Alumina and Silica Oxides as Catalysts for the Oxidation of Benzoins to Benzils under Solvent-free Conditions
Abstract
:Introduction
Results and Discussion
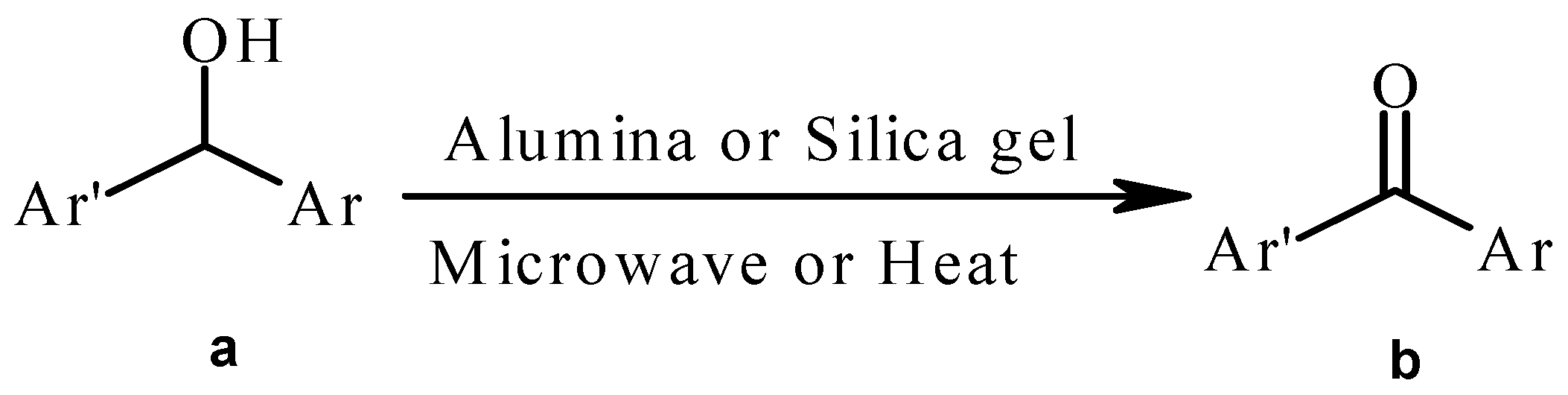
| Compound | Substituents | Time (min) | Yield (%) b |
|---|---|---|---|
| 1 | Ar′ = C6H5, Ar =C6H5-CO | 10 | 100 |
| 2 | Ar′ = C6H5, Ar = 4-Me2NC6H4-CO | 60 | 95 |
| 3 | Ar′ = 4-MeOC6H4, Ar = 4-MeOC6H4-CO | 180 | 100 |
| 4 | Ar′ = C6H5, Ar =4-MeOC6H4-CO | 65 | 90 |
| 5 | Ar′ = Ar = C6H5 | N.R.c | -- |
| 6 | Ar′ = C6H5, Ar = H | N.R. | -- |
| Compound | Time (min) | Yield (%) |
|---|---|---|
| 1 | 5 | 100 |
| 2 | 5 | 95 |
| 3 | 5 | 90 |
| 4 | 5 | 70 |
| 5 | N.R. | -- |
| 6 | N.R. | -- |
| Compound | Time (min) | Yield (%) |
|---|---|---|
| 1 | 180 | 100 |
| 2 | 180 | 70 |
| 3 | 150 | 90 |
| 4 | b | 100 |
| 5 | N.R. | -- |
| 6 | N.R. | -- |
| Compound | Time (min) | Yield (%) |
|---|---|---|
| 1 | 5 | 95 |
| 2 | 10 | 95 |
| 3 | 15 | 85 |
| 4 | 15 | 70 |
| 5 | N.R. | -- |
| 6 | N.R. | -- |
Conclusions
Experimental
General
Typical procedure for the oxidation of benzoins to benzils on alumina or silica gel.
Acknowledgements
References
- Mitra, A. K.; De, A.; Karchaudhuri, N. J. Chem. Res. (S) 1999, 246.(b)Vogel, A. Textbook of Practical Organic Chemistry including Qualitative Organic Analysis, 4th. Ed.; Longman: New York, 1978; p. 883. [Google Scholar](c)Joul, J. A.; Mills, K.; Smith, G. F. Heterocyclic Chemistry, 3rd Ed.; Chapman & Hall: London, 1995. [Google Scholar]
- Iranpoor, N.; Firouzabadi, H.; Zolfigol, M. A. Bull. Chem. Soc. Jpn. 1998, 71, 905.
- Hendrickson, J. B.; Schwartzman, S. M. Tetrahedron Lett. 1975, 65, 703.
- Morimoto, T.; Hirano, M.; Hamaguchi, T.; Shimoyama, M.; Zhuang, X. Bull. Chem. Soc. Jpn. 1992, 65, 703.
- Yamamoto, J.; Ito, S.; Tsuboi, T.; Tsuboi, T.; Tsukihara, K. Bull. Chem. Soc. Jpn. 1985, 58, 470.
- McKillop, A.; Swann, B. P.; Ford, M. E.; Taylor, E. C. J. Am. Chem. Soc. 1973, 95, 3641.
- Hatanaka, Y.; Imamoto, T.; Yokoyama, M. Tetrahedron Lett. 1983, 24, 2399.
- Hammond, G. S.; Wu, S. C. J. Am. Chem. Soc. 1973, 95, 8215.
- Kar, S. K.; Kar, A. J. Org. Chem. 1977, 42, 390.
- Dabbagh, H. A.; Mohammad Salehi, J. J. Org. Chem. 1998, 63, 7619.
- Dabbagh, H. A.; Hughes, H. G.; Davis, B. H. J. Catal. 1992, 133, 445.
- Dabbagh, H. A.; Davis, B. H. J. Org. Chem. 1990, 55, 2011.
- Dabbagh, H. A.; Davis, B. H. J. Catal. 1988, 110, 416.
- Dabbagh, H. A.; Davis, B. H. J. Mol. Catal. 1988, 47, 123.
- Hajipour, A. R.; Mallakpour, S. E.; Backnejad, H. Synth. Commun. 2000, 30, 3855.
- Hajipour, A. R.; Mallakpour, S. E.; Khoee, S. Synlett. 2000, 740.
- Hajipour, A. R.; Mallakpour, S. E.; Adibi, H. Chem. Lett. 2000, 460.
- Hajipour, A. R.; Mallakpour, S. E.; Adibi, H. Chem. Lett. 2001, 164.
- Hajipour, A. R.; Mallakpour, S. E.; Mohammadpour-Baltork, I.; Malakoutikhah, M. Tetrahedron 2002, 143.
- Varma, R. S.; Saini, R. K.; Dahiya, R. J. Chem. Res. (S) 1998, 120.
- Bolt, P. H.; Habraken, F. H. P. M.; Geus, J. W. J. Chem. Res. (S) 1998, 50.
- Varma, R. S.; Saini, R. K. Tetrahedron Lett. 1998, 39, 1481.
- Kotsuki, H.; Shimanouchi, T. Tetrahedron Lett. 1996, 37, 1845.
- Pérez, J. M.; López-Alvarado, P.; Ángel Alonso, M.; Avendaño, C.; Menéndez, J. C. Tetrahedron Lett. 1996, 37, 6955.
- Yong-Li, Z.; Tony, K. M. Shing. J. Org. Chem. 1997, 62, 2622.
- Kadilkar, B. M.; Borkar, S. D. Tetrahedron Lett. 1997, 38, 1641.
- Ide, W. S.; Buck, J. S. Organic React. (N. Y.) 1979, 4, 269.
- Firouzabadi, H.; Sardarian, A.; Badparva, H. Bull. Chem. Soc. Jpn. 1996, 69, 685.
- Sample availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Noroozi –Pesyan, N.; Dabbagh, A.H. Alumina and Silica Oxides as Catalysts for the Oxidation of Benzoins to Benzils under Solvent-free Conditions. Molecules 2005, 10, 1364-1368. https://doi.org/10.3390/10111364
Noroozi –Pesyan N, Dabbagh AH. Alumina and Silica Oxides as Catalysts for the Oxidation of Benzoins to Benzils under Solvent-free Conditions. Molecules. 2005; 10(11):1364-1368. https://doi.org/10.3390/10111364
Chicago/Turabian StyleNoroozi –Pesyan, N., and A. H. Dabbagh. 2005. "Alumina and Silica Oxides as Catalysts for the Oxidation of Benzoins to Benzils under Solvent-free Conditions" Molecules 10, no. 11: 1364-1368. https://doi.org/10.3390/10111364




