Homo-polymerization of α-Olefins and Co-polymerization of Higher α-Olefins with Ethylene in the Presence of CpTiCl2(OC6H4X-p)/MAO Catalysts (X = CH3, Cl)
Abstract
:Introduction
| Oxidation state of Ti | Substituent X (σ) | |||
| Cl (0,23) | H (0) | CH3 (-0,17) | ||
| Ti (IV) | 35 | 25 | 10 | |
| Ti (III) | 55 | 65 | 75 | |
| Ti (II) | 10 | 10 | 15 | |
Results and Discussion
Homo-polymerization of ethylene
Homo-polymerization of propylene.
| Substituent | σ | Oligomers of propylene [wt%] | ||
|---|---|---|---|---|
| C6 | C9 | C12 | ||
| CH3 | -0.17 | 50,1 | 31,3 | 18,6 |
| H | 0 | 49,9 | 31,7 | 18,4 |
| Cl | 0.23 | 55,6 | 30,2 | 14,2 |
| Substituent X | σ | Weight of the product [g] | DSC [°C] | Form of the product | ||
|---|---|---|---|---|---|---|
| 25°C | 70°C | 25°C | 70°C | |||
| CH3 | -0.17 | ~3.2* | ~0.6** | - | - | Fluid |
| H | 0 | 2.20 | 1.04 | 95.0;118.1 | 106.4;128.1;203.2 | Gum |
| Cl | 0.23 | 1,77 | 3.71 | 163.6 | ~100;119.2;164.5 | Gum |
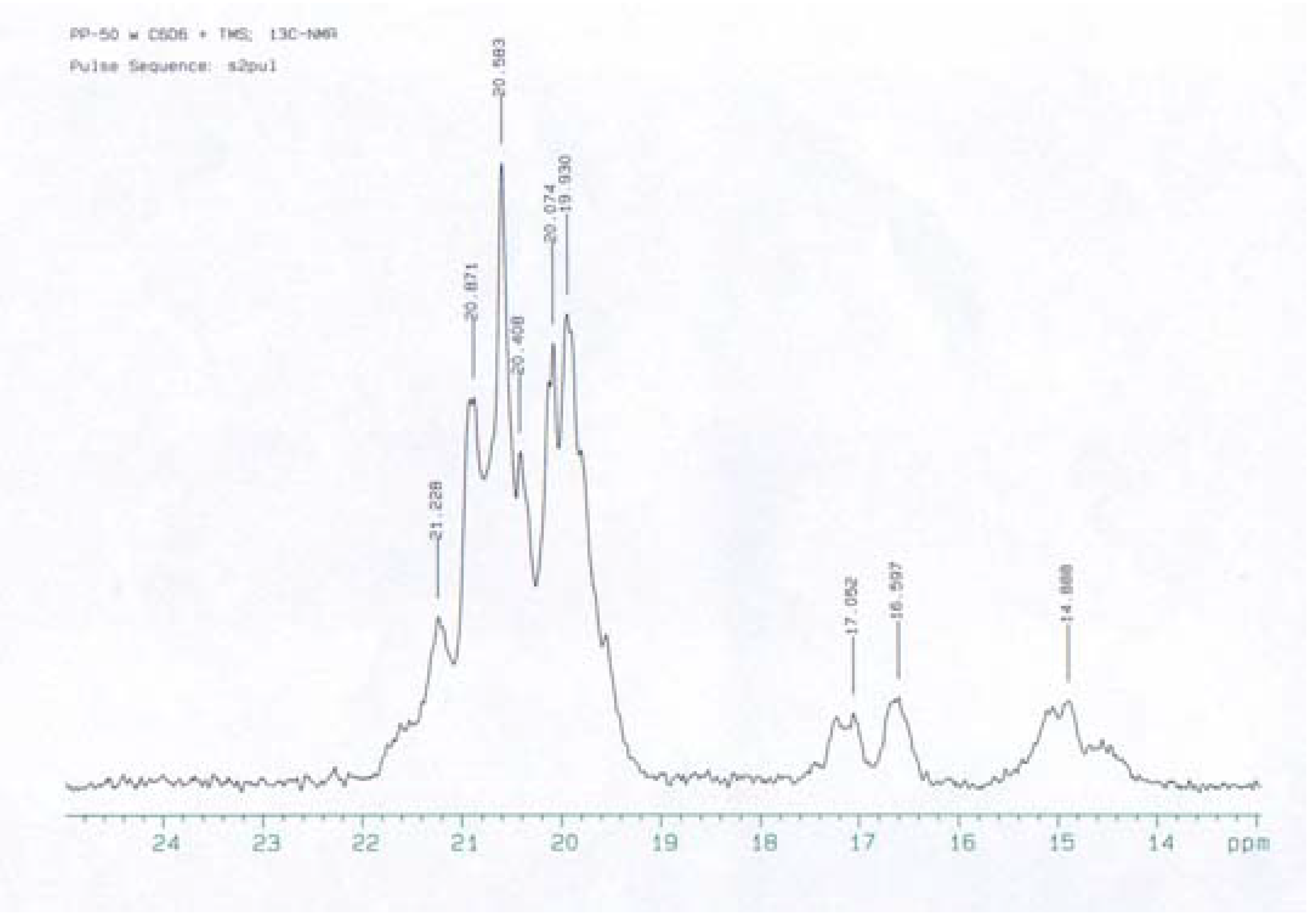
Homo-polymerization of 1-butene, 1-pentene and 1-hexene
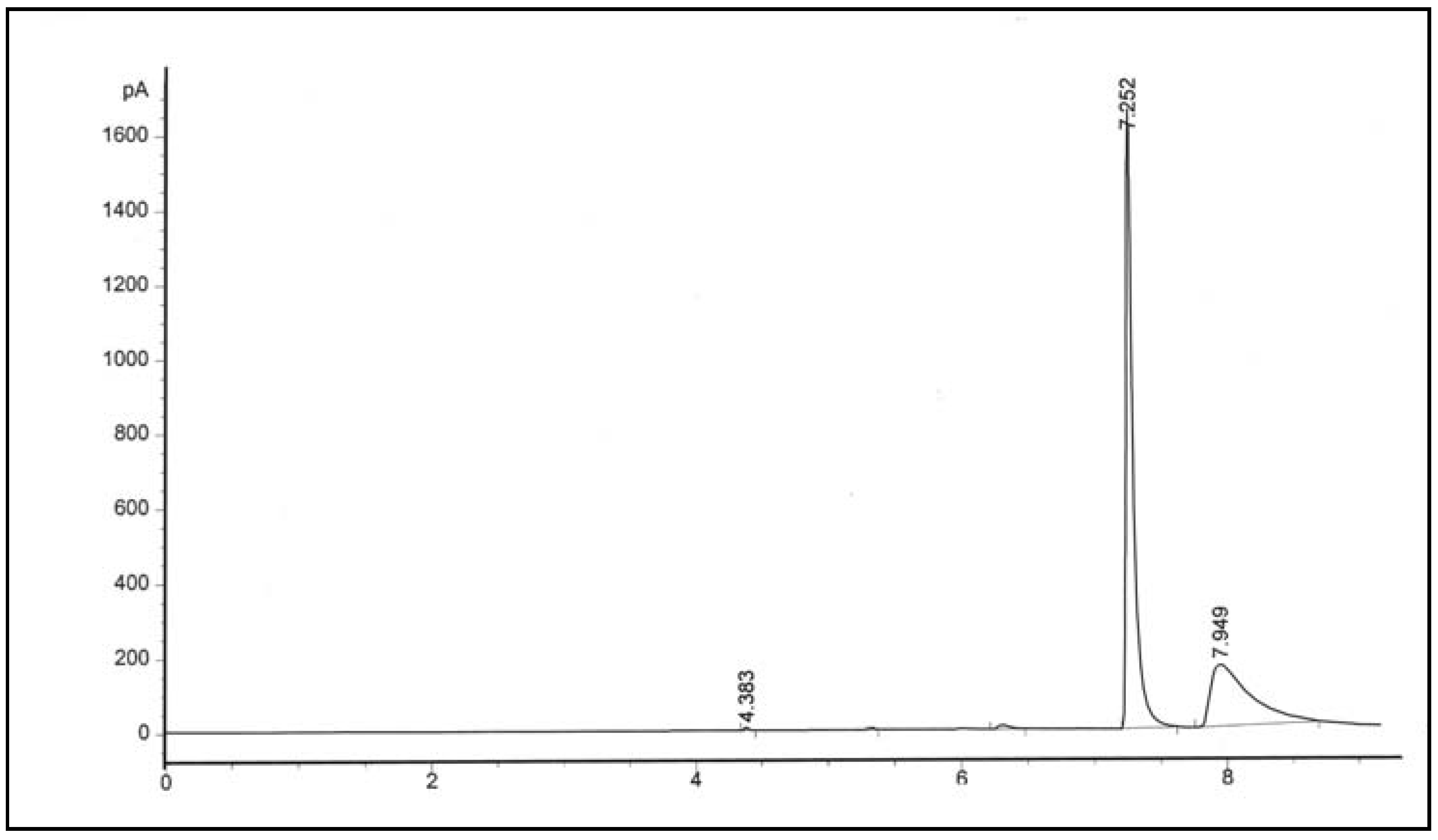
Co-polymerization of ethylene with higher α-olefins
| Sample | Ethylene Pressure [atm.] | Time [min.] | Mass of the product [g] | Activity [kg/(mol Ti∗h)] | DSC I [°C] | DSC II [°C] | Mw/1000 [g/mol] | Mn/1000 [g/mol] | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| PE | 5 | 30 | 1.67 | 167 | 133 | 129 | 667 | 279 | 2.4 |
| Pp-1 | 4 | 15 | 2.53 | 506 | 133 | 127 | 758 | 146 | 5.1 |
| B-1 | 5 | 30 | 8.69 | 869 | 114, 125 | 104 | 319 | 122 | 2.6 |
| P-2 | 3 | 30 | 0.35 | 35 | Very wide signal with max. temp. 119 | Wide signal two max. temp.102 and 116 | 234 | 69 | 3.3 |
| P-4 | 5 | 10 | 0.37 | 111 | 108 | 100 and 115 | 272 | 75 | 3.6 |
| P-5 | 5 | 20 | 0.32 | 48 | 112 | 100 and 118 | 408 | 84 | 4.8 |
| P-1 | 5 | 30 | 6.33 | 633 | 109, 138 | 105 | 301 | 86 | 3.5 |
| P-3 | 8 | 30 | 8.07 | 807 | 122 | 121 | 283 | 60 | 4.7 |
| H-2 | 5 | 30 | 0.77 | 77 | 112, 135 | 113 | 245 | 45 | 5.5 |
| H-3 | 8 | 30 | 0.63 | 63 | Wide, tattered signal from 100 to 163 | 122 | 258 | 103 | 2.5 |

Conclusions
Experimental
General
Homo-polymerization of ethylene
Homo-polymerization of propylene
Polymerization under normal pressure of propylene.
Polymerization of propylene under increased pressure (7 atm.)
Homo-polymerization of 1-butene, 1-pentene and 1-hexene
Ethylene/propylene co-polymerization
Ethylene/1-butene co-polymerization
Ethylene/1-pentene and ethylene/1-hexene co-polymerization
References
- Mashima, K.; Akayama, Y.; Nakamura, A. Recent trends in the polymerization of α-olefins catalyzed by organometallic complexes of early transition metals. Adv. Polym. Sci. 1997, 133, 1–51. [Google Scholar]
- Baird, M.C. Carbocationic alkene polymerizations initiated by organotransition metal complexes: an alternative, unusual role for soluble Ziegler-Natta catalysts. Chem. Rev. 2000, 100, 1471–1478. [Google Scholar]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K.; Imai, A. Synthesis of various nonbridged titanium(IV) cyclopentadienyl-aryloxy complexes of the type CpTi(OAr)X2 and their use in catalysis of alkene polymerization. Important role of substituents on both aryloxy and cyclopentadienyl groups. Organometallics 1998, 17, 2152–2154. [Google Scholar]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl)-(aryloxy)titanium(IV) complexes – cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar]
- Nomura, K.; Komatsu, T.; Imanishi, Y. Polymerization of 1-hexene, 1-octene, catalyzed by Cp’TiCl2(O-2,6-iPr2C6H3)-MAO system. Unexpected increase of the catalytic activity for ethylene/1-hexene co-polymerization by (1,3-tBu2C5H3)TiCl2(O-2,6-iPrC6H3)-MAO catalyst system. J. Mol. Catal. A: Chem. 2000, 152, 249–252. [Google Scholar]
- Nomura, K.; Komatsu, T.; Imanishi, Y. Ligand effect in olefin polymerization catalyzed by (cyclopentadienyl)(aryloxy)titanium(IV) complexes, Cp’TiCl2(Oar)-MAO system.: Ethylene/1-hexene co-polymerization by (1,3-tBu2C5H3)TiCl2(O-2,6-iPrC6H3)-MAO catalyst system. J. Mol. Catal. A: Chem. 2000, 159, 127–137. [Google Scholar]
- Nomura, K.; Oya, K.; Komatsu, T.; Imanishi, Y. Effect of the cyclopentadienyl fragment on monomer reactivities and monomer sequence distributions in ethylene/α-olefin co-polymerization by a nonbridged (cyclopentadienyl)(aryloxy)titanium(IV) complex - MAO catalyst system. Macromolecules 2000, 33, 3187–3189. [Google Scholar] [CrossRef]
- Nomura, K.; Komatsu, T.; Nakamura, M.; Imanishi, Y. Effect of cocatalyst in 1-hexene polymerization by Cp*TiMe2(O-2,6-iPr2C6H3) complex. J. Mol. Catal. A: Chem. 2000, 164, 131–135. [Google Scholar]
- Nomura, K.; Oya, K.; Imanishi, Y. Ethylene/α-olefin co-polymerization by various nonbridged (cyclopentadienyl)(aryloxy)titanium(IV) complexes – MAO catalyst system. J. Mol. Catal. A: Chem. 2001, 174, 127–140. [Google Scholar]
- Skupiński, W.; Wasilewski, A. The Hammett relationship in CpTiCl2OphX (X = CH3O, CH3, Cl, NO2) + BuLi(Et2AlCl) catalytic systems. J. Organomet. Chem. 1985, 282, 69–74. [Google Scholar] [CrossRef]
- Doherty, S.; Errington, R.J.; Jarvis, A.P.; Collins, S.; Clegg, W.; Elsegood, M.R.J. Polymerization of ethylene by the electrophilic mixed cyclopentadienylpyridylalkoxide complexes [CpM{NC5H4(CR2O)-2}Cl2] (M = Ti, Zr, R = Ph, Pri). Organometallics 1998, 17, 3408–3410. [Google Scholar] [CrossRef]
- Richter, J.; Edelmann, F.T.; Noltemeyer, M.; Schmidt, H.-G.; Schmulison, M.; Eisen, M.S. Metallocene analogues containing bulky heteroallylic ligands and their use as new olefin polymerization catalysts. J. Mol. Catal. A: Chem. 1998, 130, 149–162. [Google Scholar]
- Patent PL 318 774, 1997.
- Patent PL 318 775, 1997.
- Skupiński, W.; Niciński, K.; Maksimowski, P.; Wasek, M. Polarographic studies of CpTiCl2(OC6H4X-p)/MAO/styrene systems. J. Mol. Catal. A: Chem. 2002, 178, 73–77. [Google Scholar]
- Skupiński, W.; Niciński, K. Syndiotactic polymerization of styrene in the presence of CpTiCl2(OC6H4X)/MAO catalytic systems. Appl. Organometal. Chem. 2001, 15, 635–638. [Google Scholar] [CrossRef]
- Munoz-Escalona, A.; Cruz, V.; Mena, N.; Martinez, S.; Martinez-Salazar, J. A theoretical study of ethylene-styrene co-polymerization by using half-sandwich Cp-based titanium catalysts. Polymer 2002, 43, 7017–7026. [Google Scholar] [CrossRef]
- Ewen, J.A. Mechanisms of stereochemical control in propylene polymerizations with soluble Group 4B metallocene/methylaluminoxane catalysts. J. Am. Chem. Soc. 1984, 106, 6355–6364. [Google Scholar] [CrossRef]
- Kaminsky, W.; Arndt, M. Metallocenes for polymer catalysis. Adv. Polym. Sci. 1997, 127, 144–187. [Google Scholar]
- Kaminsky, W.; Drögemüller, H. Polymer Reaction Engineering; Reichert, K.H, Geiseler, W., Eds.; VCH: Berlin, 1989; p. 372. [Google Scholar]
- Czaja, K.; Białek, M. Microstructure of ethylene- 1-hexene and ethylene- 1-octene copolymers obtained over Ziegler-Natta catalysts supported on MgCl2(THF)2. Polymer 2001, 6, 2289–2297. [Google Scholar] [CrossRef]
- Po, R.; Cardi, N. Synthesis of syndiotactic polystyrene: reaction mechanisms and catalysis. Prog. Polym. Sci. 1996, 21, 47–88. [Google Scholar] [CrossRef]
- Nowakowska, M. Chemia Polimerów t.II Podstawowe polimery syntetyczne i ich zastosowania; Florjańczyk, Z., Penczek, S., Eds.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, 1997; p. 83. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Skupinski, W.; Nicinski, K.; Jamanek, D.; Wieczorek, Z. Homo-polymerization of α-Olefins and Co-polymerization of Higher α-Olefins with Ethylene in the Presence of CpTiCl2(OC6H4X-p)/MAO Catalysts (X = CH3, Cl). Molecules 2005, 10, 659-671. https://doi.org/10.3390/10060659
Skupinski W, Nicinski K, Jamanek D, Wieczorek Z. Homo-polymerization of α-Olefins and Co-polymerization of Higher α-Olefins with Ethylene in the Presence of CpTiCl2(OC6H4X-p)/MAO Catalysts (X = CH3, Cl). Molecules. 2005; 10(6):659-671. https://doi.org/10.3390/10060659
Chicago/Turabian StyleSkupinski, W., K. Nicinski, D. Jamanek, and Z. Wieczorek. 2005. "Homo-polymerization of α-Olefins and Co-polymerization of Higher α-Olefins with Ethylene in the Presence of CpTiCl2(OC6H4X-p)/MAO Catalysts (X = CH3, Cl)" Molecules 10, no. 6: 659-671. https://doi.org/10.3390/10060659




