The Syntheses of Some Novel (Naphthalen-1-yl-selenyl)acetic Acid Derivatives
Abstract
:Introduction
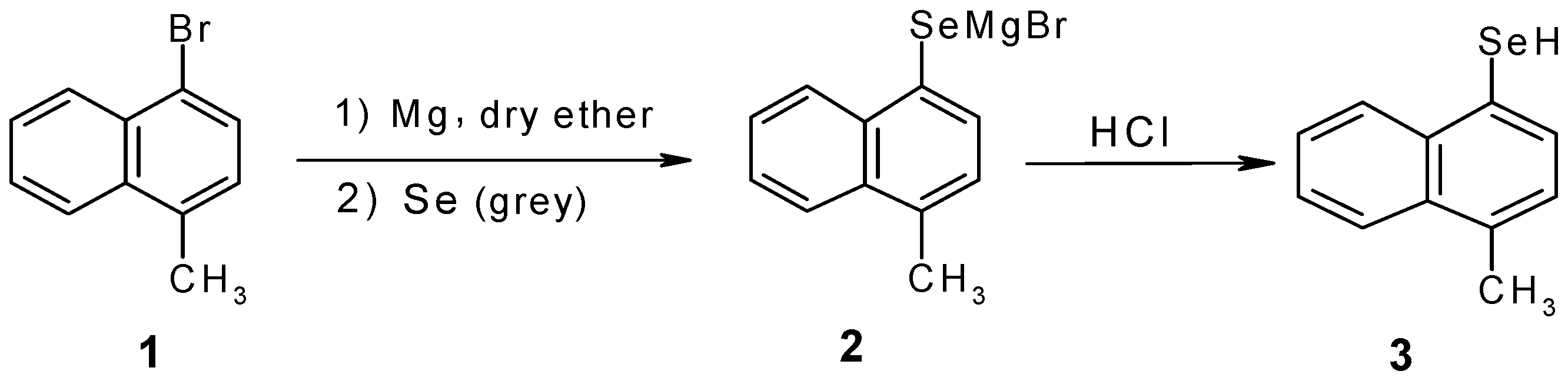
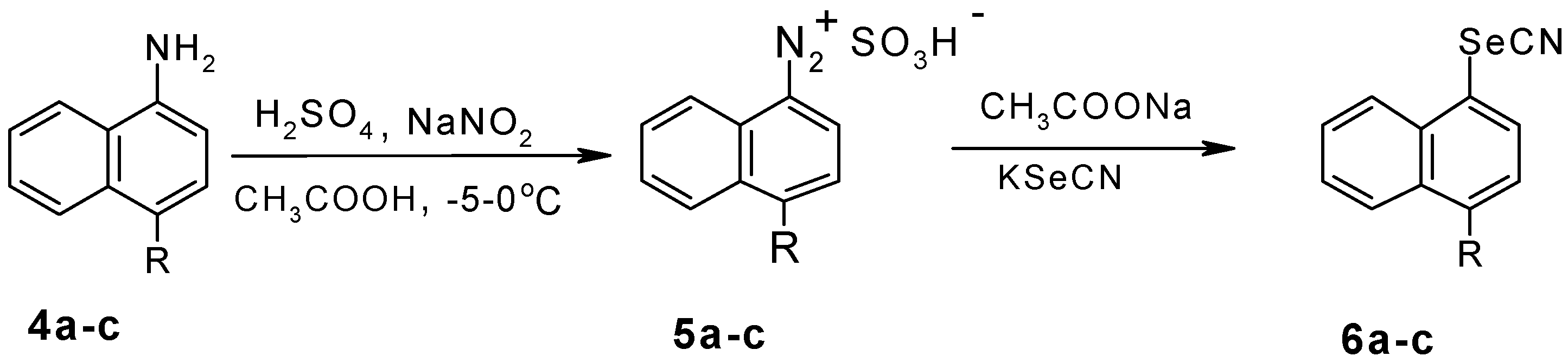
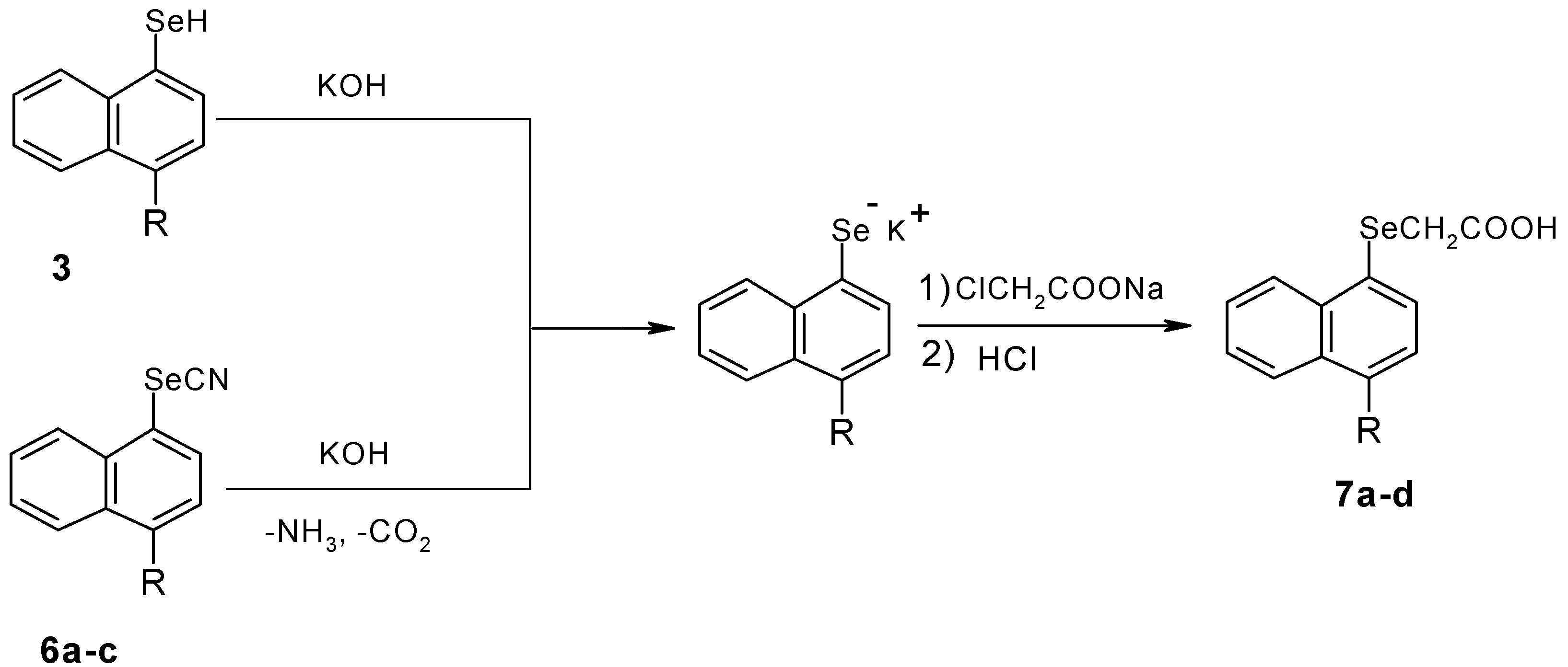
Results and Discussion
Conclusions
Experimental
General
4-Methyl-1-naphthylselenol (3)
Acknowledgements
References
- Rayman, M.P. Brit. Med. J. 2000, 356, 233.
- Günther, W.H.H. Organic Selenium Compounds Their Chemistry and Biology; Klayman, D.L., Günther, W.H.H., Eds.; Wiley: New York, 1973; p. 30. [Google Scholar]
- Xie, Y.; Short, M.D.; Cassidy, P.B.; Roberts, J.C. Bioorg. Med. Chem. Lett. 2001, 11, 2911. [CrossRef]
- Overvad, K. Bibl. Nutr. Diet. 1998, 54, 141.
- Bellinger, N.; Cagniant, P. C.R. Acad. Sc. Paris 1969, 268, 1385.
- Morgan, G.T.; Porrit, W.H. J. Chem. Soc. 1925, 1755.
- Sjöberg, B.; Herdevall, S. Acta Chem. Scand. 1958, 12, 1347. [CrossRef]
- Taboury, M. Bull. Soc. Chim. France 1903, 29, 762.
- Behagel, O.; Rollmann, M. J. Prakt. Chem. 1929, 123, 336.
- Özkan, H.; Dişli, A.; Yıldırır, Y. Org. Prep. Proc. Int. 2004, 36, 161. [CrossRef]
- Murata, S.; Suzuki, C.; Inoue, H.; Andoha, Y.; Hayashi, Y. Heterocycles 1999, 52, 621.
- Sample availability: Samples may be obtained from corresponding author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yavuz, S.; Dişli, A.; Yıldırır, Y.; Türker, L. The Syntheses of Some Novel (Naphthalen-1-yl-selenyl)acetic Acid Derivatives. Molecules 2005, 10, 1000-1004. https://doi.org/10.3390/10081000
Yavuz S, Dişli A, Yıldırır Y, Türker L. The Syntheses of Some Novel (Naphthalen-1-yl-selenyl)acetic Acid Derivatives. Molecules. 2005; 10(8):1000-1004. https://doi.org/10.3390/10081000
Chicago/Turabian StyleYavuz, Serkan, Ali Dişli, Yılmaz Yıldırır, and Lemi Türker. 2005. "The Syntheses of Some Novel (Naphthalen-1-yl-selenyl)acetic Acid Derivatives" Molecules 10, no. 8: 1000-1004. https://doi.org/10.3390/10081000



