Thermal Behaviour, Biological Activity and Conformational Study of a [Methoprene/β-Cyclodextrin] Complex in a Smoke Generating Formulation
Abstract
:Introduction

Results and Discussion
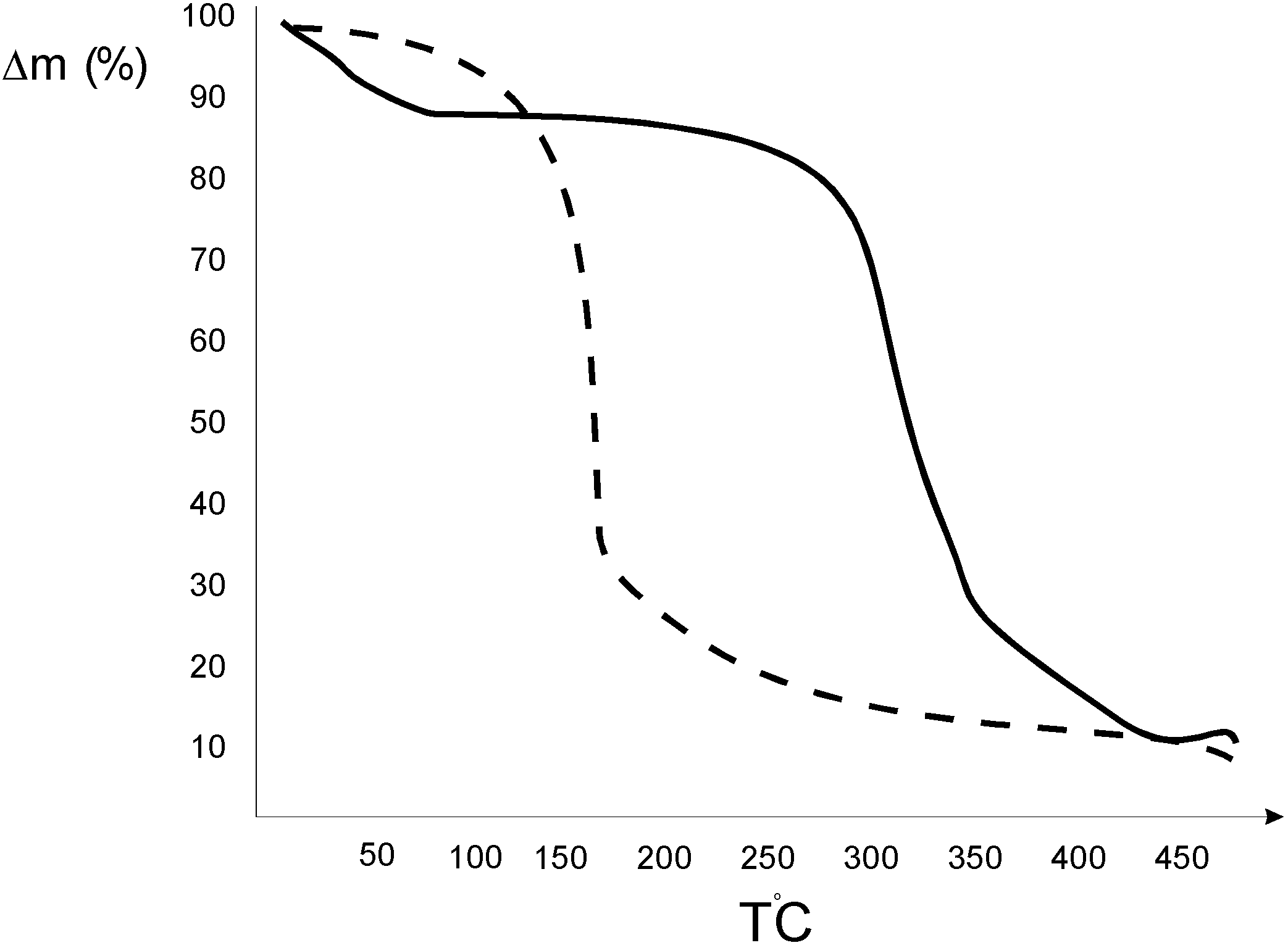
Characterisation of the [methoprene / ß-cyclodextrin] complex
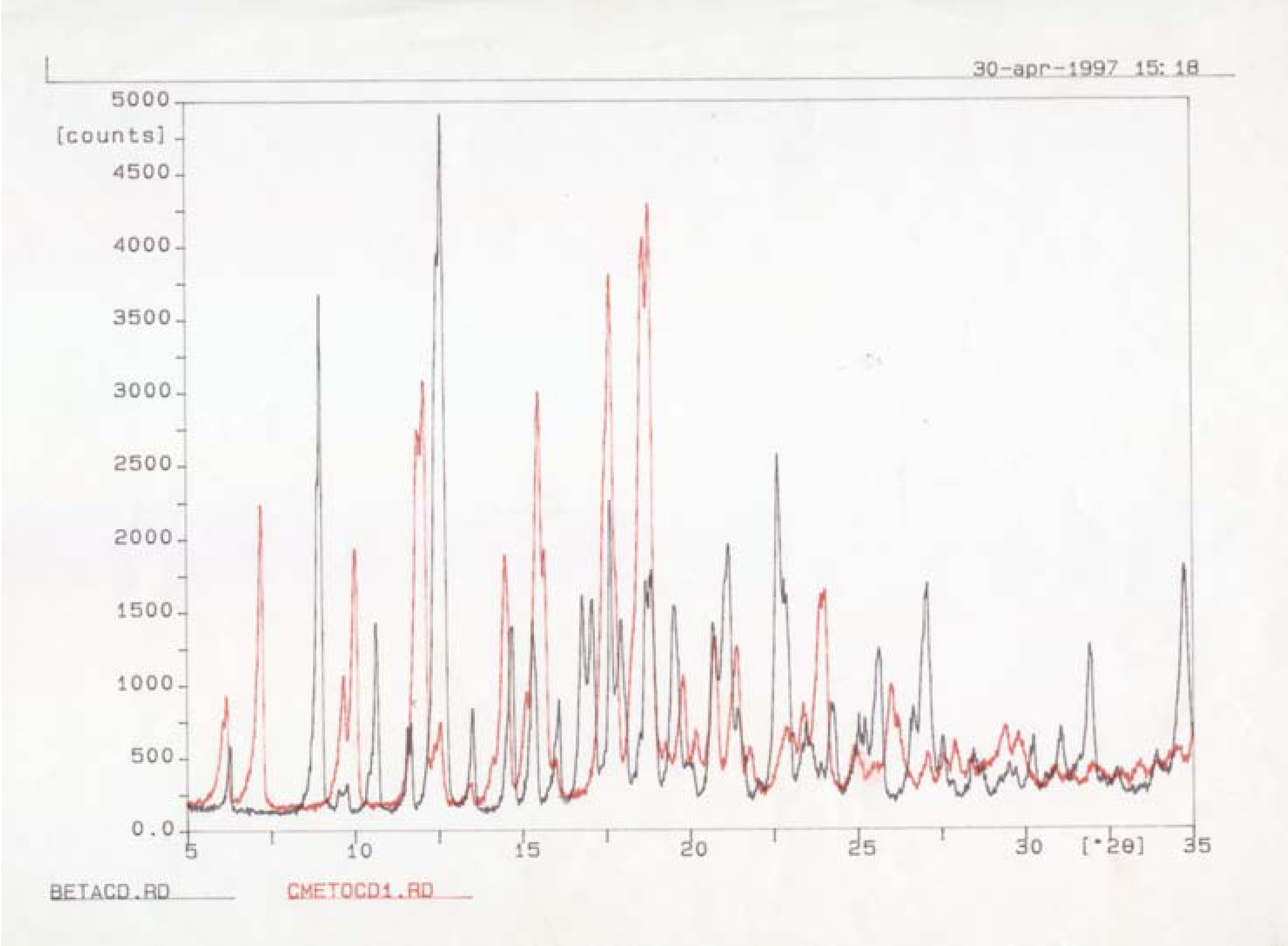
Thermo- analytical investigations
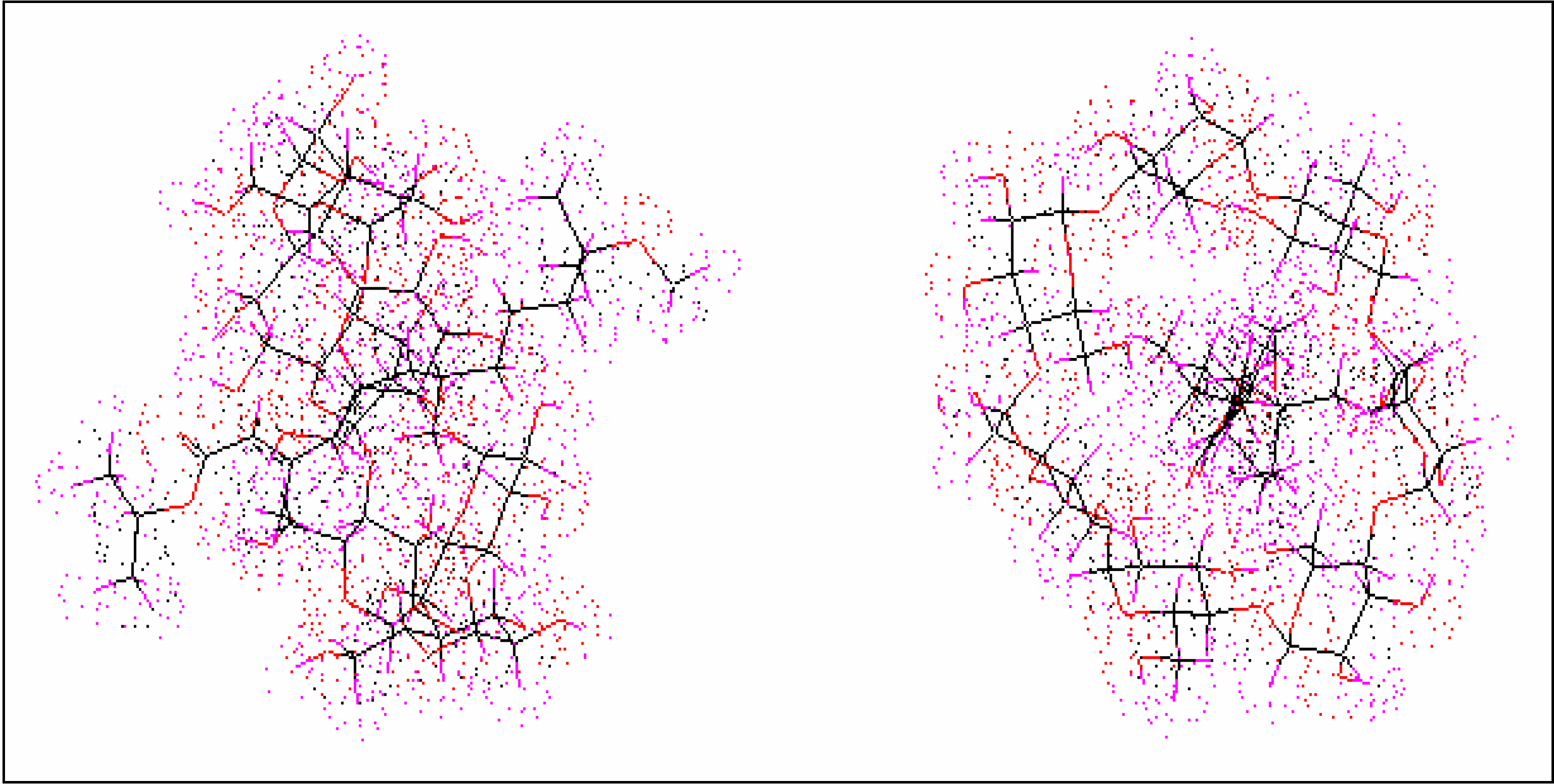
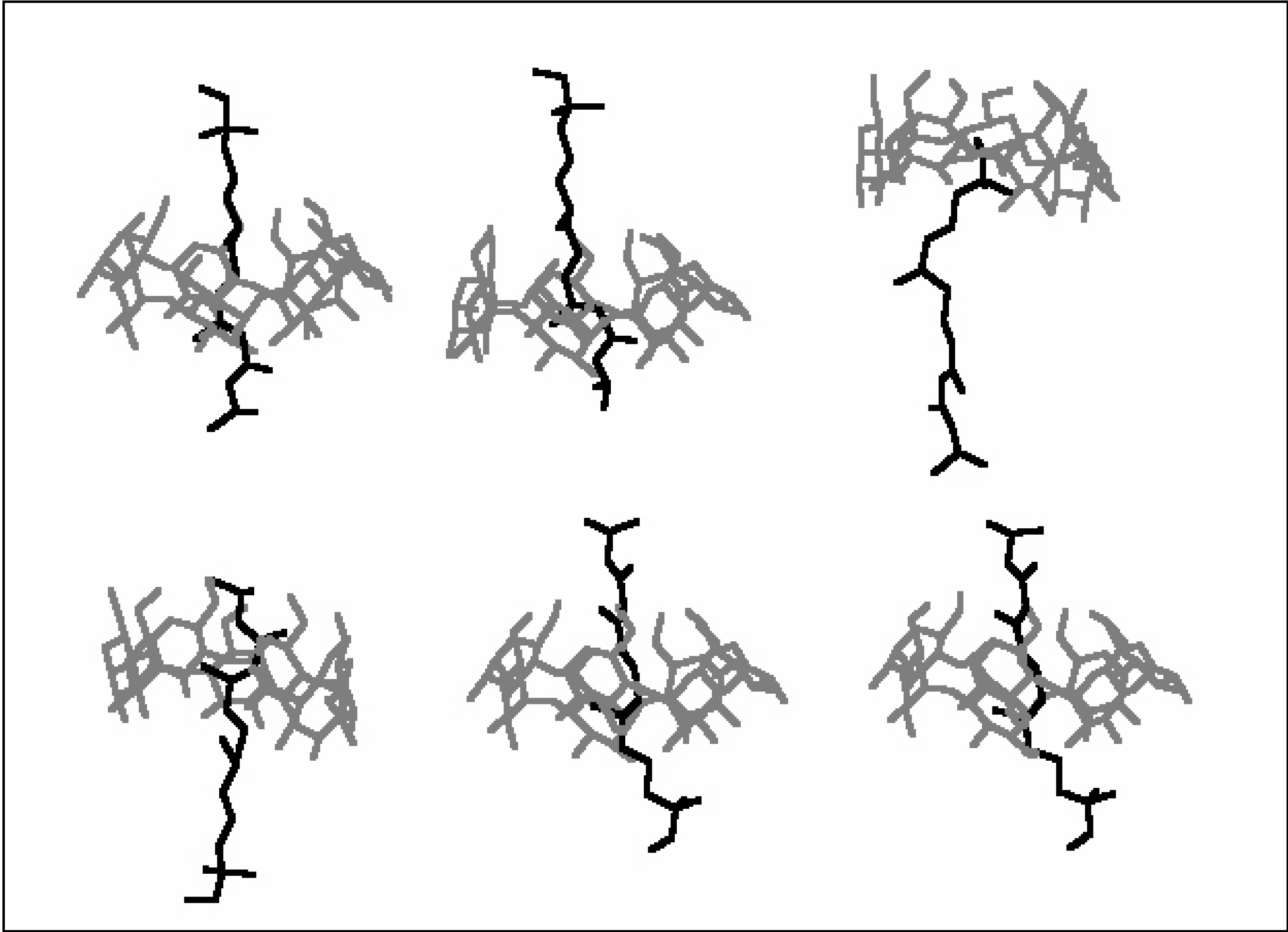
Conformational analysis
| Structure | Energy (kcal/mol) |
|---|---|
| 1 | -25.94 |
| 2 | -24.94 |
| 3 | -20.31 |
| 4 | -17.39 |
| 5 | -29.18 |
| 6 | -30.76 |
| Total interaction Energy (kcal/mol) | -31.7 |
|---|---|
| Bond | |
| Angle | |
| Dihedral | |
| Electrostatic | |
| Stretch-Bond | |
| Van der Waals |
Methoprene recovery in fumes
| Fumigant matrix composition | Methoprene formulationa | % Recovery |
|---|---|---|
| KClO3, dextrin and kaolin | Free | b.d.l.b |
| KClO3, dextrin and kaolin | complexed in ß-CD | b.d.l.b |
| plus 40% adc | Free | 25 +/-3 |
| plus 40% adc | complexed in ß-CD | 48+/- 2 |
Biological Assay
| Status of methoprene | Concentration (mg/m3) | Pupation % | Emergence % |
|---|---|---|---|
| Free | 6 | 77.5+/-3.5 | 22+/-3 |
| 20 | 42.5+/-7.5 | 18+/-3.5 | |
| In BCD | 6 | 77.5+/-4 | 0 |
| 20 | 50+/-12 | 0 | |
| Control | 0 | 92.5 +/-5 | 90+/-5 |
Experimental
Chemicals
Preparation of [methoprene / BCD] complex
Thermo-analytical Analysis
X-Ray powder diffraction
Smoke releasing mixtures
Conformational analysis
Recovery of methoprene in fumes
Biological Material
Biological Assay
Acknowledgements
References
- Copping, L.G.; Hewitt, G. Chemistry and Mode of Action of Crop Protection Agents; The Royal Society of Chemistry, Redwood Books Ltd.: London, 1998; p. 61. [Google Scholar]
- Duhnam, L.; Miller, W. Analytical methods for Pesticides and Plant Growth Regulators; Zweig, G., Sherma, J., Eds.; Academic Press Inc.: New York, 1963; Vol. 10, pp. 95–109. [Google Scholar]
- Zerba, E.; Wood, E.; Melgar, F.; Bazán, D.; Licastro, S.A.; Villar, M.I.P. de; Malkenson, N.C.; Fontán, A.; Segovia, S. Nuevas Formulaciones Fumígenas para el Control de Vectores de la Enfermedad de Chagas. Chagas. 1988, 5, 2–5. (in Spanish). [Google Scholar]
- Zerba, E. Fumigant Canisters and other Novel Insecticide Formulations. Public Health (Bayer) 1995, 12, 63–68. [Google Scholar]
- Zerba, E. Development of new Insecticides and Synergistic Formulations for the Chagas’ Disease vector Control. Rev. Arg. Microbiol. 1988, 20, 25–31. [Google Scholar]
- Zerba, E. Insecticidal Activity of pyrethroids on insects of medical importance. Parasitol. Today. 1988, 4, 53–57. [Google Scholar]
- González Audino, P.; Licastro, S.; Zerba, E. Thermal behaviour and biological activity of pyrethroids in smoke-generating formulations. Pestic. Sci. 1999, 55, 1187–1193. [Google Scholar]
- Anonymous, Cyclodextrins Kleptose®, General Information, Pharmaceutical and Cosmetic Department, ROQUETTE.
- Stella, V.J.; Rajewsky, R. A. Cyclodextrins: Their future in Drug Formulation and Delivery. Pharmaceut. Res. 1997, 14, 556–567. [Google Scholar]
- Kostense, A.S.; van Helden, S.P.; Janssen, L.H.M. Modelling and Conformation analysis of β-cyclodextrin complexes. J. Comput. Aid. Mol. Des. 1991, 5, 525–543. [Google Scholar]
- Carney, R.L. Processo para preparacao de un complexo de inclusao de ciclodextrina, tal complexo, composicao insecticida, e proceso para o controle de insetos. Brasil Patent 401448 A, 1985. (In Portuguese)[Google Scholar]
- Gammon, D.W.; Brown, M.A.; Casida, J.E. Pestic. Biochem. Physiol. 1981, 15, 81–191.
- Katsuta, Y. Stable insecticidal fumigant containing pyrethroids, antioxidant and organic foaming agent for cockroach control. Japanese Patent 0209605 A2, 1990. [Google Scholar]
- Allinger, N. L. J. Am. Chem. Soc. 1977, 99, 8127–8234.
- Manunza, B.; Deiana, S.; Pintore, M.; Delogu, G.; Gessa, L. A Molecular Modelling Study of interaction between β-cyclodextrin and Synthetic Pyrethroids. Carbohyd. Res. 1997, 300, 89–93. [Google Scholar]
- Loukas, Y.L.; Antoniadou-Vyza, E.; Papadaki-Valirak, A.; Machera, K.G. γ-Cyclodextrin inclusion complex of a new organophosphorous insecticide. Determination of stability constant with HPLC. J. Agric. Food Chem. 1994, 44–48. [Google Scholar]
- Szente, L.; Magisztrak, H.; Szejtli, J. Formulation of insect controlling agents with β-cyclodextrin. J. Pestic. Sci. 1990, 28, 7–16. [Google Scholar]
- Qureschi, R.A.; Quadri, S.; Anwarullah, M.; Navqui, S.N.H. Effect of neo pesticides (JHA’S) on the morphology , emergence and sterility in Musca domestica PCSIR strain and its relation to phosphatases. Sonderdruck aus Bd. 1983, 95, 304–309. [Google Scholar]
- Sample availability: Contact the authors
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Audino, P.G.; Masuh, H.; Zerba, E. Thermal Behaviour, Biological Activity and Conformational Study of a [Methoprene/β-Cyclodextrin] Complex in a Smoke Generating Formulation. Molecules 2005, 10, 534-544. https://doi.org/10.3390/10030534
Audino PG, Masuh H, Zerba E. Thermal Behaviour, Biological Activity and Conformational Study of a [Methoprene/β-Cyclodextrin] Complex in a Smoke Generating Formulation. Molecules. 2005; 10(3):534-544. https://doi.org/10.3390/10030534
Chicago/Turabian StyleAudino, Paola González, Hector Masuh, and Eduardo Zerba. 2005. "Thermal Behaviour, Biological Activity and Conformational Study of a [Methoprene/β-Cyclodextrin] Complex in a Smoke Generating Formulation" Molecules 10, no. 3: 534-544. https://doi.org/10.3390/10030534




