Results and Discussion
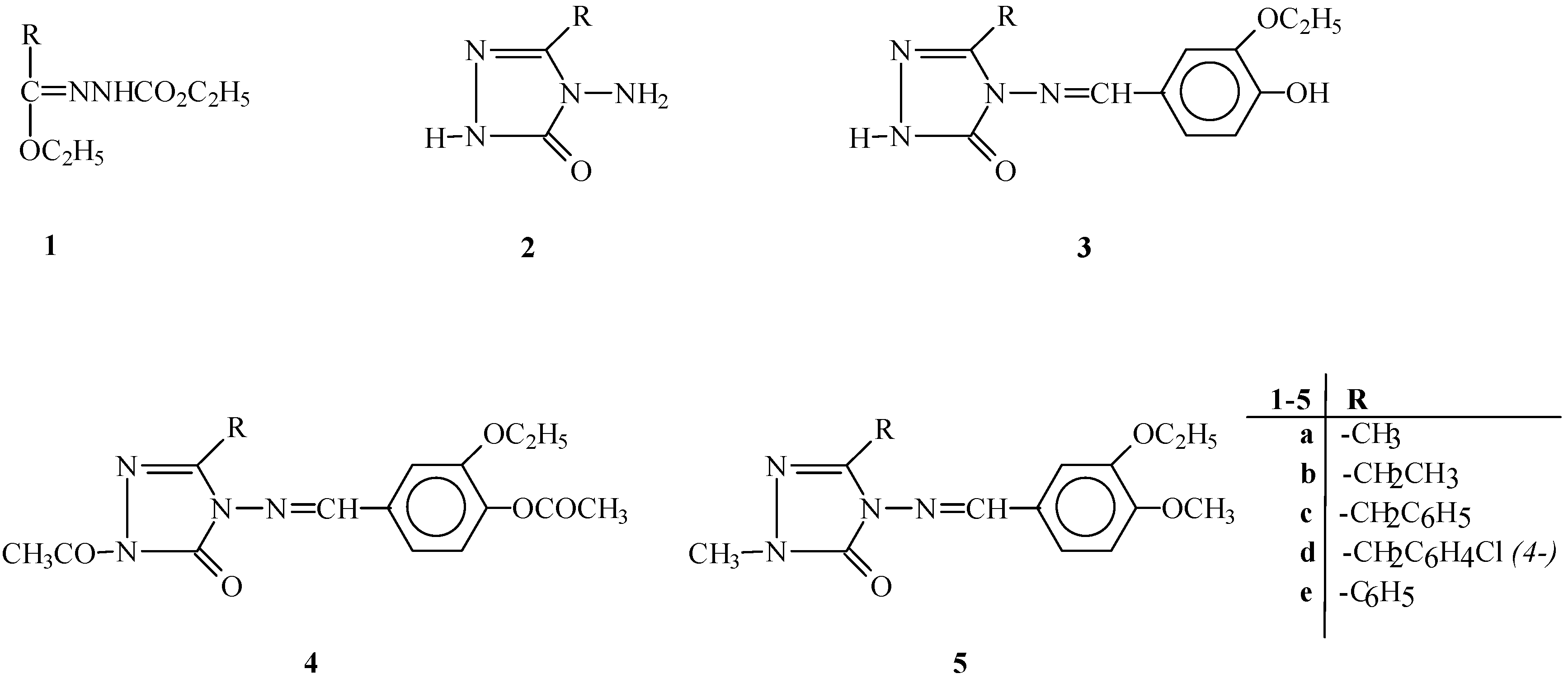
In this study, the structures of five new 3-alkyl(aryl)-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1
H-1,2,4-triazol-5-ones,
3a-e, four new 1-acetyl-3-alkyl(aryl)-4-(3-ethoxy-4-acetoxy-benzylidenamino)-4,5-dihydro-1
H-1,2,4-triazol-5-ones,
4a-c and
e, and two new 1-methyl-3-alkyl-(aryl)-4-(3-ethoxy-4-methoxybenzylidenamino)-4,5-dihydro-1
H-1,2,4-triazol-5-ones,
5c,e, were identified using elemental analysis and IR,
1H-,
13C-NMR and UV spectral data, and the observed spectral values were seen to be compatible with literature values [
11,
12,
13,
14,
15,
16,
25].
After the potentiometric titrations of compounds
3 with TBAH in non-aqueous solvents, the mV values from each titration were plotted against TBAH volumes used (mL) and the potentiometric titration curves were obtained for all the cases. From the titration curves, the HNP values and the corresponding p
Ka values were obtained. As an example, the potentiometric titration curves for 0.001 M solutions of 3-(4-chloro-benzyl)-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (
3d) titrated with 0.05 N TBAH in isopropyl alcohol,
tert-butyl alcohol, acetonitrile and
N,N-dimethylformamide are presented in
Figure 1.
Figure 1.
Potentiometric titration curves of 0.001 M solutions of compound 3d titrated with 0.05 M TBAH in isopropyl alcohol (▲), tert-butyl alcohol (х), acetonitrile (◆) and N,N-dimethylformamide (■) at 25°C.
Figure 1.
Potentiometric titration curves of 0.001 M solutions of compound 3d titrated with 0.05 M TBAH in isopropyl alcohol (▲), tert-butyl alcohol (х), acetonitrile (◆) and N,N-dimethylformamide (■) at 25°C.
The half-neutralization potential (HNP) values and the corresponding p
Ka values of compounds
3a-3e, obtained from the potentiometric titrations with 0.05 M TBAH in isopropyl alcohol,
tert-butyl alcohol, acetonitrile and
N,N-dimethylformamide, are presented in
Table 1,
Table 2,
Table 3 and
Table 4.
Table 1.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in isopropyl alcohol at 25 °C.
Table 1.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in isopropyl alcohol at 25 °C.
| Compd. no | HNP1 (mV) | pKa1 | HNP2 (mV) | pKa2 |
|---|
| 3a | -363 | 13.22 | -495 | - |
| 3b | -367 | 13.92 | - | - |
| 3c | -369 | 13.78 | -474 | - |
| 3d | -355 | 13.63 | -460 | 16.00 |
| 3e | -344 | 12.85 | -449 | 14.98 |
Table 2.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in tert-butyl alcohol at 25 °C.
Table 2.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in tert-butyl alcohol at 25 °C.
| Compd. no | HNP1 (mV) | pKa1 | HNP2 (mV) | pKa2 |
|---|
| 3a | -384 | 13.65 | -501 | - |
| 3b | -443 | 15.62 | -558 | - |
| 3c | -416 | 14.00 | -544 | - |
| 3d | -472 | - | - | - |
| 3e | -448 | 14.96 | - | - |
Table 3.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in acetonitrile at 25 °C.
Table 3.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in acetonitrile at 25 °C.
| Compd. no | HNP1 (mV) | pKa1 | HNP2 (mV) | pKa2 |
|---|
| 3a | -441 | 15.41 | -536 | - |
| 3b | -493 | 15.63 | - | - |
| 3c | -482 | - | -578 | - |
| 3d | -474 | 15.28 | - | - |
| 3e | -507 | - | - | - |
Table 4.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in N,N-dimethylformamide at 25 °C.
Table 4.
The half-neutralization potentials (HNP) and the corresponding pKa values of compounds 3a-3e in N,N-dimethylformamide at 25 °C.
| Compd. no | HNP1 (mV) | pKa1 | HNP2 (mV) | pKa2 |
|---|
| 3a | -503 | - | - | - |
| 3b | -496 | - | -655 | - |
| 3c | -499 | - | -628 | - |
| 3d | -493 | - | -621 | - |
| 3e | -536 | - | - | - |
The
pH of weak acids can be calculated using the following equation:
where
pH =
pKa when [A
-] is equal to [HA] at the half-neutralization points. Therefore, the
pH values at the half-neutralization points were taken as
pKa. Taking into consideration the dielectric permittivity of the solvents, the acidity ranking might be expected to be as follows:
N,N‑dimethylformamide (ε=37) > acetonitrile (ε=36) > isopropyl alcohol (ε=19.4) >
tert-butyl alcohol (ε=12). However, as seen in
Table 1,
Table 2,
Table 3 and
Table 4, the observed acidity ranking for all compounds is isopropyl alcohol >
tert-butyl alcohol > acetonitrile >
N,N-dimethylformamide. This result thus matches the theoretical arrangement, except for acetonitrile and
N,N-dimethylformamide. In
N,N-dimethylformamide, all these compounds show the weakest acidic properties, but they show the strongest acidic properties in isopropyl alcohol. This situation may be attributed to the hydrogen bonding between the negative ions formed and the solvent molecules in the amphiprotic neutral solvents.
As seen
Scheme 1, there is one weak acidic N-H group in the 4,5-dihydro-1H-1,2,4-triazol-5-one ring and one phenolic group on the aryl substituent in compounds
3a-3e. Thus, these compounds give two end-points as well as two half-neutralization potential (HNP) values. Thus, as expected, the potentiometric titration curves for these compounds
3a-3e titrated with TBAH in isopropyl alcohol,
tert-butyl alcohol, acetonitrile and
N,N-dimethylformamide resemble the titration curves for diprotic acids.
For compound 3b in isopropyl alcohol, compounds 3d and 3e in tert-butyl alcohol, compounds 3b, 3d and 3e in acetonitrile and compounds 3a and 3e in N,N-dimethylformamide, the second half-neutralization potential (HNP2) values and the corresponding pKa values have not been obtained. In addition, the pKa values bigger than 16.00 have not been determined due to the fact that this value is outside the range of the pH meter.
As it is well known, the acidity of a compound depends on several factors. The two most important ones are the solvent effect and molecular structure [
15,
16,
18,
19,
20,
21,
22,
23].
Table 1,
Table 2,
Table 3 and
Table 4 and
Figure 1 show that the HNP values and corresponding p
Ka values obtained from the potentiometric titrations depend on the non-aqueous solvents used and the substituents at C-3 in 4,5-dihydro-1
H-1,2,4-triazol-5-one ring.
Experimental
General
Melting points were taken on a Electrothermal 9100 digital melting point apparatus and are uncorrected. IR spectra were registered on a Perkin-Elmer 1600 FTIR spectrometer.
1H-NMR and
13C-NMR spectra were recorded in deuterated dimethyl sulfoxide with TMS as internal standard on a Varian Mercury spectrometer at 200 MHz and 50 MHz, respectively. UV absorption spectra were measured in 10-mm quartz cells between 200 and 400 nm using a Shimadzu UV-1201 spectrophotometer. In this study, a Jenway 3040 ion analyser pH meter equipped with an Ingold pH electrode was used for potentiometric titrations. For each compound titrated, a 0.001 M solution was separately prepared in each non-aqueous solvent. A 0.05 M solution of TBAH in isopropyl alcohol, which is widely used in the titration of acids, was used as titrant. The mV values obtained on the pH meter were recorded. Finally, the half-neutralization potential (HNP) values were determined by plotting the volume (mL) (TBAH)-mV graph. The starting compounds
2a-e were prepared from the reactions of the corresponding ester ethoxycarbonylhydrazones
1a-e with an aqueous solution of hydrazine hydrate as described in the literature [
17,
24].
General Method for the Preparation of 3-Alkyl(aryl)-4-(3-ethoxy-4-hydroxy-benzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones 3a-e
The corresponding compound 2 (0.01 mole) was dissolved in acetic acid (15 mL) and treated with 3-ethoxy-4-hydroxybenzaldehyde (1.66 g, 0.01 mole). The mixture was refluxed for 1 h and then evaporated at 50-55 °C in vacuo. Several recrystallizations of the residue from an appropriate solvent (AcOH-H2O, 1:3) gave pure compounds 3a-e as colourless crystals.
3-Methyl-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3a). Yield 67%; mp. 205 °C; Calculated for C12H14N4O3 (262.27): 54.96% C, 5.38% H, 21.36% N; found: 54.96% C, 5.29% H, 21.07% N; 1H-NMR: δ 1.40 (t, 3H, CH3), 2.30 (s, 3H, CH3), 4.11 (q, 2H, CH2), 6.92 (d, 1H, Ar-H), 7.23 (d, 1H, Ar-H), 7.41 (s, 1H, Ar-H), 9.54 (s, 1H, N=CH), 9.79 (s, 1H, OH), 11.81 (s,1H, NH); 13C-NMR: δ 11.12, 14.61, 63.77 (aliphatic carbons), 111.17, 115.60, 122.49, 124.67, 144.17, 151.30 (aromatic carbons), 147.12 (triazole C3), 150.30 (N=CH), 154.73 (triazole C5); IR: 3529 (OH), 3162 (NH), 1716 (C=O), 1597 (C=N) cm-1; UV λmax (ε, L·mol-1·cm-1): 320 (18262), 235 (11377), 212 (14098) nm.
3-Ethyl-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3b). Yield 79%; mp. 174 °C; Calculated for C13H16N4O3 (276.30): 54.51% C, 5.84% H, 20.28% N; found: 54.38% C, 5.38% H, 19.70% N; 1H-NMR: δ 1.16 (t, 3H, CH3), 1.32 (t, 3H, CH3), 2.61 (q, 2H, CH2), 4.02 (q, 2H, OCH2), 6.86 (d, 1H, Ar-H, J=8.1 Hz), 7.20 (d, 1H, Ar-H, J=8.2 Hz), 7.32 (s, 1H, Ar-H), 9.46 (s, 1H, N=CH), 9.69 (s, 1H, OH), 11.76 (s, 1H, NH); 13C-NMR: δ 9.90, 14.61, 18.54, 63.78 (aliphatic carbons), 111.18, 115.63, 122.39, 124.79, 147.96, 151.49 (aromatic carbons), 147.13 (triazole C3), 150.32 (N=CH), 154.64 (triazole C5); IR: 3527 (OH), 3222 (NH), 1701 (C=O), 1597 (C=N) cm-1; UV λmax (ε, L·mol-1·cm-1): 321 (27690), 235 (17345), 213 (22069) nm.
3-Benzyl-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3c). Yield 91%; mp. 211 °C; Calculated for C18H18N4O3 (338.37): 63.89% C, 5.36% H, 16.56% N; found: 63.66% C, 5.74% H, 16.22% N; 1H-NMR: δ 1.44 (t, 3H, CH3), 4.09 (s, 2H, CH2Ph), 4.11 (q, 2H, CH2), 6.92 (d, 1H, Ar-H, J=8.0 Hz), 7.25 (d, 1H, Ar-H, J=8.1 Hz), 7.36 (s, 5H, Ar-H), 7.38 (s, 1H, Ar-H), 9.53 (s, 1H, N=CH), 9.93 (s, 1H, OH), 12.02 (s, 1H, NH); 13C-NMR: δ 14.57, 31.20, 63.63 (aliphatic carbons), 110.30, 115.45, 122.85, 124.70, 126.61, 128.36 (2C), 128.66 (2C), 135.85, 146.05, 151.20 (aromatic carbons), 147.08 (triazole C3), 150.30 (N=CH), 153.90 (triazole C5); IR: 3165 (OH), 3055 (NH), 1712 (C=O), 1584 (C=N), 755, 699 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 265 (16250), 221 (29650) nm.
3-(4-Chlorobenzyl)-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3d). Yield 71%; mp. 232 °C; Calculated for C18H17N4O3Cl (373.82): 57.99% C, 4.60% H, 15.03% N; found: 57.29% C, 4.36% H, 14.79% N; 1H-NMR: δ 1.42 (t, 3H, CH3), 4.08 (s, 2H, CH2Ph), 4.09 (q, 2H, CH2), 6.92 (d, 1H, Ar-H), 7.24 (d, 1H, Ar-H), 7.30-7.55 (m, 5H, Ar-H), 9.52 (s, 1H, N=CH), 9.92 (s, 1H, OH), 12.02 (s, 1H, NH); 13C-NMR: δ 14.57, 30.80, 63.63 (aliphatic carbons), 110.31, 115.46, 122.89, 124.59, 128.57 (2C), 130.54 (2C), 131.45, 134.88, 145.80, 151.30 (aromatic carbons), 147.10 (triazole C3), 150.28 (N=CH), 153.98 (triazole C5); IR: 3170 (OH), 3110 (NH), 1712 (C=O), 1587 (C=N), 805 (1,4-disubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol‑1·cm-1): 357 (5794), 321 (18320), 213 (28038) nm.
3-Phenyl-4-(3-ethoxy-4-hydroxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (3e). Yield 82%; mp. 206 °C (AcOH-water, 1:3); Calculated for C17H16N4O3 (324.34): 62.95% C, 4.97% H, 17.27% N; found: 62.43% C, 4.95% H, 16.90% N. 1H-NMR: δ 1.41 (t, 3H, CH3), 4.11 (q, 2H, CH2), 6.97 (d, 1H, Ar-H, J=8.2 Hz), 7.32 (d, 1H, Ar-H, J=8.3 Hz), 7.42 (s, 1H, Ar-H), 7.56-7.60 (m, 3H, Ar-H), 7.95-8.05 (m, 2H, Ar-H), 9.48 (s, 1H, N=CH), 9.89 (s, 1H, OH), 12.42 (s, 1H, NH); 13C-NMR: δ 14.54, 63.63 (aliphatic carbons), 111.10, 115.70, 122.80, 124.48, 126.85, 127.76 (2C), 128.39 (2C), 130.00, 144.40, 151.40 (aromatic carbons), 147.09 (triazole C3), 150.55 (N=CH), 157.30 (triazole C5); IR: 3373 (OH), 3076 (NH), 1701 (C=O), 1607,1584 (C=N), 758,680 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1) : 325 (17886), 282 (12032), 215 (21952) nm.
General Method for the Preparation of 1-Acetyl-3-alkyl(aryl)-4-(3-ethoxy-4-acetoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-ones 4a-c and e
The corresponding compound 3 (0.01 mol) was refluxed with acetic anhydride (15 mL) for 0.5 h. After addition of absolute ethanol (50 mL), the mixture was refluxed for 1 h. more. Evaporation of the resulting solution at 40-45 °C in vacuo and several recrystallizations of the residue from EtOH gave pure compounds 4a-c and e as colourless crystals.
1-Acetyl-3-methyl-4-(3-ethoxy-4-acetoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4a). Yield 83%; mp. 189 °C; Calculated for C16H18N4O5 (346.34): 55.49% C, 5.24% H, 16.18% N; found: 54.98% C, 5.29% H, 16.00% N; 1H-NMR: δ 1.37 (t, 3H, CH3), 2.33 (s, 3H, CH3), 2.40 (s, 3H, NCOCH3), 2.54 (s, 3H, OCOCH3), 4.17 (q, 2H, CH2), 7.29 (d, 1H, Ar-H), 7.54 (d, 1H, Ar-H), 7.64 (s, 1H, Ar-H), 9.60 (s, 1H, N=CH); 13C-NMR: δ 11.20, 14.41, 20.30, 23.45, 64.14 (aliphatic carbons), 112.38, 120.39, 123.51, 131.77, 142.38, 146.77 (aromatic carbons), 147.85 (triazole C3), 150.52 (N=CH), 155.17 (triazole C5), 166.08 (CO), 168.34 (CO); IR: 1776, 1760 (C=O), 1625, 1580 (C=N) cm-1; UV λmax (ε, L·mol-1·cm-1): 306 (13788), 257 (32072), 215 (24697) nm.
1-Acetyl-3-ethyl-4-(3-ethoxy-4-acetoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4b). Yield 88%; mp. 162 °C; Calculated for C17H20N4O5 (360.37): 56.66% C, 5.59% H, 15.55% N; found: 56.44% C, 5.46% H, 15.20% N; 1H-NMR: δ 1.33 (t, 3H, CH3), 1.37 (t, 3H, CH3), 2.34 (s, 3H, NCOCH3), 2.55 (s, 3H, OCOCH3), 2.81 (q, 2H, CH2), 4.20 (q, 2H, OCH2), 7.30 (d, 1H, Ar-H), 7.54 (d, 1H, Ar-H), 7.65 (s, 1H, Ar-H), 9.65 (s, 1H, N=CH); 13C-NMR: δ 9.35, 14.37, 18.52, 20.27, 23.43, 64.08 (aliphatic carbons), 112.34, 120.76, 123.48, 131.76, 142.34, 150.17 (aromatic carbons), 148.03 (triazole C3), 150.47 (N=CH), 155.03 (triazole C5), 165.96 (CO), 168.28 (CO); IR: 1776, 1766 (C=O), 1621, 1579 (C=N) cm-1; UV λmax (ε, L·mol-1·cm-1): 321 (16396), 215 (32072) nm.
1-Acetyl-3-benzyl-4-(3-ethoxy-4-acetoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4c). Yield 87%; mp. 173 °C; Calculated for C22H22N4O5 (422.44): 62.55% C, 5.25% H, 13.26% N; found: 62.20% C, 5.23% H, 12.95% N; 1H-NMR: δ 1.37 (t, 3H, CH3), 2.31 (s, 3H, NCOCH3), 2.54 (s, 3H, OCOCH3), 4.12 (q, 2H, OCH2), 4.17 (s, 2H, CH2Ph), 7.18-7.60 (m, 8H, Ar-H), 9.55 (s, 1H, N=CH); 13C-NMR: δ 14.35, 20.26, 23.49, 31.08, 64.03 (aliphatic carbons), 111.40, 121.46, 123.42, 126.91, 128.46 (2C), 128.81 (2C), 131.73, 134.71, 142.35, 148.19 (aromatic carbons), 147.97 (triazole C3), 150.42 (N=CH), 154.16 (triazole C5), 165.98 (CO), 168.29 (CO); IR: 1782, 1752 (C=O), 1616, 1578 (C=N), 741, 708 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol‑1·cm‑1): 263 (15053), 215 (32368) nm.
1-Acetyl-3-phenyl-4-(3-ethoxy-4-acetoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (4e). Yield 99%; mp. 160 °C; Calculated for C21H20N4O5 (408.41): 61.76% C, 4.94% H, 13.72% N; found: 61.68% C, 4.64% H, 13.59% N; 1H-NMR: δ 1.36 (t, 3H, CH3), 2.34 (s, 3H, NCOCH3), 2.63 (s, 3H, OCOCH3), 4.13 (q, 2H, OCH2), 7.28 (d, 1H, Ar-H), 7.46 (d, 1H, Ar-H), 7.53-7.92 (m, 4H, Ar-H), 7.98 (m, 2H, Ar-H), 9.55 (s, 1H, N=CH); 13C-NMR: δ 14.32, 20.26, 23.51, 63.95 (aliphatic carbons), 112.08, 121.15, 123.56, 125.13, 128.56 (2C), 128.61 (2C), 131.28, 131.58, 142.50, 145.90 (aromatic carbons), 148.07 (triazole C3), 150.46 (N=CH), 157.31 (triazole C5), 166.18 (CO), 168.28 (CO); IR: 1768, 1743, 1723 (C=O), 1605, 1582 (C=N), 758, 692 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 323 (14286), 266 (20714), 215 (30408) nm.
General Method for the Preparation of 1-Methyl-3-alkyl(aryl)-4-(3-ethoxy-4-methoxybenzyliden-amino)-4,5-dihydro-1H-1,2,4-triazol-5-ones 5c,e
The corresponding compound 3 (0.01 mol) was dissolved in 2N NaOH (10 mL) and treated dimethyl sulphate (3.2 mL). After stirring of the mixture at room temperature for 1 hr, the solid formed was filtered, washed with cold water (15 mL) and dried in vacuo. Several recrystallizations of crude product from 1:3 AcOH-water gave pure compounds 5c,e as colourless crystals.
1-Methyl-3-benzyl-4-(3-ethoxy-4-methoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (5c). Yield 55%; mp. 198 °C; Calculated for C20H22N4O3 (366.42): 65.56% C, 6.05% H, 15.29% N; found: 65.99% C, 5.72% H, 15.26% N; 1H-NMR: δ 1.42 (t, 3H, CH3), 3.42 (s, 3H, NCH3), 3.87 (s, 3H, OCH3), 4.11 (m, 4H, OCH2 + CH2Ph), 7.05-7.60 (m, 8H, Ar-H), 9.55 (s, 1H, N=CH); 13C-NMR: δ 14.81, 31.25, 32.12, 55.74, 63.81 (aliphatic carbons), 109.27, 111.64, 123.26, 125.99, 126.94, 128.65 (2C), 128.95 (2C), 135.90, 144.82, 152.05 (aromatic carbons), 148.40 (triazole C3), 149.72 (N=CH), 153.88 (triazole C5); IR: 1709 (C=O), 1624, 1577 (C=N), 756, 712 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol-1·cm-1): 323 (12195), 215 (14268) nm.
1-Methyl-3-phenyl-4-(3-ethoxy-4-methoxybenzylidenamino)-4,5-dihydro-1H-1,2,4-triazol-5-one (5e). Yield 64%; mp. 152 °C; Calculated for C19H20N4O3 (352.39): 64.76% C, 5.72% H, 15.90% N; found: 64.89% C, 5.94% H, 15.82% N; 1H-NMR: δ 1.36 (t, 3H, CH3), 3.50 (s, 3H, NCH3), 3.85 (s, 3H, OCH3), 4.13 (q, 2H, OCH2), 7.10 (d, 1H, Ar-H), 7.34 (d, 1H, Ar-H), 7.36 (s, 1H, Ar-H), 7.55 (m, 3H, Ar-H), 7.90-7.96 (m, 2H, Ar-H), 9.46 (s, 1H, N=CH); 13C-NMR: δ 14.51, 32.24, 55.47, 63.43 (aliphatic carbons), 109.59, 111.43, 122.77, 125.56, 126.14, 127.79 (2C), 128.36 (2C), 130.08, 142.66, 151.93 (aromatic carbons), 148.12 (triazole C3), 149.58 (N=CH), 156.49 (triazole C5); IR: 1702 (C=O), 1602, 1577 (C=N), 761, 691 (monosubstituted benzenoid ring) cm-1; UV λmax (ε, L·mol‑1·cm‑1): 322 (10286), 280 (10529), 215 (14912) nm.