Copper(II) Complexes with Ligands Derived from 4-Amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and Biological Activity
Abstract
:Introduction
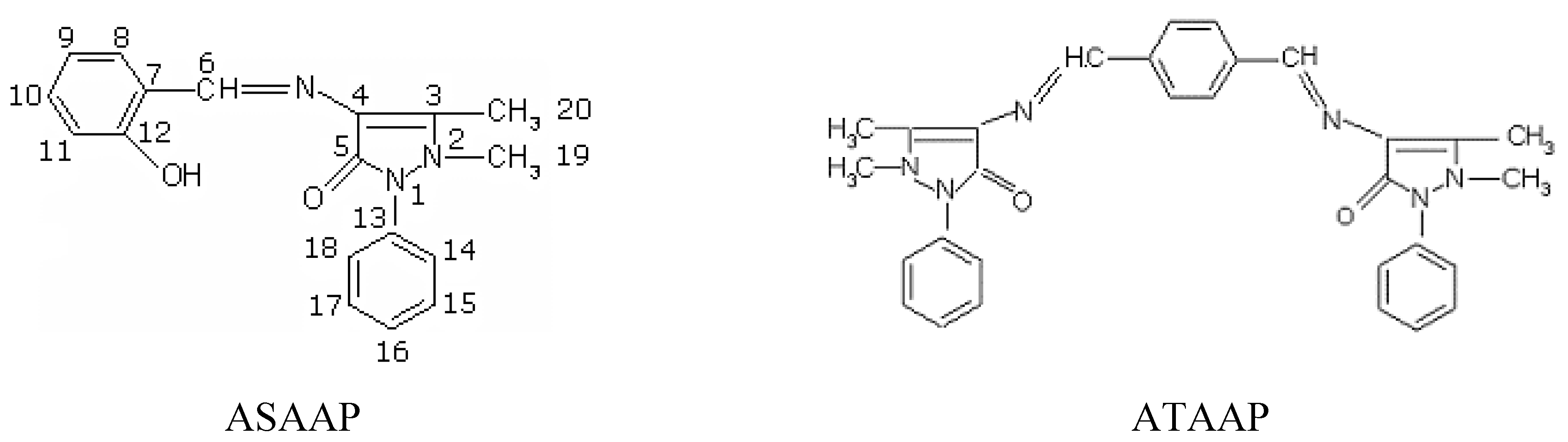
Results and Discussion
Synthesis
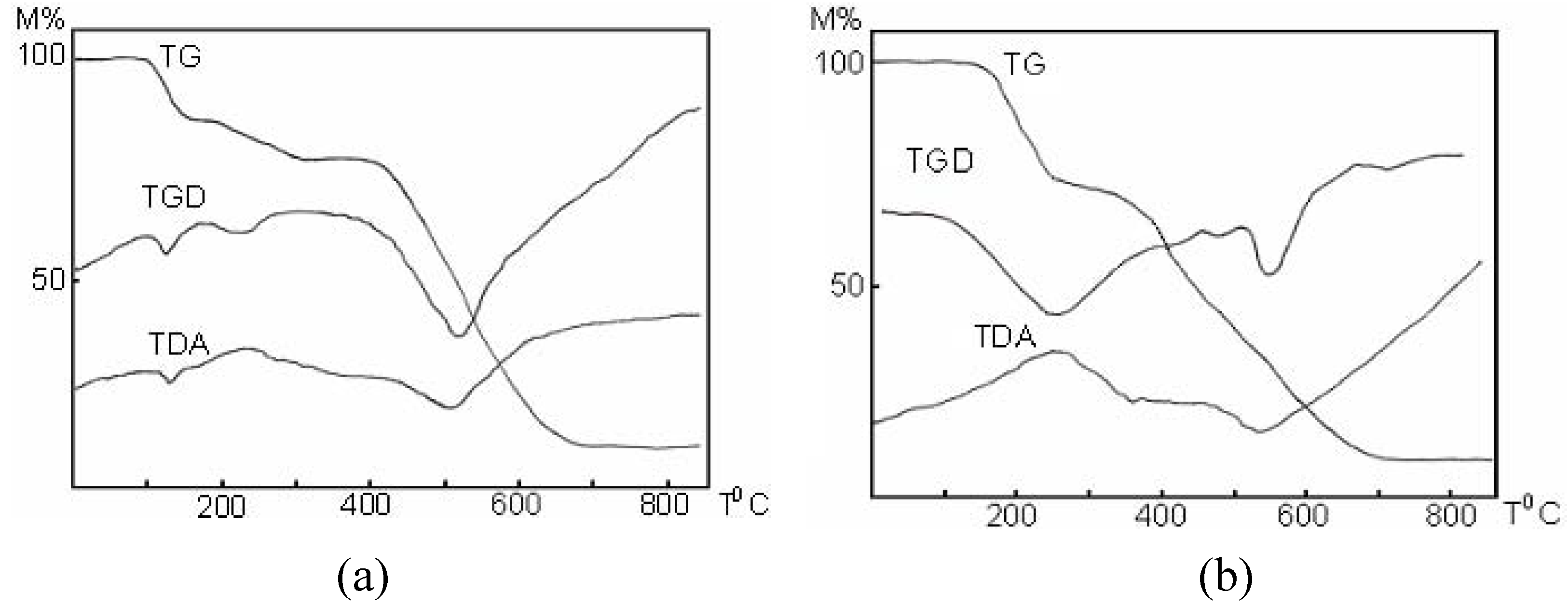
Properties
IR Spectra
| Compound | νC=N | νAr-OH | ν>C=O | ρr | ρw | νSO42- | |||
|---|---|---|---|---|---|---|---|---|---|
| ν1 | ν2 | ν3 | ν4 | ||||||
| C18H17N3O2 | 1653 | 3210- 3370 1140 | 1615 | - | - | - | - | - | - |
| [Cu(C18H16N3O2)(H2O)2]Cl (a) | 1640 | - 1120 | 1594 | 897 | 572 | - | - | - | - |
| C30H28N6O2 | 1654 | - | 1595 | - | - | - | - | - | |
| [Cu2(C30H28N6O2)(SO4)2] (b) | 1639 | - | 1565 | - | 995 | 462 | 1050 1105 1170 | 574 610 641 | |
Electronic spectra.
| Compound | Transitions d-d (cm-1) | Geometry | ||
|---|---|---|---|---|
| [Cu(C18H16N3O2)(H2O)2]Cl | x2-y2 → xz; yz; xy | C2v | ||
| 14600 | ||||
| [Cu2(C30H28N6O2)(SO4)2] | - | xy→xz; yz 13570 | xy→z2; x2-y2 14830 | Td deformed |
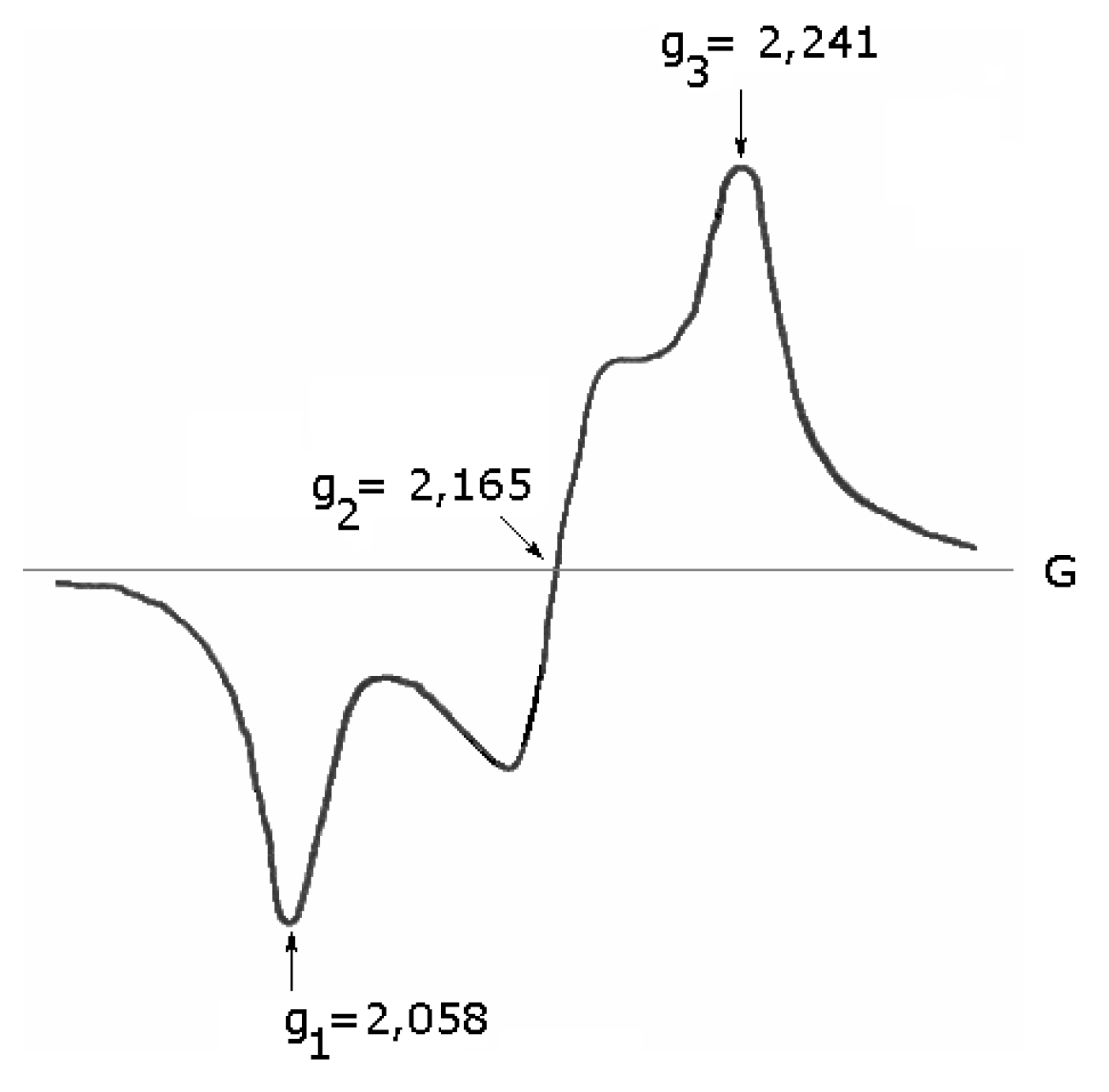
ESR spectra

Antimicrobial activity assays

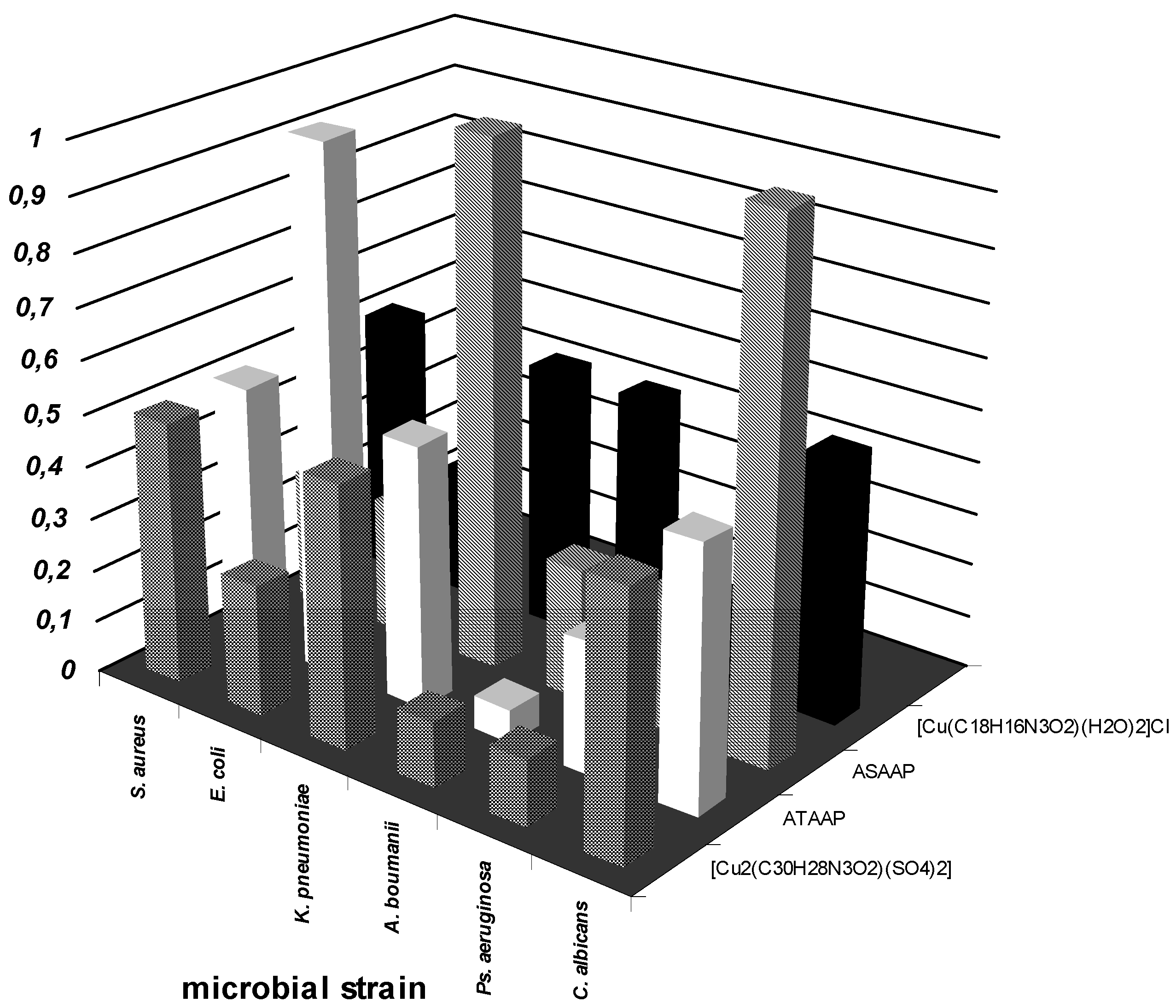
Conclusions
- a)
- the qualitative anti-microbial activity screening results of the tested compounds proved that the most efficient test method was the spot method.
- b)
- the quantitative anti-microbial activity test results proved that both the ligands and the complex combinations have specific anti-microbial activity, depending on the microbial species tested.
Experimental
General
Synthesis of the Schiff base 1-phenyl-2,3-dimethyl-4-(N-salicylidene)-3-pyrazolin-5-one (ASAAP)
Synthesis of the Schiff base bis(1-phenyl-2,3-dimethyl-3-pyrazolin-5-one-4-imino) terephthalic aldehyde (ATAAP)
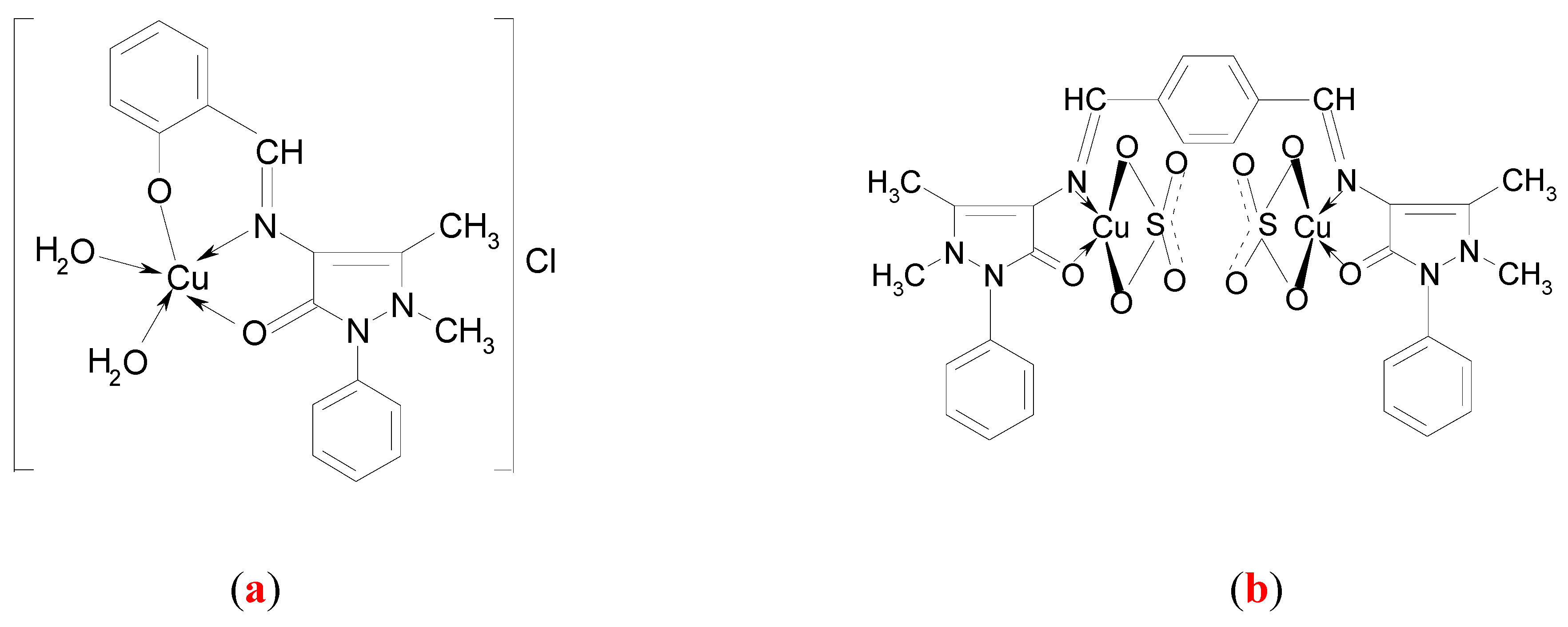
Synthesis of the complex [Cu(C18H16N3O2)(H2O)2]Cl (a)
Synthesis of the complex [Cu2(C30H28N6O2)(SO4)2] (b)
Biological assays
Acknowledgements
References
- Karlin, K.D.; Gultneh, Y. Bioinorganic chemical modeling of dioxygen-activating copper proteins. J. Chem. Ed. 1985, 62, 983–992. [Google Scholar] [CrossRef]
- Halfen, J.A.; Mahapatra, S.; Wilkinson, E. C.; Kaderli, S.; Young, V. G., Jr.; Que, L., Jr.; Zuberbühler, A.D.; Tolman, W.B. Reversible cleavage and formation of dioxygen O-O bond within a copper complex. Science 1996, 271, 1397–1400. [Google Scholar]
- (a)Berdanier, C.D.; Groff, J.L.; Gropper, S.S. Advanced Nutrition and Human Metabolism, 3rd. ed.; Wadsworth/Thomson Learning: Belmont, CA, 1999; pp. 87–105. [Google Scholar](b)Gropper, S.S.; Smith, J.L.; Groff, J.L. Advanced Nutrition and Human Metabolism, 4th ed.; Thomson Wadsworth Publishing Co.: Belmont, CA, 2005. [Google Scholar]
- Brill, A.S.; Martin, R.B.; Williams, R.J.P. Electronic Aspects of Biochemistry; Academic Press Inc.: New-York, 1964; pp. 519–557. [Google Scholar]
- Frieden, E.; Osaki, S.; Kobayashi, H. Copper proteins and oxygen. Correlations between structure and function of the copper oxidases. J. Gen. Physiol. 1965, 49 (1 Suppl.), 213–252. [Google Scholar] [CrossRef]
- Sugiura, Y.; Hirayama, Y.; Tanaka, H.; Ishizu, K. Copper(II) complex of sulfur-containing peptides. Characterization and similarity of electron spin resonance spectrum to the chromophore in blue copper proteins. J. Am. Chem. Soc. 1975, 97, 5577–5581. [Google Scholar] [CrossRef]
- Fee, J.A. Copper proteins - Systems containing the "blue" copper center. In Structure and Bonding; Springer-Verlag: Heidelberg, 1975; Vol. 23, pp. 1–60. [Google Scholar]
- Sakaguchi, U.; Addison, A.W. Structural implications for blue protein copper centers from electron spin resonance spectra of copper sulfide (CuIIS4) chromophores. J. Am. Chem. Soc. 1977, 99, 5189–5190. [Google Scholar] [CrossRef]
- Raman, N.; Kulandaisamy, A.; Shunmugasundaram, A.; Jeyasubramanian, K. Synthesis, spectral, redox and antimicrobial activities of Schiff base complexes derived from 1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one and acetoacetanilide. Transit. Metal Chem. 2001, 26, 131–135. [Google Scholar] [CrossRef]
- Raman, N.; Kulandaisamy, A.; Jeyasubramanian, K. Synthesis, spectral, redox and antimicrobial activity of Schiff base transition metal(II) complexes derived from 4-aminoantipyrine and benzil. Synth. React. Inorg. Met-Org. Nano-Met. Chem. 2002, 32, 1583–1610. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, D.K.; Singh, U.; Kumar, A. Synthesis and spectral studies of Cu(II) coordination compounds of 4[N-(cinnamalidene)amino]antipyrine semicarbazone. Asian J. Chem. 2004, 16, 577–580. [Google Scholar]
- Raman, N.; Thangaraja, C.; Johnsonraja, S. Synthesis, spectral characterization, redox and antimicrobial activity of Schiff base transition metal(II) complexes derived from 4-aminoantipyrine and 3-salicylideneacetylacetone. Centr. Eur. J. Chem. 2005, 3, 537–555. [Google Scholar] [CrossRef]
- Pandey, O.P.; Sengupta, S.K.; Dwivedi, A. Organophosphorus derivatives containing antipyrine ring as chemotherapeutics against fungal pathogens of sugarcane. Electron. J. Environ. Agr. Food Chem. 2005, 4, 886–891. [Google Scholar]
- Agarwal, R.K.; Singh, L.; Sharma, D. K. Synthesis, spectral and biological properties of copper (II) complexes of thiosemicarbazones of Schiff bases derived from 4-aminoantipyrine and aromatic aldehydes. Bioinorg. Chem. Appl. 2006, article ID 59509. [Google Scholar]
- Agarwal, R.K.; Gargb, R.K.; Sindhub, S.K. Synthesis and magneto-spectral investigations of some six and nine coordinated complexes of lanthanides(III) derived from 4[N-(2'-hydroxy-1'-napthalidene)amino]antipyrine thiosemicarbazone. J. Iran. Chem. Soc. 2005, 2, 203–211. [Google Scholar] [CrossRef]
- Agarwal, R. K.; Prasad, S. Synthesis and spectral investigations of some platinum metals ions coordination compounds of 4[N-(furan-2'-carboxalidene)amino]antipyrine thiosemicarbazone and 4[N-(3',4',5'-trimethoxybenzalidene)amino]antipyrine thiosemicarbazone. Turk. J. Chem. 2005, 29, 289–297. [Google Scholar]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; Wiley and Sons: New York, 1986; pp. 248–249. [Google Scholar]
- Tyagi, S.; Hathaway, B.J. Crystal structure and electronic properties of bis(2,2-bipyridyl)-cyanocopper(II) nitrate dihydrate: a correlation of the in-plane angular distortion with the splitting of the electronic spectrum. J. Chem. Soc. Dalton Trans. 1983, 199–203. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier Science: New York, 1984; pp. 560–571. [Google Scholar]
- Bates, C.A.; Smoore, W.; Standley, K.J.; Stevens, K.W.H. Paramagnetic resonance of a Cu2+ ion in a tetrahedral crystal field. Proc. Phys. Soc. 1962, 79, 73–83. [Google Scholar] [CrossRef]
- Sharnoff, M. Electron paramagnetic resonance and the primarily 3d wavefunctions of the tetrachlorocuprate ion. J. Chem. Phys. 1965, 42, 3383–3395. [Google Scholar] [CrossRef]
- Forster, D.; Weiss, V.W. An electron paramagnetic resonance study of cupric ion tetrahedrally coordinated by nitrogen atoms. J. Phys. Chem. 1968, 72, 2669–2671. [Google Scholar] [CrossRef]
- Yokoi, H.; Addison, A.W. Spectroscopic and redox properties of pseudotetrahedral copper(II) complexes. Their relation to copper proteins. Inorg. Chem. 1977, 16, 1341–1349. [Google Scholar] [CrossRef]
- Wasson, J.U.R.; Klassen, D.M.; Richardson, H.W.; Hatfield, W.E. Low-symmetry copper(II) complexes. Spectral properties of dihalo[2,6-di(2'-quinolyl)pyridine]copper(II) complexes. Inorg. Chem. 1977, 16, 1906–1911. [Google Scholar] [CrossRef]
- Sample availability: Samples of the ligands ASAAP and ATAAP and the complexes (a) and (b) are available from MDPI.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rosu, T.; Pasculescu, S.; Lazar, V.; Chifiriuc, C.; Cernat, R. Copper(II) Complexes with Ligands Derived from 4-Amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and Biological Activity. Molecules 2006, 11, 904-914. https://doi.org/10.3390/11110904
Rosu T, Pasculescu S, Lazar V, Chifiriuc C, Cernat R. Copper(II) Complexes with Ligands Derived from 4-Amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and Biological Activity. Molecules. 2006; 11(11):904-914. https://doi.org/10.3390/11110904
Chicago/Turabian StyleRosu, Tudor, Simona Pasculescu, Veronica Lazar, Carmen Chifiriuc, and Raluca Cernat. 2006. "Copper(II) Complexes with Ligands Derived from 4-Amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one: Synthesis and Biological Activity" Molecules 11, no. 11: 904-914. https://doi.org/10.3390/11110904





