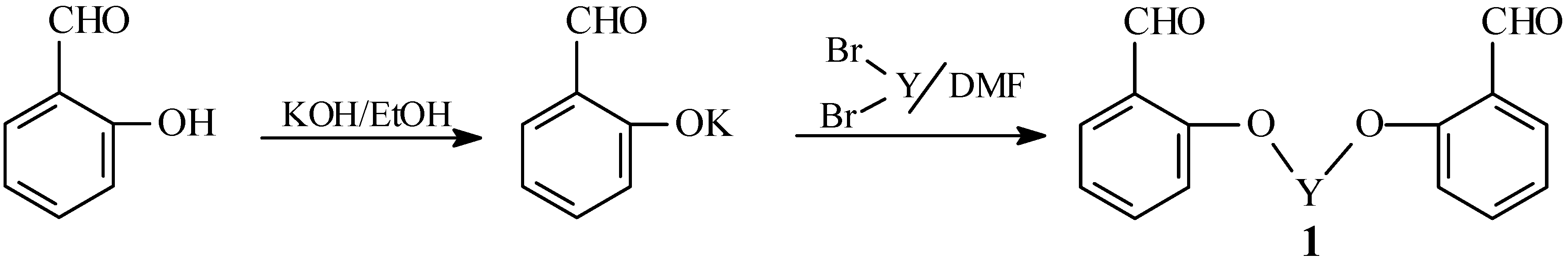Experimental
General
Melting points were determined on a Barnstead Electrothermal melting point apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra (δ, ppm) were recorded on a Varian-Mercury 200 MHz spectrophotometer using tetramethylsilane as the internal reference. The IR spectra (υ, cm-1) were obtained with a Perkin-Elmer 1600 FTIR spectrometer in KBr pellets. The mass spectra were recorded on a MicroMass Quattro LC-MS/MS (70 eV) spectrometer. The necessary chemicals were purchased from Merck and Fluka.
Synthesis of bis-aldehydes 1a-b
Salicylaldehyde (0.01 mol) was dissolved in hot ethanolic KOH (prepared by dissolving 0.01 mol of KOH in 100 mL of absolute ethanol) and the solvent was then removed in vacuo. The residue was dissolved in DMF (25 mL) and the appropriate dihalide (0.005 mol) was added. The reaction mixture was refluxed for 5 minutes, during which KCl separated out. The solvent was then removed in vacuo and the remaining material was washed with water and crystallized from an appropriate solvent to give compounds 1a-b.
1,2-Bis(o-formylphenoxy)ethane (
1a). Yield 68%; m.p. 129-130 °C (from ethanol; lit. [
27] m.p. 129 °C).
1,3-Bis(o-formylphenoxy)propane (
1b). Yield 70%; m.p. 99-100 °C (from ethanol; lit. [
27] m.p. 99 °C).
Synthesis of hydrazones 2a-c
A solution of an appropriate hydrazide (0.01 mol) in absolute ethanol (25 mL) was added to a solution of iminoester hydrochloride (0.01 mol) in absolute ethanol (25 mL). The mixture was stirred for 6 h at 0-5 °C and subsequently for 2 h at room temperature. The reaction mixture was then poured into a beaker containing cold water (40 mL) and ice (10 g). The precipitate formed was washed with ice-water (50 mL), dried and the product was recrystallized from from 2:1 benzene-petroleum ether to give compounds 2a-c.
Ethyl benzoate benzoylhydrazone (
2a). Yield 79%; m.p. 120-121 °C (lit. [
28] m.p. 121°C).
Ethyl p-methylbenzoate benzoylhydrazone (
2b). Yield 84%; m.p. 77-78 °C (lit. [
26] m.p. 78 °C).
Ethyl p-chlorobenzoate benzoylhydrazone (2c). Yield 72%; m.p. 80-81 °C; IR: 3199 (N-H), 1637 (C=O), 1613 (C=N), 702, 730, 759, 839 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.37 (t, 3H, CH3), 4.10 (q, 2H, CH2), 7.44-7.75 (m, 5H, Ar-H), 8.13 (m, 4H, Ar-H),10.79 (s, 1H, NH).
Synthesis of amino compounds 3a-c
Compounds 2 (0.005 mol) were added to a solution of hydrazine hydrate (0.01 mol) in 1-propanol (50 mL) and the mixture was refluxed for 24 h. On cooling, a precipitate was formed. This product was filtered and, after drying, was washed with benzene (20 mL). The product was then recrystallized from an appropriate solvent to give compounds 3a-c.
4-Amino-3,5-diphenyl-4H-1,2,4-triazole (
3a). Yield 80%; m.p. 264-265 °C (from 1-propanol; lit. [
28], m.p. 265 °C).
4-Amino-3-p-tolyl-5-phenyl-4H-1,2,4-triazole (
3b). Yield 85%; m.p. 283-284 °C (from 1-propanol; lit. [
26], m.p. 284 °C).
4-Amino-3-p-chlorophenyl-5-phenyl-4H-1,2,4-triazole (
3c). Yield 68%; m.p. 284-285 °C (from ethyl acetate; lit. [
28], m.p. 285 °C).
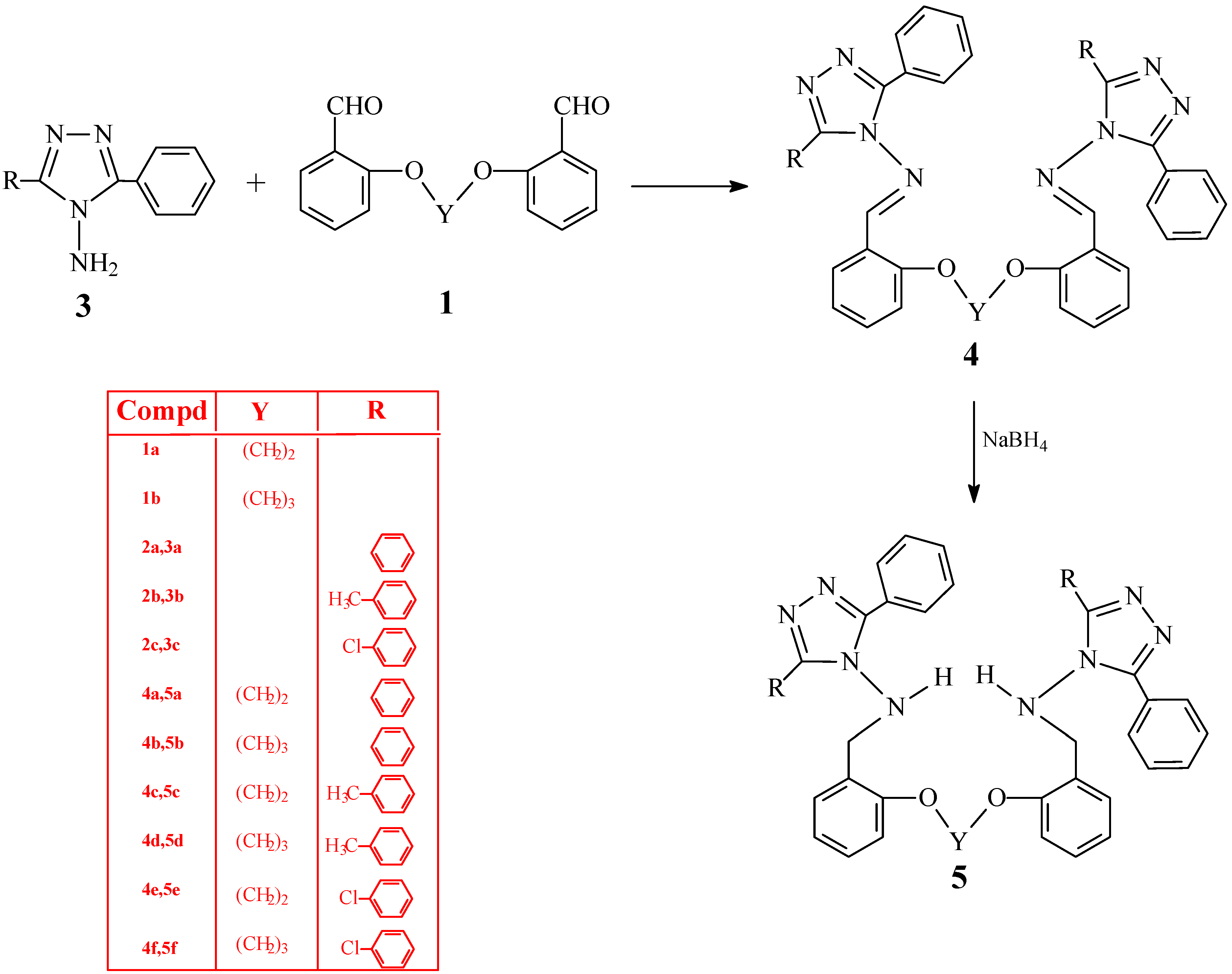
Synthesis of bis-Schiff bases 4a-f
The corresponding bis-aldehyde (0.01 mol) was added to a solution of compound 3 (0.005 mol) in glacial acetic acid (20 mL) and the mixture was refluxed for 16 h. After cooling, the mixture was poured into a beaker containing ice-water (100 mL). The precipitate formed was filtered. After drying in vacuo, the product was recrystallized from 1:2 benzene-petroleum ether to give the desired compound.
1,2-Bis[o-(N-methylidenamino-3,5-diphenyl-4H-1,2,4-triazole-4-yl)-phenoxy]ethane (4a). Yield 70%; m.p. 212-213 °C; IR: 1598 (C=N), 693, 770 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 3,98 (s, 4H, OCH2), Ar-H: [6,89-7,04 (m, 4H), 7,33 (m, 10H), 7,70 (m, 6H), 7.44-7.56 (m, 4H), 7,92-8,06 (m, 4H)], 8,23 (s, 2H, CH); 13C-NMR (DMSO-d6) δ (ppm): 164.44 (2C, N=CH), 149.87 (2C, triazole C3), 149.87 (2C, triazole C5), Ar-C: [158.20 (2C), 134.75 (2C), 129.55 (2C), 128.76 (8C), 128.27 (8C), 126.88 (4C), 126.23 (4C), 121.33 (2C), 119.39 (2C), 113.48 (2C)], 66.86 (2C, OCH2); LC-MS/MS, m/z (I, %) for C44H34N8O2 (m.w.: 706.81 g/mol): 729.26 [M+Na]+ (50), 707.25 [M+1]+ (30), 221.97 (100), 103.86 (60).
1,3-Bis[o-(N-methylidenamino-3,5-diphenyl-4H-1,2,4-triazole-4-yl)-phenoxy]propane (4b). Yield 72%; m.p. 189-190 °C; IR: 1595 (C=N), 694, 754 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1,65 (q, 2H, -CH2-), 3,77 (t, 4H, OCH2), Ar-H: [6,97-7,16 (m, 6H), 7,51 (m, 12H), 7,80 (m, 8H), 7,97 (m, 2H)], 8,67 (s, 2H, CH); 13C-NMR (DMSO-d6) δ (ppm): 164.73 (2C, N=CH), 150.00 (2C, triazole C3), 150.00 (2C, triazole C5), Ar-C: [158.36 (2C), 134.93 (2C), 129.60 (2C), 128.66 (8C), 128.23 (8C), 126.95 (4C), 126.48 (4C), 120.97 (2C), 119.29 (2C), 112.93 (2C)], 64.29 (2C, OCH2), 27.75 (C, CH2); LC-MS/MS, m/z (I, %) for C45H36N8O2 (m.w.: 720.83 g/mol): 743.18 [M+Na]+ (25), 721.23 [M+1]+ (60), 500.16 (12), 221.96 (100), 114.83 (28), 103.82 (32)
1,2-Bis[o-(N-methylidenamino-3-p-tolyl-5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]ethane (4c).Yield 72%; m.p. 271-272 °C; IR: 1597 (C=N), 696, 721, 757, 823 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 2,52 (s, 6H, CH3), 4,00 (s, 4H, OCH2), Ar-H: [7,12 (m, 8H), 7,32 (bs, 6H), 7,49-7,68 (m, 10H), 7,94(m, 2H)], 8,25 (s, 2H, CH); 13C-NMR (DMSO-d6) δ (ppm): 164.34 (2C, N=CH), 149.90 (2C, triazole C3), 149.72 (2C, triazole C5), Ar-C: [158.45 (2C), 139.30 (2C), 134.72 (2C), 129.50 (2C), 129.16 (4C), 128.56 (4C), 128.09 (4C), 128.00 (4C), 126.93 (2C), 126.31 (2C), 123.42 (2C), 121.36 (2C), 119.50 (2C), 113.56 (2C)], 66.86 (2C, OCH2), 20.73 (2C, CH3); LC-MS/MS, m/z (I, %) for C46H38N8O2 (m.w.: 734.86 g/mol): 757.18 [M+Na]+ (100), 735.14 [M+1]+ (70), 555.17 (22), 503.05 (14), 288.99 (98), 251.00 (35), 104.75 (27).
1,3-Bis[o-(N-methylidenamino-3-p-tolyl-5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]propane (4d). Yield 62%; m.p. 229-230 °C; IR: 1597 (C=N), 697, 723, 765, 822 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 2.51 (s, 6H, CH3), 1,69 (q, 2H, -CH2-), 3,78 (t, 4H, OCH2), Ar-H: [6.98-7.13 (m, 4H), 7.24 (m, 4H), 7.49 (m, 10H), 7.78 (m, 3H), 7.82 (m, 3H), 7.98 (m, 2H)], 8.69 (s, 2H,CH); 13C-NMR (DMSO-d6) δ (ppm): 164.65 (2C, N=CH), 150.04 (2C, triazole C3), 149.87 (2C, triazole C5), Ar-C: [158.33 (2C), 139.25 (2C), 134.90 (2C), 129.52 (2C), 129.20 (4C), 128.62 (4C), 128.17 (4C), 128.11 (4C), 126.96 (2C), 126.54 (2C), 123.61 (2C), 120.94 (2C), 119.31 (2C), 112.88 (2C)], 64.26 (2C, OCH2), 27.72 (C, CH2), 20.68 (2C, CH3); LC-MS/MS, m/z (I, %) for C47H40N8O2 (m.w.: 748.89 g/mol): 771.20 [M+Na]+ (55), 749.24 [M+1]+ (40), 475.31 (42), 235.88 (15), 155.88 (26), 148.83 (32), 117.86(100).
1,2-Bis[o-(N-methylidenamino-3-p-chlorophenyl-5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy] ethane (4e). Yield 75%; m.p. 213-214 °C; IR υ (cm-1): 1598 (C=N), 695, 723, 758, 834 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 4,02 (s, 4H, OCH2), Ar-H: [7,01-7,10 (m, 3H), 7,30-7,37 (m, 6H), 7,42-7,55 (m, 10H), 7,64-7,73 (m, 3H), 7,81 (d, 3H), 7,94 (m, 1H)], 8,30(s, 2H, CH); 13C-NMR (DMSO-d6) δ (ppm): 164.68 (2C, N=CH), 149.85 (2C, triazole C3), 149.10 (2C, triazole C5), Ar-C: [158.47(2C), 134.85 (2C), 134.46 (2C), 129.74 (4C), 129.56 (4C), 128.74 (4C), 128.57 (4C), 128.17 (2C), 127.01 (2C), 126.07 (2C), 125.11 (2C), 121.36 (2C), 119.35 (2C), 113.51 (2C)], 66.87 (2C, OCH2); LC-MS/MS, m/z (I, %) for C44H32Cl2N8O2 (m.w.: 775.70 g/mol): 798.11 [M+Na]+ (20), 775.13 [M]+ (18), 255.93 (10), 166.84 (15), 164.84 (43), 134.83(100).
1,3-Bis[o-(N-methylidenamino-3-p-chlorophenyl-5-phenyl-4H-1,2,4-triazol-4-yl)-phenoxy]propane (4f). Yield 77%; m.p. 189-190 °C; IR: 1597 (C=N), 690, 723, 757, 833 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1,69 (bs, 2H, -CH3-), 3,80 (bs, 4H, OCH2), Ar-H: [6,99-7,14 (m, 6H), 7,44-7,56 (m, 12H), 7,83-7,99 (m, 8H)], 8,70 (s, 2H, CH); 13C-NMR (DMSO-d6) δ (ppm): 163.88 (2C, N=CH), 149.08 (2C, triazol C3), 148.21 (2C, triazol C5), Ar-C: [157.41 (2C), 133.99 (2C), 133.47 (2C), 128.89 (4C), 127.78 (4C), 127.66 (4C), 127.24 (4C), 126.03 (2C), 125.35 (2C), 124.35 (2C), 124.23 (2C), 119.99 (2C), 118.25 (2C), 111.91 (2C)], 63.31 (2C, OCH2), 26.79 (CH2); LC-MS/MS, m/z (I, %) for C45H34Cl2N8O2 (m.w.: 789.72 g/mol): 811.19 [M+Na]+ (60), 789.31 [M]+ (56), 559.18 (13), 256.03 (53), 134.93 (84), 104.86 (100).
Synthesis of reduced rompounds 5a-f
The corresponding compound 4a-f (0.005 mol) was dissolved in dried methanol (50 mL) and NaBH4 (0.01 mol) was added in small portions to this solution. The mixture was refluxed for 20 min and then allowed to cool. After evaporation at 30-35 °C under reduced pressure, the solid residue was washed with cold water. After drying in vacuo, the solid product was recrystallized from an appropriate solvent (1:1 ethanol-water, unless otherwise noted) to afford the desired compound.
1,2-Bis[o-(N-methylamino-3,5-diphenyl-4H-1,2,4-triazole-4-yl)-phenoxy]ethane (5a). Yield 85%; m.p. 242-243 °C; IR: 3245 (NH), 1600 (C=N), 692, 720, 756 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 3.63 (d, 4H, -NH-CH2), 3.86 (s, 4H, OCH2), 6.95 (t, 2H, NH), Ar-H: [6.70 (d, 6H), 7.09-7.16 (m, 2H), 7.42 (m, 12H), 7.85-7.90 (m, 8H); 13C-NMR (DMSO-d6) δ (ppm): 153.57 (2C, triazole C3), 153.57 (2C, triazole C5), Ar-C: [156.20 (2C), 130.02 (2C), 129.47 (4C), 129.05 (2C), 128.29 (8C), 127.68 (8C), 126.97 (4C), 123.41 (2C), 120.11 (2C), 111.47 (2C)], 65.96 (2C, OCH2), 48.78 (2C,CH2-NH); LC-MS/MS, m/z (I, %) for C44H38N8O2 (m.w.: 710.84 g/mol): 733.22 [M+Na]+ (15), 711.22 [M+1]+ (35), 490.18 (8), 269.02 (11), 221.96 (28), 147.85 (100).
1,3-Bis[o-(N-methylamino-3,5-diphenyl-4H-1,2,4-triazole-4-yl)-phenoxy]propane (5b). Yield 74%; m.p. 216-217 °C; IR: 3253 (NH), 1601 (C=N), 694, 717, 745 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.88 (q, 2H, -CH2-), 3.55 ( bs, 4H, OCH2 + 4H, -NH-CH2 ), 7.04 ( m, 2H, 2NH + 2H, Ar-H ), Ar-H: [6.66 (m, 4H), 7.47 (bs, 14H), 7.94 (bs, 8H); 13C-NMR (DMSO-d6) δ (ppm): 153.65 (2C, triazole C3), 153.65 (2C, triazole C5), Ar-C: [156.24 (2C), 129.96 (2C), 129.54 (4C), 129.07 (2C), 128,34 (8C), 127.76 (8C), 127.07 (4C), 123,31 (2C), 119.81 (2C), 111.09 (2C)], 63.90 (2C, OCH2), 48.87 (2C, CH2-NH), 28.17 (CH2); LC-MS/MS, m/z (I, %) for C45H40N8O2 (m.w.: 724.87 g/mol): 747.26 [M+Na]+ (95), 725.28 [M+1]+ (100), 272.98 (52), 234.97 (100), 104.86 (16).
1,2-Bis[o-(N-methylamino-3-p-tolyl,5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]ethane (5c). Yield 79%; m.p. 192-193 °C; IR: 3261(NH), 1601(C=N), 690, 729, 748, 820 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 2.29 (s, 6H, CH3), 3.64 (d, 4H, -NH-CH2-), 3.88 (s, 4H, OCH2), 6.92 (t, 2H, NH), Ar-H: [6.72 (d, 4H), 7.22 (d, 4H), 7.37-7.43 (m, 10H), 7.78-7.87 (m, 8H); 13C-NMR (DMSO-d6) δ (ppm): 153.51 (2C, triazole C3), 153.47 (2C, triazole C5), Ar-C: [156.23 (2C), 139.15 (2C), 130.03 (2C), 129.40 (2C), 129.04 (2C), 128.92 (4C), 128.23 (4C), 127.71 (4C), 127.53 (4C), 127.05 (2C), 124.20 (2C), 123.48 (2C), 120.14 (2C), 111.48 (2C)], 65.98 (2C, OCH2), 48.76 (2C, CH2-NH), 20.80 (2C, CH3); LC-MS/MS, m/z (I, %) for C46H42N8O2 (m.w.: 738.65 g/mol): 740.30 [M+2]+ (55), 739.23 [M+1]+ (100), 414.95 (12), 370.13 (15), 216.93 (16), 156.88 (38).
1,3-Bis[o-(N-methylamino-3-p-tolyl,5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]propane (5d). Yield 78%; m.p. 181-182 °C; IR: 3249 (NH), 1601 (C=N), 692, 729, 749, 823 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 2.33 (s, 6H, CH3), 1.89 (q, 2H, -CH2-), 3.76 ( bs, 4H, OCH2 + 4H, -NH-CH2), 7.04 (t, 2H, NH), Ar-H: [6.63-6.74 (m, 6H), 7.11-7.16 (m, 2H), 7.26-7.37 (m, 5H), 7.45 (m, 5H), 7.82-7.93 (m, 8H)]; 13C-NMR (DMSO-d6) δ (ppm): 153.57 (2C, triazole C3), 153.48 (2C, triazole C5), Ar-C: [156.25 (2C), 139.22 (2C), 129.97 (2C), 129.44 (2C), 129.05 (2C), 128.94 (4C), 128.28 (4C), 127.77 (4C), 127.62 (4C), 127.12 (2C), 124.29 (2C), 123.37 (2C), 119.82 (2C), 111.06 (2C)], 63.93 (2C, OCH2), 28.19 (C, CH2), 48.82 (2C, CH2-NH), 20.83 (2C, CH3); LC-MS/MS, m/z (I, %) for C47H44N8O2 (m.w.: 752.85 g/mol): 775.23 [M+Na]+ (65), 753.33 [M+1]+ (100), 235.94 (5), 105.02 (5).
1,2-Bis[o-(N-methylamino-3-p-chlorophenyl,5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]ethane (5e). Yield 69%; m.p. 219-220 °C (from 1:2 ethanol-water); IR: 3249 (NH), 1601 (C=N), 689, 729, 749, 833 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 3.63 (bs, 4H, -NH-CH2-), 3.89 (bs, 4H, -OCH2), 6.99 (bs, 2H, NH), Ar-H: [6.71 (bs ,6H), 7.14 (bs, 2H), 7.45 (bs, 10H), 7.88 (m, 8H); 13C-NMR (DMSO-d6) δ (ppm): 153.81 (2C, triazole C3), 152.65 (2C, triazole C5), Ar-C: [156.21 (2C), 134.27 (2C), 130.20 (2C), 129.65 (2C), 129.35 (4C), 129.17 (4C), 128.41 (4C), 128.30 (4C), 127.66 (2C), 126.79 (2C), 125.69 (2C), 123.29 (2C), 120.14 (2C), 111.40 (2C)], 65.95 (2C, OCH2), 48.79 (2C, CH2-NH); LC-MS/MS, m/z (I, %) for C44H36Cl2N8O2 (m.w.: 778.24 g/mol): 801.12 [M+Na]+(48), 779.15 [M+1]+ (18), 747.26 (21), 725.22 (20), 214.93 (13), 164.84 (43), 134.83 (100).
1,3-Bis[o-(N-methylamino-3-p-chlorophenyl,5-phenyl-4H-1,2,4-triazole-4-yl)-phenoxy]propane (5f). Yield 72%; m.p. 126-127 °C (from 1:2 ethanol-water); IR: 3291(NH), 1601(C=N), 690, 734, 754, 835 cm-1 (aromatic ring); 1H-NMR (DMSO-d6) δ (ppm): 1.89 (bs, 2H, -CH2-), 3.75 ( bs, 4H, OCH2 + 4H, -NH-CH2 ), 7.08 ( m,2H, 2NH + 2H, Ar-H ), Ar-H: [6.65(m, 6H), 7.50 (m, 10H), 7.93 (m, 8H)]; 13C-NMR (DMSO-d6) δ (ppm): 153.81 (2C, triazole C3), 152.78 (2C, triazole C5), Ar-C: [156.21 (2C), 134.29 (2C), 130.11 (2C), 129.68 (2C), 129.44 (4C), 129.15 (2C), 128.45 (4C), 128.30 (4C), 127.69 (4C), 126.90 (2C), 125.78 (2C), 123.17 (2C), 119.78 (2C), 110.94 (2C)], 64.20 (2C, OCH2), 48.82 (2C, CH2-NH), 28.19 (C, CH2); LC-MS/MS, m/z (I, %) for C45H38Cl2N8O2 (m.w.: 792.25 g/mol): 815.26 [M+Na]+ (100), 793.25 [M+1]+ (45), 563.18 (28), 283.10 (12), 34.93 (15).