Experimental and Theoretical Studies on the Functionalization Reactions of 4-Benzoyl-1,5-Diphenyl-1H-Pyrazole-3-Carboxylic Acid and Acid Chloride with 2,3-Diaminopyridine
Abstract
:Introduction

Results and Discussion
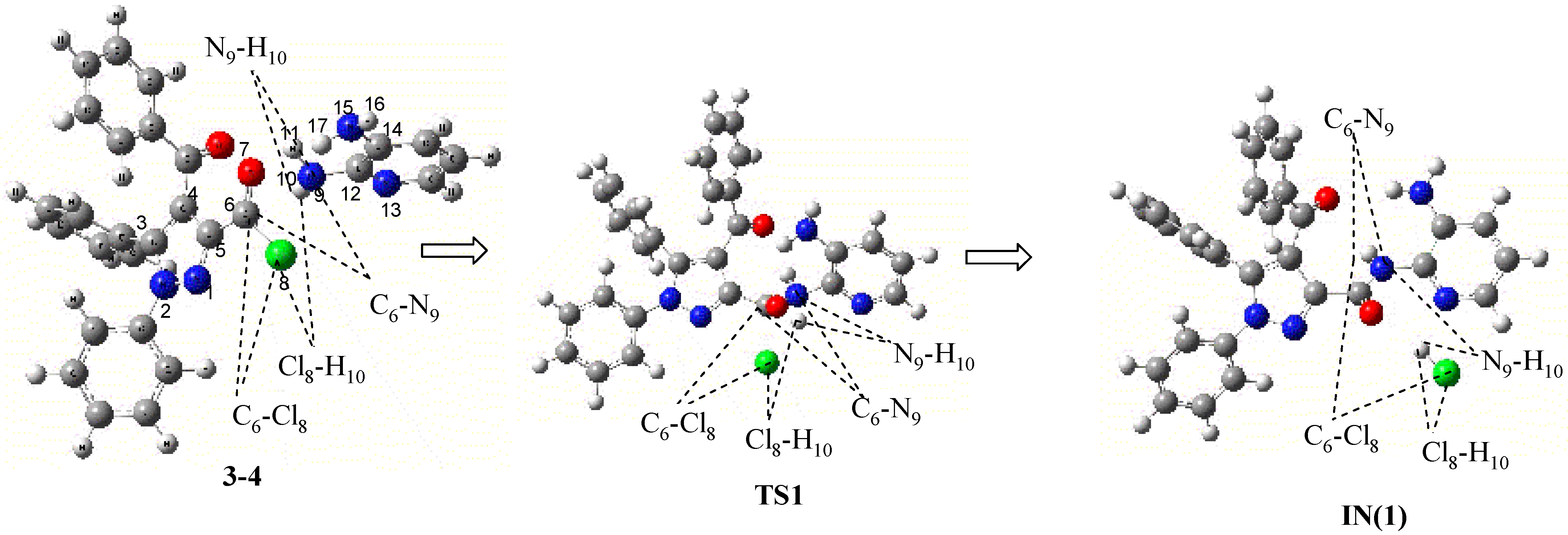
| Atoms/Bonds | 3-4 | TS1 | IN(1) | ||||||
| AM1 | RHF STO-3G | RHF 3-21G | AM1 | RHF STO-3G | AM1 | RHF STO-3G | RHF 3-21G | ||
| INTERATOMIC DISTANCES (in Å) | C6-N9 | 3.11 | 3.65 | 3.22 | 1.55 | 1.61 | 1.38 | 1.38 | 1.35 |
| C6- O7 | 1.23 | 1.21 | 1.19 | 1.24 | 1.21 | 1.24 | 1.25 | 1.22 | |
| C6-Cl8 | 1.73 | 1.82 | 1.85 | 2.23 | 2.34 | 4.49 | 3.49 | 4.12 | |
| N9-H10 | 1.00 | 1.03 | 1.00 | 1.07 | 1.06 | 3.79 | 2.68 | 3.44 | |
| N10-H11 | 1.00 | 1.03 | 1.00 | 1.02 | 1.04 | 1.00 | 1.04 | 1.00 | |
| N9-C12 | 1.41 | 1.44 | 1.38 | 1.46 | 1.48 | 1.42 | 1.45 | 1.41 | |
| C12-C14 | 1.40 | 1.44 | 1.39 | 1.39 | 1.43 | 1.40 | 1.44 | 1.37 | |
| C12-N13 | 1.36 | 1.34 | 1.31 | 1.36 | 1.34 | 1.36 | 1.35 | 1.30 | |
| N15-H16 | 1.00 | 1.03 | 1.00 | 1.00 | 1.03 | 1.00 | 1.03 | 1.00 | |
| N15-H17 | 1.00 | 1.03 | 1.00 | 1.00 | 1.03 | 1.00 | 1.03 | 1.00 | |
| C6-C5 | 1.46 | 1.50 | 1.45 | 1.49 | 1.52 | 1.49 | 1.52 | 1.50 | |
| C5-C4 | 1.45 | 1.43 | 1.41 | 1.45 | 1.42 | 1.45 | 1.44 | 1.43 | |
| C5-N1 | 1.39 | 1.33 | 1.30 | 1.36 | 1.33 | 1.36 | 1.33 | 1.30 | |
| Cl8-H10 | 4.32 | 5.37 | 4.39 | 2.00 | 1.97 | 1.30 | 1.45 | 1.33 | |
| Frequencies(cm-1) | -351 | -281 | |||||||
| Atoms/Bonds | 3-4 | TS1 | IN(1) | ||||||
| AM1 | RHF STO-3G | RHF 3-21G | AM1 | RHF STO-3G | AM1 | RHF STO-3G | RHF 3-21G | ||
| CHARGES (in electronoc units ē) | N1 | -0.01 | -0.12 | -0.34 | -0.01 | -0.20 | -0.01 | -0.12 | -0.32 |
| C4 | -0.17 | -0.06 | -0.33 | -0.24 | -0.05 | -0.22 | -0.07 | -0.49 | |
| C5 | -0.16 | 0.05 | 0.37 | -0.14 | 0.08 | -0.14 | 0.05 | 0.35 | |
| C6 | 0.34 | 0.28 | 0.44 | 0.41 | 0.37 | 0.40 | 0.36 | 0.99 | |
| O7 | -0.26 | -0.19 | -0.53 | -0.35 | -0.24 | -0.32 | -0.26 | -0.63 | |
| Cl8 | -0.06 | -0.18 | 0.05 | -0.56 | -0.69 | -0.23 | -0.53 | -0.40 | |
| N9 | -0.31 | -0.41 | -0.95 | -0.10 | -0.32 | -0.32 | -0.38 | -1.06 | |
| H10 | 0.19 | 0.17 | 0.33 | 0.31 | 0.32 | 0.23 | 0.27 | 0.31 | |
| H11 | 0.19 | 0.19 | 0.37 | 0.25 | 0.27 | 0.26 | 0.26 | 0.42 | |
| C12 | 0.05 | 0.18 | 0.74 | -0.06 | 0.17 | 0.06 | 0.18 | 0.78 | |
| N13 | -0.18 | -0.27 | -0.78 | -0.10 | -0.22 | -0.13 | -0.24 | -0.75 | |
| N15 | -0.32 | -0.40 | -0.96 | -0.32 | -0.39 | -0.32 | -0.38 | -1.03 | |
| H16 | 0.20 | 0.20 | 0.35 | 0.21 | 0.19 | 0.21 | 0.18 | 0.38 | |
| H17 | 0.17 | 0.16 | 0.32 | 0.19 | 0.19 | 0.18 | 0.18 | 0.34 | |

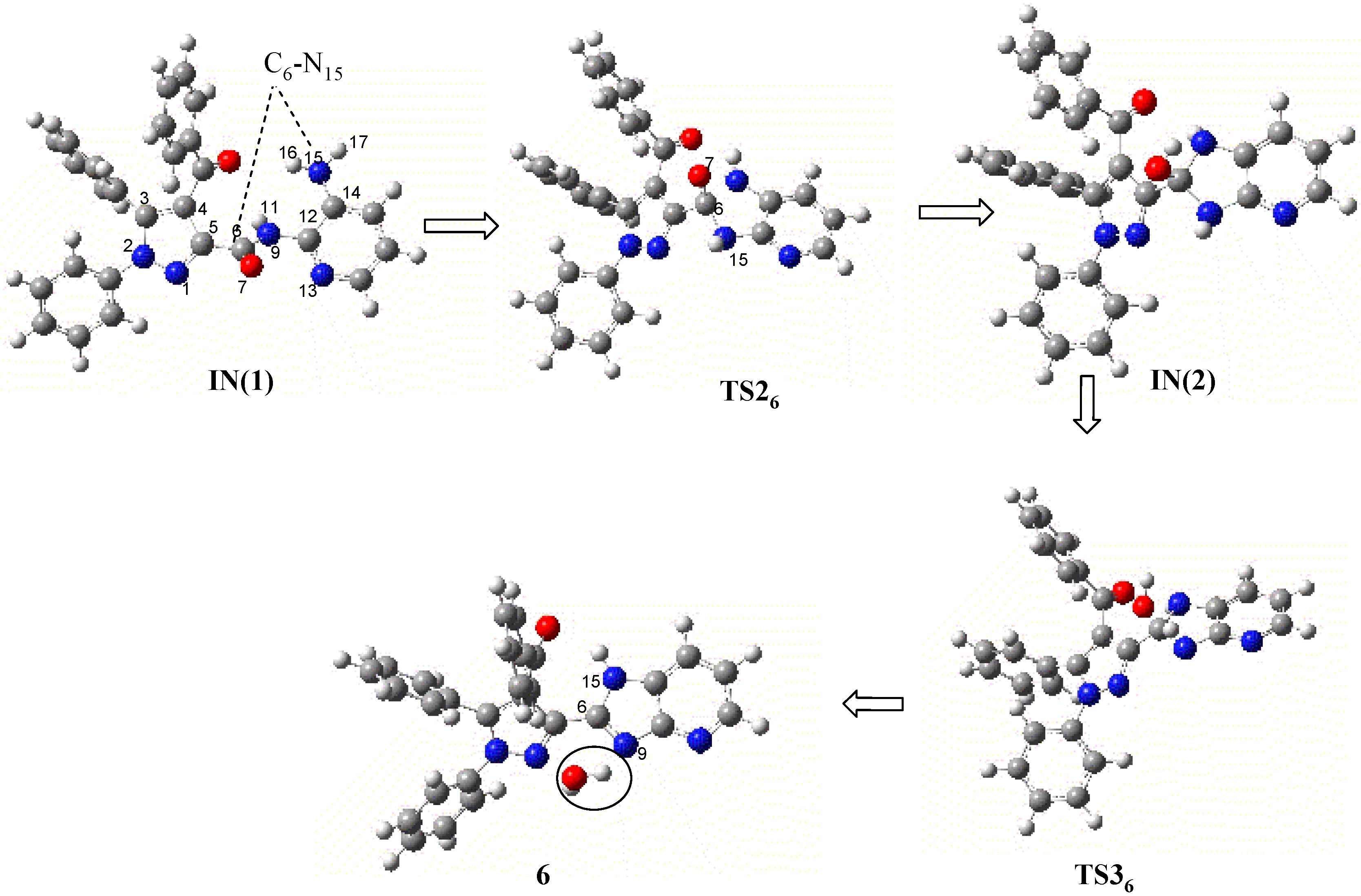
| Atoms/Bonds | IN(1) | TS25 | 5 | |
|---|---|---|---|---|
| INTERATOMIC DISTANCES (in Å) | C14-N15 | 1.40 | 1.44 | 1.41 |
| N15-H17 | 1.00 | 1.02 | 1.00 | |
| N15-H16 | 1.00 | 1.10 | 3.12 | |
| N15-C19 | 2.89 | 1.54 | 1.38 | |
| C19-O20 | 1.23 | 1.23 | 1.25 | |
| C21-Cl18 | 1.74 | 2.40 | 3.79 | |
| Cl18-H19 | 3.04 | 1.90 | 1.30 | |
| C19-C21 | 1.46 | 1.47 | 1.48 | |
| C21-N23 | 1.37 | 1.37 | 1.36 | |
| C21-C22 | 1.45 | 1.45 | 1.45 | |
| C12-N9 | 1.42 | 1.39 | 1.40 | |
| N9- C6 | 1.39 | 1.40 | 1.39 | |
| N9-H11 | 1.00 | 1.04 | 1.00 | |
| C6-O7 | 1.24 | 1.24 | 1.24 | |
| C6- C5 | 1.48 | 1.28 | 1.48 | |
| Frequencies(cm-1) | -274 | |||
| CHARGES (in electronoc units ē) | Atoms/Bonds | 5I | TS2 | 5 |
| N1 | -0.04 | -0.04 | -0.03 | |
| N2 | -0.16 | -0.07 | -0.06 | |
| C4 | -0.20 | -0.18 | -0.19 | |
| C5 | -0.09 | -0.11 | -0.10 | |
| C6 | 0.46 | 0.39 | 0.40 | |
| O7 | -0.37 | -0.30 | -0.34 | |
| N9 | -0.45 | -0.33 | -0.30 | |
| H11 | 0.33 | 0.32 | 0.26 | |
| C12 | 0.12 | 0.22 | 0.16 | |
| N13 | -0.19 | -0.18 | -0.17 | |
| C14 | 0.01 | -0.20 | -0.05 | |
| N15 | -0.47 | -0.10 | -0.33 | |
| H16 | 0.26 | 0.32 | 0.22 | |
| H17 | 0.25 | 0.22 | 0.26 | |
| Cl18 | -0.09 | -0.65 | -0.22 | |
| C19 | 0.38 | 0.40 | 0.40 | |
| O20 | -0.28 | -0.28 | -0.37 | |
| C21 | -0.14 | -0.13 | -0.13 | |
| C22 | -0.20 | -0.18 | -0.20 | |
| N23 | -0.02 | -0.01 | -0.04 |
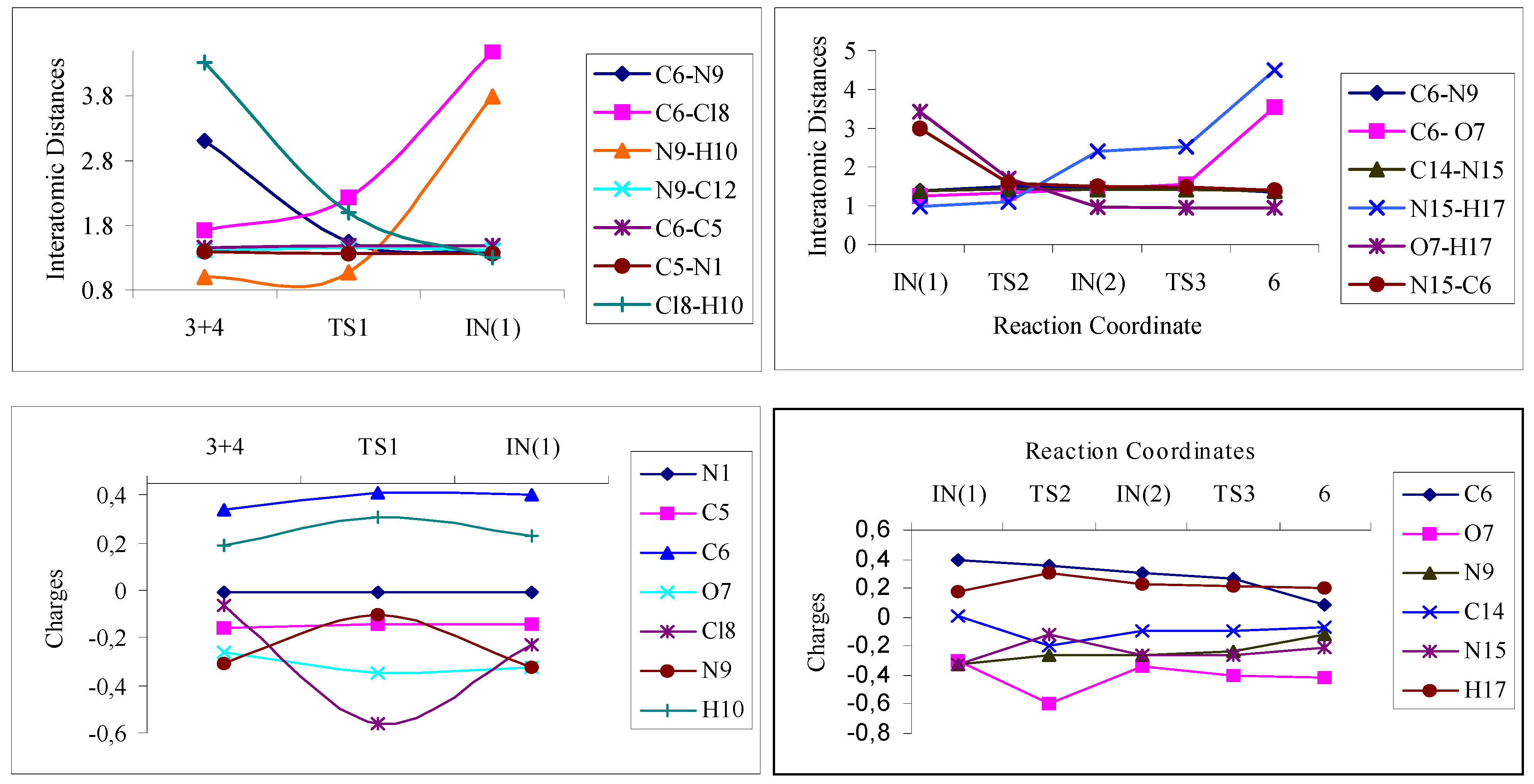
| Atoms/Bonds | IN(1) | TS26 | IN(2) | |||||||
| AM1 | RHFSTO-3G | RHF3-21G | AM1 | RHFSTO-3G | RHF3-21G | AM1 | RHFSTO-3G | RHF3-21G | ||
| INTERATOMIC DISTANCES (in Å) | C6-N9 | 1.38 | 1.45 | 1.36 | 1.51 | 1.52 | 1.46 | 1.51 | 1.47 | 1.44 |
| C6-O7 | 1.25 | 1.22 | 1.21 | 1.33 | 1.27 | 1.33 | 1.44 | 1.42 | 1.42 | |
| N10-H11 | 0.99 | 1.03 | 1.00 | 1.00 | 1.03 | 1.00 | 1.00 | 1.03 | 0.99 | |
| N9-C12 | 1.42 | 1.45 | 1.42 | 1.41 | 1.42 | 1.36 | 1.42 | 1.44 | 1.38 | |
| C12-N13 | 1.36 | 1.35 | 1.31 | 1.36 | 1.35 | 1.31 | 1.35 | 1.33 | 1.29 | |
| C12-C14 | 1.45 | 1.41 | 1.41 | 1.46 | 1.45 | 1.40 | 1.46 | 1.41 | 1.41 | |
| C14-N15 | 1.38 | 1.44 | 1.37 | 1.44 | 1.45 | 1.44 | 1.42 | 1.43 | 1.38 | |
| N15-H16 | 0.99 | 1.03 | 1.00 | 1.01 | 1.03 | 1.01 | 1.00 | 1.03 | 1.00 | |
| N15-H17 | 0.99 | 1.03 | 0.99 | 1.10 | 1.04 | 1.05 | 2.40 | 2.48 | 1.00 | |
| C6-C5 | 1.49 | 1.52 | 1.50 | 1.51 | 1.53 | 1.49 | 1.52 | 1.54 | 1.50 | |
| C5-C4 | 1.45 | 1.43 | 1.42 | 1.45 | 1.43 | 1.41 | 1.45 | 1.43 | 1.41 | |
| C5-N1 | 1.36 | 1.33 | 1.30 | 1.36 | 1.33 | 1.30 | 1.36 | 1.33 | 1.30 | |
| O7-H17 | 3.42 | 4.06 | 5.58 | 1.70 | 1.69 | 1.65 | 0.97 | 0.99 | 0.97 | |
| N15-C6 | 3.00 | 3.24 | 2.81 | 1.59 | 1.81 | 1.63 | 1.51 | 1.50 | 1.45 | |
| Frequencies(cm-1) | 2031 | -1854 | -1804 | |||||||
| Atoms/Bonds | IN(1) | TS26 | IN(2) | |||||||
| AM1 | RHFSTO-3G | RHF3-21G | AM1 | RHFSTO-3G | RHF3-21G | AM1 | RHFSTO-3G | RHF3-21G | ||
| CHARGES (in electronoc units ē) | N1 | -0.01 | -0.16 | -0.33 | -0.02 | -0.12 | -0.40 | -0.01 | -0.17 | -0.40 |
| C4 | -0.23 | -0.06 | -0.41 | -0.23 | 0.08 | -0.34 | -0.22 | -0.22 | -0.38 | |
| C5 | -0.13 | 0.04 | 0.36 | -0.10 | 0.06 | 0.46 | -0.10 | 0.06 | 0.53 | |
| C6 | 0.39 | 0.29 | 0.91 | 0.35 | 0.28 | 0.76 | 0.30 | 0.31 | 0.76 | |
| O7 | -0.30 | -0.24 | -0.58 | -0.59 | -0.30 | -0.74 | -0.34 | -0.30 | -0.68 | |
| N9 | -0.33 | -0.36 | -1.02 | -0.26 | -0.38 | -0.97 | -0.26 | -0.34 | -0.94 | |
| H11 | 0.26 | 0.21 | 0.40 | 0.23 | 0.27 | 0.39 | 0.23 | 0.20 | 0.38 | |
| C12 | 0.06 | 0.17 | 0.73 | 0.13 | 0.15 | 0.89 | 0.04 | 0.18 | 0.79 | |
| N13 | -0.11 | -0.25 | -0.71 | -0.16 | -0.26 | -0.78 | -0.14 | -0.26 | -0.77 | |
| C14 | 0.01 | 0.10 | 0.33 | -0.20 | 0.06 | 0.17 | -0.09 | 0.07 | 0.34 | |
| N15 | -0.33 | -0.38 | -1.01 | -0.12 | -0.38 | -1.01 | -0.26 | -0.36 | -1.01 | |
| H16 | 0.21 | 0.19 | 0. 37 | 0.23 | 0.23 | 0.42 | 0.22 | 0.20 | 0.39 | |
| H17 | 0.18 | 0.17 | 0.34 | 0.30 | 0.20 | 0.27 | 0.23 | 0.20 | 0.39 | |
Conclusions
Experimental
General
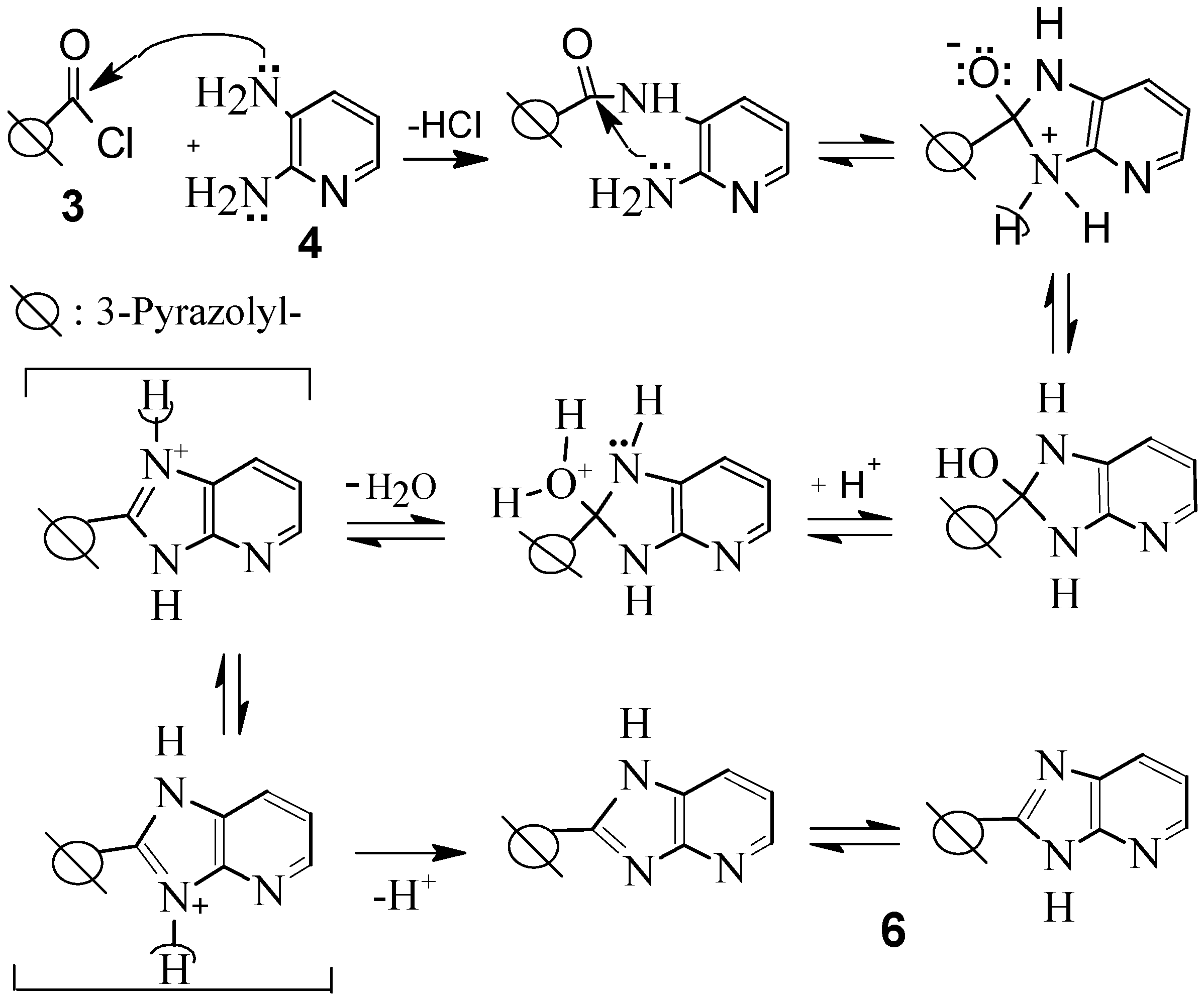
4-Benzoyl-1,5-diphenyl-N-(2-{[(4-benzoyl-1,5-diphenyl-1H-pyrazol-3-yl)carbonyl]amino}pyridin-3-yl)-1H-pyrazole-3-carboxamide (5).
Compound 5 was prepared by two methods as follows:
2-(4-Benzoyl-1,5-diphenyl-1H-pyrazol-3-yl)-3H-imidazo[4,5-b]pyridine-1,4-diiumdichloride (6).
Acknowledgements
References and Notes
- Ziegler, E.; Eder, M.; Belegratis, C.; Prewedourakis, E. Synthesen von heterocyclen, 103. Mitt.: Über reaktionen mit oxalylchlorid. Monatsh. Chem. 1967, 98, 2249–2251. [Google Scholar]
- Saalfrank, R.W.; Lutz, T.; Hörner, B.; Gündel, J.; Peters, K.; von Schnering, H.G. Vielseitige synthese von 2,3-dioxo-2,3-dihydrofuranen und alkylidenbutenoliden. Kristall- und molekülstruktur von 5-(4-chlorphenyl)-4-methoxycarbonyl-2,3-dioxo-2,3-dihydrofuran. Chem. Ber. 1991, 124, 2289–2295. [Google Scholar]
- Hökelek, T.; Sarıpınar, E.; Yıldırım, I.; Akkurt, M.; Akçamur, Y. 4-(4-Methoxybenzoyl)-5-(4-methoxyphenyl)-2,3-furandione. Acta Cryst. 2002, E58, 30–32. [Google Scholar]
- Ziegler, E.; Kollenz, G.; Eder, M.; Igel, H. Synthesen von heterocyclen, 160. Mitt.: Über reaktionen mit cyclischen oxalylverbindungen. Monatsh. Chem. 1971, 102, 1769–1773. [Google Scholar]
- Kollenz, G.; Kappe, C.O.; El-Nabi, H.A.A. On the reaction of 2,2,6,6,-tetramethyl-3,5-heptanedione “Dipivaloylmethane” with oxalyl chloride. Heterocycles 1991, 32, 669–673. [Google Scholar] [CrossRef]
- Ott, W.; Ziegler, E.; Kollenz, G. Umsetzung von 4-benzoyl-2,3-dioxo-5-phenyl-2,3-dihydrofuran mit o-phenylendiamin. Synthesis 1976, 477–478. [Google Scholar] [CrossRef]
- Kollenz, G.; Penn, G.; Dolenz, G.; Akçamur, Y.; Peters, K.; Peters, E.-M.; von Schnering, H.G. Untersuchungen von reaktionsmechanismen durch isotopenmarkierung, VIII. Zum bildungsweg der pyrrolo[2,3-d]pyrimidine aus 4-benzoyl-5-phenyl-2,3-furandion und arylisocyanaten. Chem. Ber. 1984, 117, 1299–1309. [Google Scholar]
- Ziegler, E.; Kollenz, G.; Ott, W. Reaktionen von 4-benzoyl-2,3-dioxo-5-phenyl-2,3-dihydro-furan mit Schiffschen basen. Synthesis 1973, 679–680. [Google Scholar] [CrossRef]
- Review: Kollenz G., Heilmayer W. Unexpected ring-rearrangements in hetero-Diels-Alder reactions. Trends Heterocycl. Chem. 1993, 3, 379–395. [Google Scholar]
- Altural, B.; Akçamur, Y.; Sarıpınar, E.; Yıldırım, I.; Kollenz, G. A simple synthesis of functionalized 1H-pyrimidines. Monatsh. Chem. 1989, 120, 1015–1020. [Google Scholar] [CrossRef]
- Yıldırım, I.; Sarıpınar, E.; Güzel, Y.; Patat, Ş.; Akçamur, Y. Theoretical investigations on the mechanism of interaction of 4-formyl furan-2,3-dion and urea. J. Mol. Struct. 1995, 334, 165–171. [Google Scholar]
- Yıldırım, I.; Tezcan, M.; Güzel, Y.; Sarıpınar, E.; Akçamur, Y. Quantum-chemical investigations on the functionalized 1H-pyrimidines. Turk J. Chem. 1996, 20, 27–32. [Google Scholar]
- Sarıpınar, E.; Yıldırım, I.; Güzel, Y.; Akçamur, Y. Investigation of the electronic structures of 1,4,5-substituted derivatives of 1H-pyrimidin-2-thione. Monatsh. Chem. 1996, 127, 505–512. [Google Scholar] [CrossRef]
- Yıldırım, I.; Ilhan, I.Ö. Functionalization and cyclization reactions of various acetanilides with 4-benzoyl-5-phenyl-2,3-furandion. Experimental and theoretical investigations. J. Heterocycl. Chem. 1997, 34, 1047–1051. [Google Scholar]
- Yıldırım, I.; Kandemirli, F. Experimental and theoretical studies on some new pyrrol-2,3-diones formation. Heteroatom. Chem. 2004, 15, 9–14. [Google Scholar] [CrossRef]
- Akçamur, Y.; Penn, G.; Ziegler, E.; Sterk, H.; Kollenz, G.; Peters, K.; Peters, E.-M.; von Schnering, H.G. Zur reaktion von 4-benzoyl-5-phenyl-furan-2,3-dion mit phenylhydrazonen bzw. phenylhydrazin. Monatsh. Chem. 1986, 117, 231–245. [Google Scholar] [CrossRef]
- Akçamur, Y.; Sener, A.; Ipekoglu, A.M.; Kollenz, G. Functionalization and cyclization reactions of 4-benzoyl-1,5-diphenyl-1H-pyrazole-3-carboxylic acid. J. Heterocycl. Chem. 1997, 34, 221–224. [Google Scholar] [CrossRef]
- Sener, A.; Kasımogulları, R.; Sener, M.K.; Bildirici, I.; Akçamur, Y. Studies on the reactions of cyclic oxalyl compounds with hydrazines or hydrazones: Synthesis and reactions of 4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxylic acid. J. Heterocycl. Chem. 2002, 39, 869–875. [Google Scholar] [CrossRef]
- Sener, A. One Step synthesis and some reactions of 7-hydrazino-3,4-diphenyl-2H-pyrazolo[3,4-d]pyridazine. Turk. J. Chem. 2004, 28, 39–45. [Google Scholar]
- Joule, J.A.; Mills, K.; Smith, G. F. Heterocyclic Chemistry, 3rd ed.; Chapman & Hall: London, UK, 1995; Chap. 22; pp. 402–405. [Google Scholar]
- Weissberger, A. (Ed.) Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings. In The Chemistry of Heterocyclic Compounds; Weissberger, A. (Ed.) Interscience Publishers: New York, 1967; Vol. 22, pp. 180–278.
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, 1984; Vol. 5, pp. 167–302. [Google Scholar]
- Daidone, G.; Raffa, D.; Plescia, F.; Maggio, B.; Roccaro, A. Synthesis of pyrazole-4-carbohydrazide derivatives of pharmaceutical interest. ARKIVOC 2002, XI, 227–235. [Google Scholar]
- Mahajan, R.N.; Havaldar, F.H; Fernandes, P.S. Syntheses and biological activity of heterocycles derived from 3-methoxy-1-phenyl-1H-pyrazole-5-carboxylate. J. Indian Chem. Soc. 1991, 68, 245–246. [Google Scholar]
- Baraldi, P.G.; Manfredini, S.; Romagnoli, R.; Stevanato, L.; Zaid, A.N.; Manservigi, R. Synthesis and anti-HSV-1 activity of 6 substituted pyrazolo[3,4-d]pyridazin-7-one nucleosides. Nucleos. Nucleot. 1998, 17, 2165–2173. [Google Scholar] [CrossRef]
- Hatheway, G.J.; Hansch, C.; Kim, K.H.; Milstein, S.R.; Schimidt, C.L.; Smith, R.N.; Quin, F.R. Antitumor 1-(X-aryl)-3,3-dialkyltriazenes. 1. Quantitative structure-activity relationships vs L1210 leukemia in mice. J. Med. Chem. 1978, 21, 563–574. [Google Scholar]
- Tewari, A.K.; Mishra, A. Synthesis and anti-inflammatory activities of N4,N5-disubstituted-3-methyl-1H-pyrazolo[3,4-c]pyridazines. Bioorg. Med. Chem. 2001, 9, 715–718. [Google Scholar] [CrossRef]
- Londershausen, M. Review: Approaches to New Parasiticides. Pestic. Sci. 1996, 48, 269–292. [Google Scholar] [CrossRef]
- Chen, H.S.; Li, Z.M. Chem. J. Chinese Univ. 1998, 19, 572–576.
- Lepage, F.; Hublot, B. Eur. Pat. Appl. EP 459 887; [Chem. Abstr. 1992, 116, 128917],
- Harnden, M.R.; Bailey, S.; Boyd, M.R.; Taylor, D.R.; Wright, N.D. Thiazolinone analogs of indolmycin with antiviral and antibacterial activity. J. Med. Chem. 1978, 21, 82–87. [Google Scholar] [CrossRef]
- Matyus, P. 3(2H)-Pyridazinones: Some recent aspects of synthetic and medicinal chemistry. J. Heterocycl. Chem. 1998, 35, 1075–1089, and references therein. [Google Scholar]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.J., Jr.; Burant, C.; Dapprich, S.; Millam, J.M.; Daniels, A.D.; Kudin, K.N.; Strain, M.C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G.A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A.G.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.P.; Gill, M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Andres, J.L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E.S.; Pople, J.A. Gaussian 98, Revision A.9; Gaussian, Inc.: Pittsburgh, PA, 1998. [Google Scholar]
- Polanyi, J.C.; Zewail, A.H. Direct Observation of the Transition State. Acc. Chem. Res. 1995, 28, 119–132. [Google Scholar] [CrossRef]
- Pedersen, S.; Herek, J.L.; Zevail, A.H. Science 1994, 266, 1293.
- Bassler, G.C.; Morrill, T.C.; Silverstein, R.M. Spectrometric Identification of Organic Compounds; John Wiley and Sons: New York; NY, 1991; pp. 108–282. [Google Scholar]
- Sample Availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yıldırım, I.; Kandemirli, F.; Demir, E. Experimental and Theoretical Studies on the Functionalization Reactions of 4-Benzoyl-1,5-Diphenyl-1H-Pyrazole-3-Carboxylic Acid and Acid Chloride with 2,3-Diaminopyridine. Molecules 2005, 10, 559-571. https://doi.org/10.3390/10030559
Yıldırım I, Kandemirli F, Demir E. Experimental and Theoretical Studies on the Functionalization Reactions of 4-Benzoyl-1,5-Diphenyl-1H-Pyrazole-3-Carboxylic Acid and Acid Chloride with 2,3-Diaminopyridine. Molecules. 2005; 10(3):559-571. https://doi.org/10.3390/10030559
Chicago/Turabian StyleYıldırım, Ismail, Fatma Kandemirli, and Elif Demir. 2005. "Experimental and Theoretical Studies on the Functionalization Reactions of 4-Benzoyl-1,5-Diphenyl-1H-Pyrazole-3-Carboxylic Acid and Acid Chloride with 2,3-Diaminopyridine" Molecules 10, no. 3: 559-571. https://doi.org/10.3390/10030559




