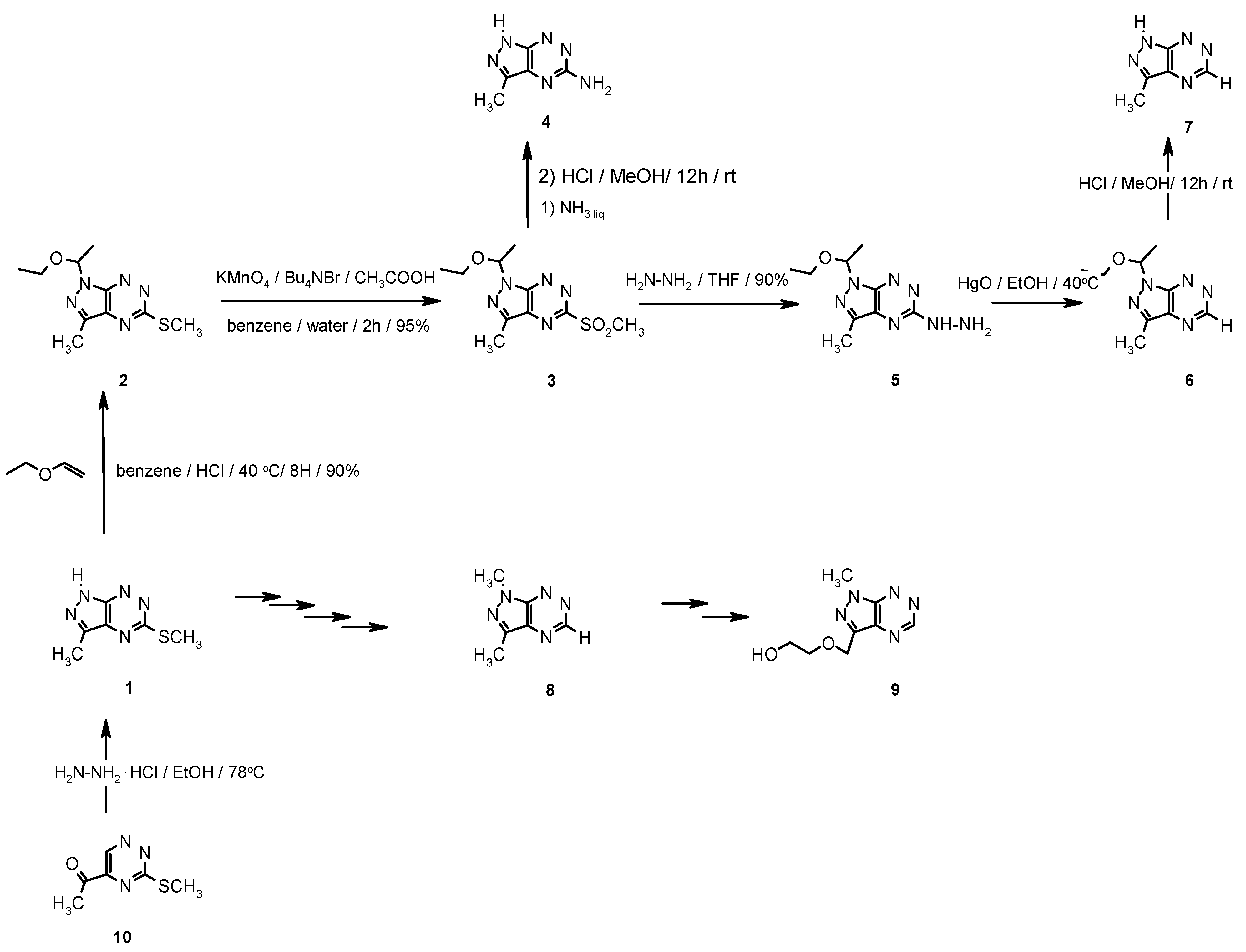
Results and Discussion
Chemistry
Spectral properties
| Compound | pH | λmax [nm] | εmax [cm-1M-1] | fluorescence λmax [nm] |
|---|---|---|---|---|
| 7 | 7 | 346 | 2270 | 480wa |
| 12 | 418 | 1450 | nfb | |
| 4 | 7 | 412 | 1570 | 490w |
| 12 | 453 | 780 | nf | |
| 1 | 7 | 408 | 2300 | nf |
| 12 | 438 | 1370 | nf | |
| 9 | 7 - 12 | 356 | 2460 | 490w |
| 8 | 7 - 12 | 370 | ~2400 | ndc |
Enzymatic assays
| Compound | Inhibition of the E. coli PNP | Inhibition of Xox | ||||
|---|---|---|---|---|---|---|
| substrate | substrate | |||||
| 7 | m7Guo | 32 | ~700 | Hx | 48 | ~800 |
| 131 | ~700 | |||||
| 4 | m7Guo | 8.8 | ~900 | Hx | 145 | NIa |
| 65.7 | >1000 | |||||
| 1 | m7Guo | 32 | ~40 | Hx | 145 | ~300 |
| 131 | ~30 | |||||
| 8 | - | - | ndb | Hx | 48 | NI |
| 9 | m7Guo | 32 | NI | - | - | nd |
| 131 | NI | |||||
Conclusions
Experimental
General
Syntheses
1-(1-Ethoxyethyl)-3-methyl-5-methylsulfanyl-1H-pyrazolo[4,3-e][1,2,4]triazine (2)
1-(1-Ethoxyethyl)-3-methyl-5-methylsulfanyl-1H-pyrazolo[4,3-e][1,2,4]triazine (3)
5-Amino-3-methyl-1H-pyrazolo[4,3-e][1,2,4]triazine (4)
1-(1-Ethoxyethyl)-3-methyl-5-hydrazino-1H-pyrazolo[4,3-e][1,2,4]triazine (5)
1-(1-Ethoxyethyl)-3-methyl-1H-pyrazolo[4,3-e][1,2,4]triazine (6)
3-Methyl-1H-pyrazolo[4,3-e][1,2,4]triazine (7)
Acknowledgements
References
- Montgomery, J. A. Studies on the biologic activity of purine and pyrimidine analogs. Med. Res. Rev. 1982, 2, 271–308. [Google Scholar] [CrossRef]
- Plunkett, W.; Saunders, PP. Metabolism and action of purine nucleoside analogs. Pharmacol. Therapeut. 1991, 49, 239–68. [Google Scholar]
- Parker, W. B.; Secrist, J. A., 3rd.; Waud, W. R. Purine nucleoside antimetabolites in development for the treatment of cancer. Curr. Op. Invest. Drugs 2004, 5, 592–596. [Google Scholar]
- Albert, A. A. Chemistry of 8-azapurines. Adv. Heterocycl. Chem. 1986, 39, 117–178. [Google Scholar]
- Wierzchowski, J.; Wielgus-Kutrowska, B.; Shugar, D. Fluorescence emission properties of 8-azapurines and their nucleosides, and application to the kinetics of the reverse synthetic reaction of purine nucleoside phosphorylase. Biochim. Biophys. Acta 1996, 1290, 9–17. [Google Scholar]
- Sugiyama, T.; Schweinberger, E.; Kazimierczuk, Z.; Ramzaeva, N.; Rosemeyer, H.; Seela, F. 2-aza-2'-deoxyadenosine: synthesis, base-pairing selectivity, and stacking properties of oligo-nucleotides. Chem. Eur. J. 2000, 6, 369–78. [Google Scholar]
- Smirnov, V. V.; Kiprianova, E. A.; Garagulya, A. D.; Esipov, S. E.; Dovjenko, S. A. Fluviols, bicyclic nitrogen-rich antibiotics, produced by Pseudomonas Fluorescens. FEMS Microbiol. Lett. 1997, 53, 357–361. [Google Scholar]
- Hirata, K.; Nakagami, H.; Takashina, J.; Mahmud, T.; Kobayashi, M.; In, Y.; Ishida, T.; Miyamoto, K. Novel violet pigment, nostocine A, an extracellular metabolite from cyanobacterium Nostoc spongiaeforme. Heterocycles 1996, 43, 1513–1519. [Google Scholar]
- Mąkosza, M.; Wojciechowski, K. Nucleophilic aromatic substitution of hydrogen as a tool for the synthesis of indole and quinoline derivatives. Heterocycles 2001, 54, 445–474. [Google Scholar] [CrossRef]
- Rykowski, A.; Olender, E.; Branowska, D.; van der Plas, H. C. A novel synthesis of 2-acylpyridines via inverse electron demand diels-alder reaction of 5-acyl-1,2,4-triazines. Org. Prep. Proced. Int. 2001, 33, 501–504. [Google Scholar]
- Branowska, D.; Ostrowski, S.; Rykowski, A. Tandem vicarious nucleophilic substitution of hydrogen/intramolecular Diels-Alder reaction of 1,2,4-triazines into functionalized cycloalkenopyridines. Chem. Pharm. Bull. 2002, 50, 463–466. [Google Scholar]
- Mojzych, M.; Rykowski, A. One-step synthesis and regioselective alkylation of substituted 1H-pyrazolo-[4,3-e][1,2,4]triazine. Polish J. Chem. 2003, 77, 1797–1804. [Google Scholar]
- A. Rykowski, A.; Lipińska, T. A concise router to a key intermediate In the total synthesis of sempervirine. Synth. Commun. 1996, 26, 4409. [Google Scholar]
- Vasilevsky, S. F.; Klyatskaya, S. V.; Tretyakov, E. V.; Elguero, J. Ethyl Vinyl Ether - an Agent for Protection of the Pyrazole NH-Fragment. A Convenient Method for the Preparation of N-Unsubstituted 4-Alkynylpyrazoles. Heterocycles 2003, 60, 879–886. [Google Scholar]
- Lindner, H. J.; Schaden, G. Pyrazolo-[4,3-e]-as-triazine, ein neues heterocyclisches System aus Pseudomonas Fluorescens var. pseudo-iodinum. Chem. Ber. 1970, 105, 1949–1955. [Google Scholar]
- Kulikowska, E.; Bzowska, A.; Wierzchowski, J.; Shugar, D. Properties of two unusual, and fluorescent substrates of purine-nucleoside phosphorylase: 7-methylguanosine and 7-methyl-inosine. Biochim. Biophys. Acta 1986, 874, 355–366. [Google Scholar]
- Kierdaszuk, B.; Modrak-Wójcik, A.; Wierzchowski, J.; Shugar, D. Formycin A and its N-methyl analogues, specific inhibitors of E. coli purine nucleoside phosphorylase: induced tautomeric shift on binding to enzyme, and enzyme → ligand fluorescence resonance energy transfer. Biochim. Biophys. Acta 2000, 1476, 109–128. [Google Scholar]
- Neet, K. E. Cooperativity in enzyme function: equilibrium and kinetic aspects. Methods Enzymol. 1980, 64, 139–192. [Google Scholar]
- Bzowska, A.; Kulikowska, E.; Shugar, D. Purine nucleoside phosphorylases: properties, functions and clinical aspects. Pharmacol. Therapeut. 2000, 88, 349–425. [Google Scholar]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar]
- Curlee, K. V.; Parker, W. B.; Sorscher, E. J. Tumor sensitization to purine analogs by E. coli PNP. Method. Mol. Med. 2004, 90, 223–45. [Google Scholar]
- Heinz, F.; Reckel, S. Xanthine oxidase. In Methods of Enzymatic Analysis, 3rd Edition; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, 1983; Vol. III, pp. 210–216. [Google Scholar]
- Mojzych, M.; Rykowski, A. Polish Pat. Appl. P372614, 2005.
- Sample Availability: Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mojzych, M.; Rykowski, A.; Wierzchowski, J. Pyrazolo[4,3-e][1,2,4]triazines: Purine Analogues with Electronic Absorption in the Visible Region. Molecules 2005, 10, 1298-1306. https://doi.org/10.3390/10101298
Mojzych M, Rykowski A, Wierzchowski J. Pyrazolo[4,3-e][1,2,4]triazines: Purine Analogues with Electronic Absorption in the Visible Region. Molecules. 2005; 10(10):1298-1306. https://doi.org/10.3390/10101298
Chicago/Turabian StyleMojzych, Mariusz, Andrzej Rykowski, and Jacek Wierzchowski. 2005. "Pyrazolo[4,3-e][1,2,4]triazines: Purine Analogues with Electronic Absorption in the Visible Region" Molecules 10, no. 10: 1298-1306. https://doi.org/10.3390/10101298




