[Hydroxy(tosyloxy)iodo]benzene Mediated α-Azidation of Ketones
Abstract
:Introduction
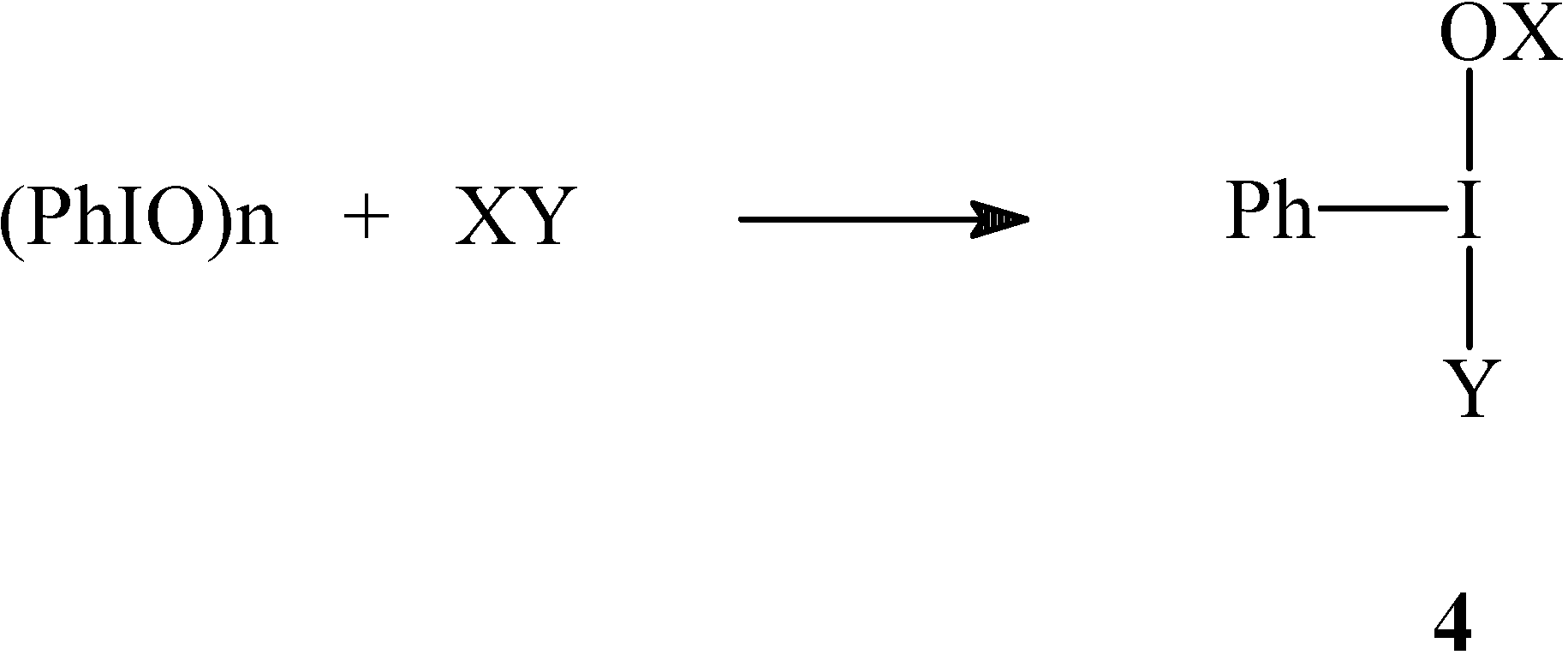
Results and Discussion
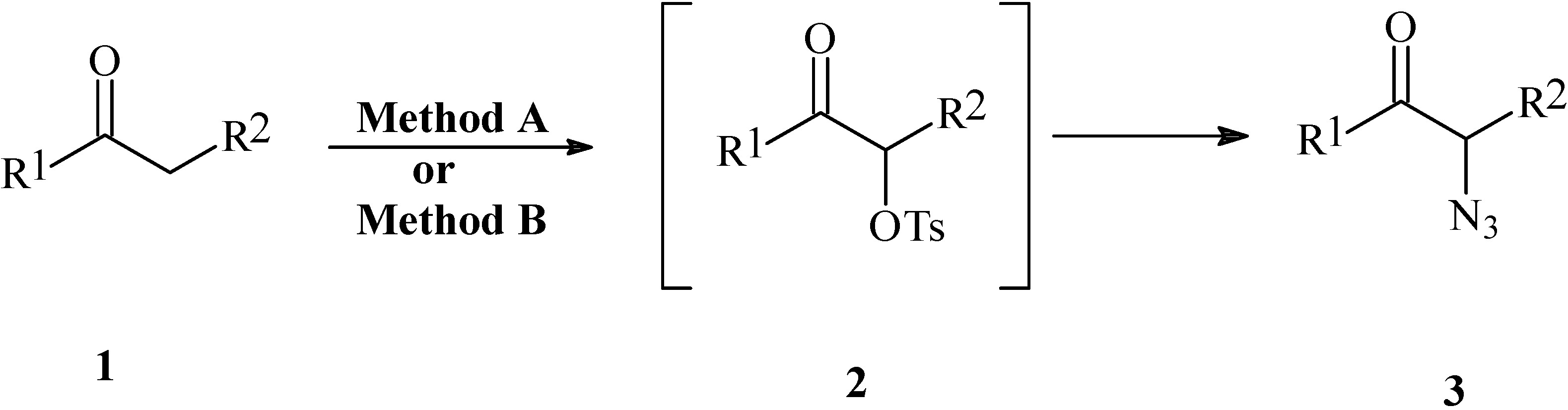
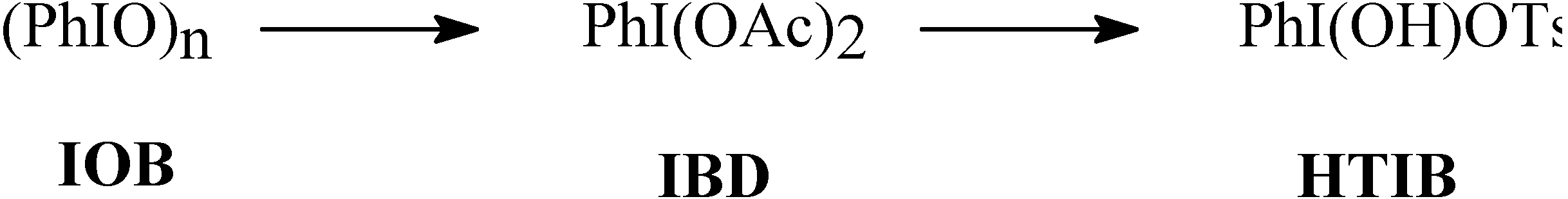
| Compound | R1 | R2 | Yield (%)a | |
|---|---|---|---|---|
| Method A | Method B | |||
| 2a | H | H | 80 | 76 |
| 2b | 4-CH3C6H4 | H | 69 | 74 |
| 2c | 4-CH3OC6H4 | H | 72 | 70 |
| 2d | 4-BrC6H4 | H | 78 | 71 |
| 2e | 4-ClC6H4 | H | 70 | 69 |
| 2f | H | CH3 | 81 | 71 |
| 2g | -(CH2)4- | 81 | 83 | |
Conclusions
Acknowledgements
Experimental
General
Representative one-pot procedure for the preparation of α-azido ketones: α-azidoacetophenone (2-azido-1-phenylethanone)(3a):
Method A. Using HTIB/ NaN3:
Method B. Using (PhIO)n + p-TsOH/ NaN3:
References
- Zhdankin, V.V.; Stang, P.J. Chem. Rev 2002, 102, 2523–2584. Varvoglis, A. Hypervalent iodine in organic synthesis; Academic Press: New York, 1997. [Google Scholar]
- Moriarty, R.M.; Chany II, C.J.; Kosmeder II, J.W. Encyclopedia of Reagents in Organic Synthesis; Paquette, L.A., Ed.; John Wiley & Sons Ltd.: Chichester, 1995; Vol 2, pp. 1479–1484. [Google Scholar] Prakash, O.; Singh, S.P. Aldrichim. Acta 1994, 27, 15–22. Varvoglis, A. Chem. Soc. Rev. 1981, 10, 377–407.
- Koser, G.F. Aldrichim. Acta 2001, 34, 89–101. Moriarity, R.M.; Vaid, R.K.; Koser, G.F. Synlett 1990, 365–383.
- Schardt, B.C.; Hill, C.L. Inorg. Chem. 1983, 22, 1563–1567.
- Ochiai, M.; Ukita, S.; Lawki, S.; Nagao, Y.; Fujita, E. J. Org. Chem. 1989, 54, 4832–4840.
- Moriarty, R.M.; Hu, H.; Gupta, S.C. Tetrahedron lett. 1981, 22, 1283–1286.
- Tohma, H.; Takizawa, S.; Maegawa, T.; Kita, Y. Angew Chem. Int. Edit. 2000, 39, 1306–1308.
- Yang, R.-Y.; Dai, L.–X. Synth. Comm. 1994, 24, 2229–2234.
- Prakash, O.; Rani, N.; Sharma, P.K. Synlett 1994, 221–226. Prakash, O. Aldrichim. Acta 1995, 28, 63–71. Prakash, O.; Saini, N.; Sharma, P.K. Heterocycles 1994, 38, 409–431. Mohan, J.; Singh, V.; Kumar, V.; Kataria, S. Indian J. Chem. 1994, 33B, 686–689. Singh, S.P.; Batra, H.; Sharma, P.K. J. Chem. Res. (S) 1997, 468–471.
- Furniss, B.S.; Hannaford, A.J. Vogel’s Textbook of Practical Organic Chemistry, 5th Edition; Longman: New York, 1989; pp. 869–870. [Google Scholar]
- Koser, G.F.; Wettach, R.H. J. Org. Chem. 1977, 42, 1476–1483.
- Boyer, J.H.; Straw, D.J. J. Am. Chem. Soc. 1952, 74, 4506–4508. Behringer, H.; Tuerck, U. Chem. Ber. 1966, 99, 1815–1815. Edwards, O.E.; Purushothaman, K.K. Can. J. Chem. 1964, 42, 712–716. Patonay, T.; Hoffman, R.V. J. Org. Chem. 1995, 60, 2368–2377.
- Lee, J.C.; Kim, S.; Shin, W.C. Synth. Comm. 2000, 30, 4271–4274.
- Sample availability: Contact the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Prakash, O.; Pannu, K.; Prakash, R.; Batra, A. [Hydroxy(tosyloxy)iodo]benzene Mediated α-Azidation of Ketones. Molecules 2006, 11, 523-527. https://doi.org/10.3390/11070523
Prakash O, Pannu K, Prakash R, Batra A. [Hydroxy(tosyloxy)iodo]benzene Mediated α-Azidation of Ketones. Molecules. 2006; 11(7):523-527. https://doi.org/10.3390/11070523
Chicago/Turabian StylePrakash, Om, Kamaljeet Pannu, Richa Prakash, and Anita Batra. 2006. "[Hydroxy(tosyloxy)iodo]benzene Mediated α-Azidation of Ketones" Molecules 11, no. 7: 523-527. https://doi.org/10.3390/11070523




