1,4-Benzodiazepine N-Nitrosoamidines: Useful Intermediates in the Synthesis of Tricyclic Benzodiazepines
Abstract
:Introduction
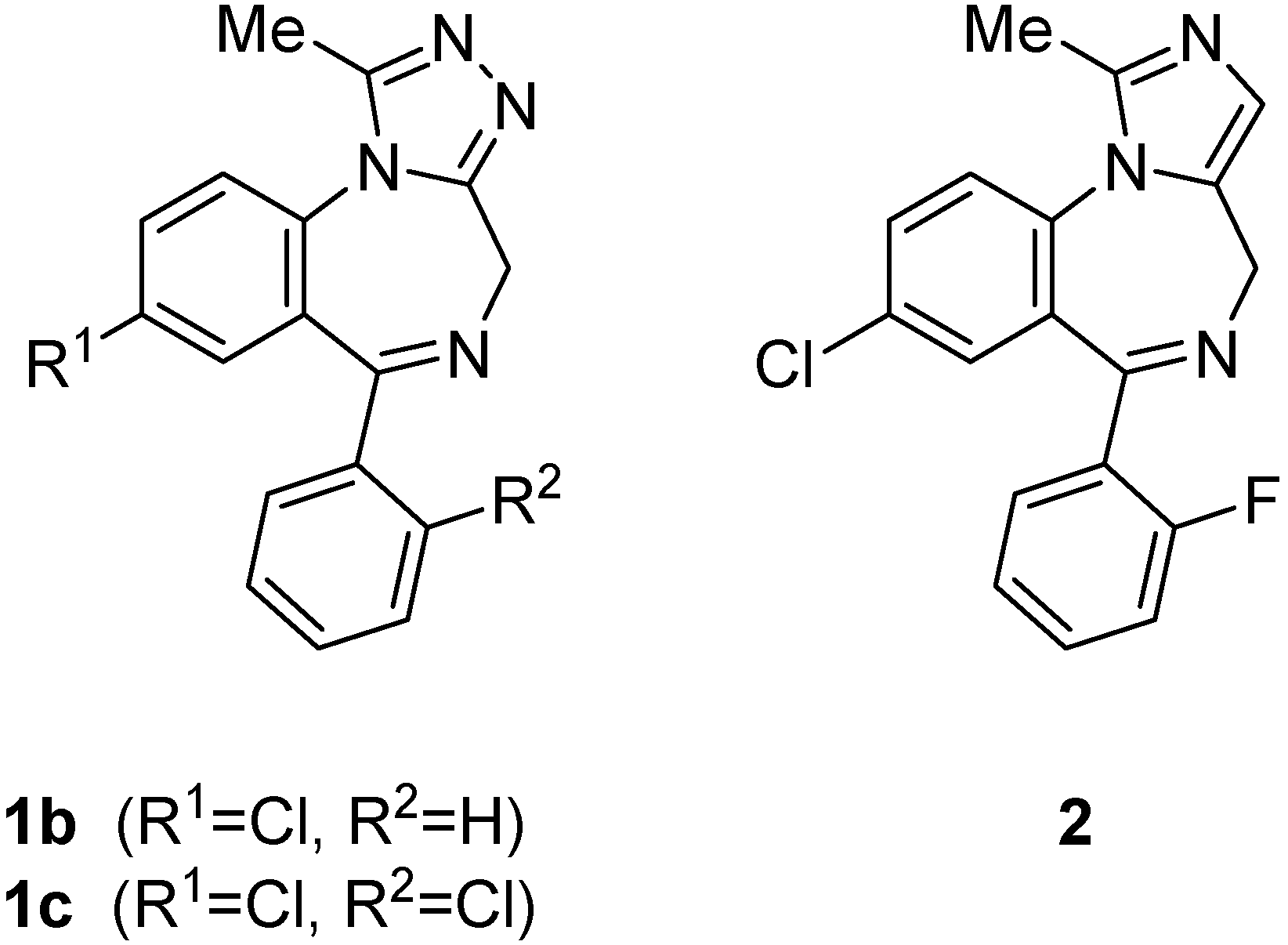
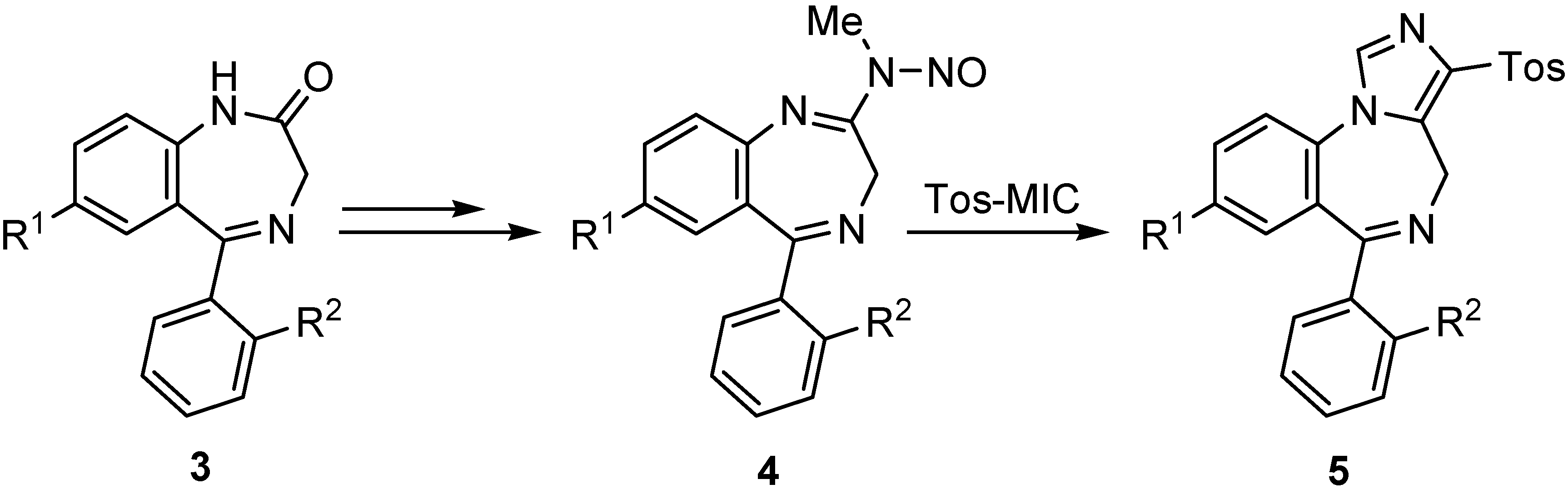
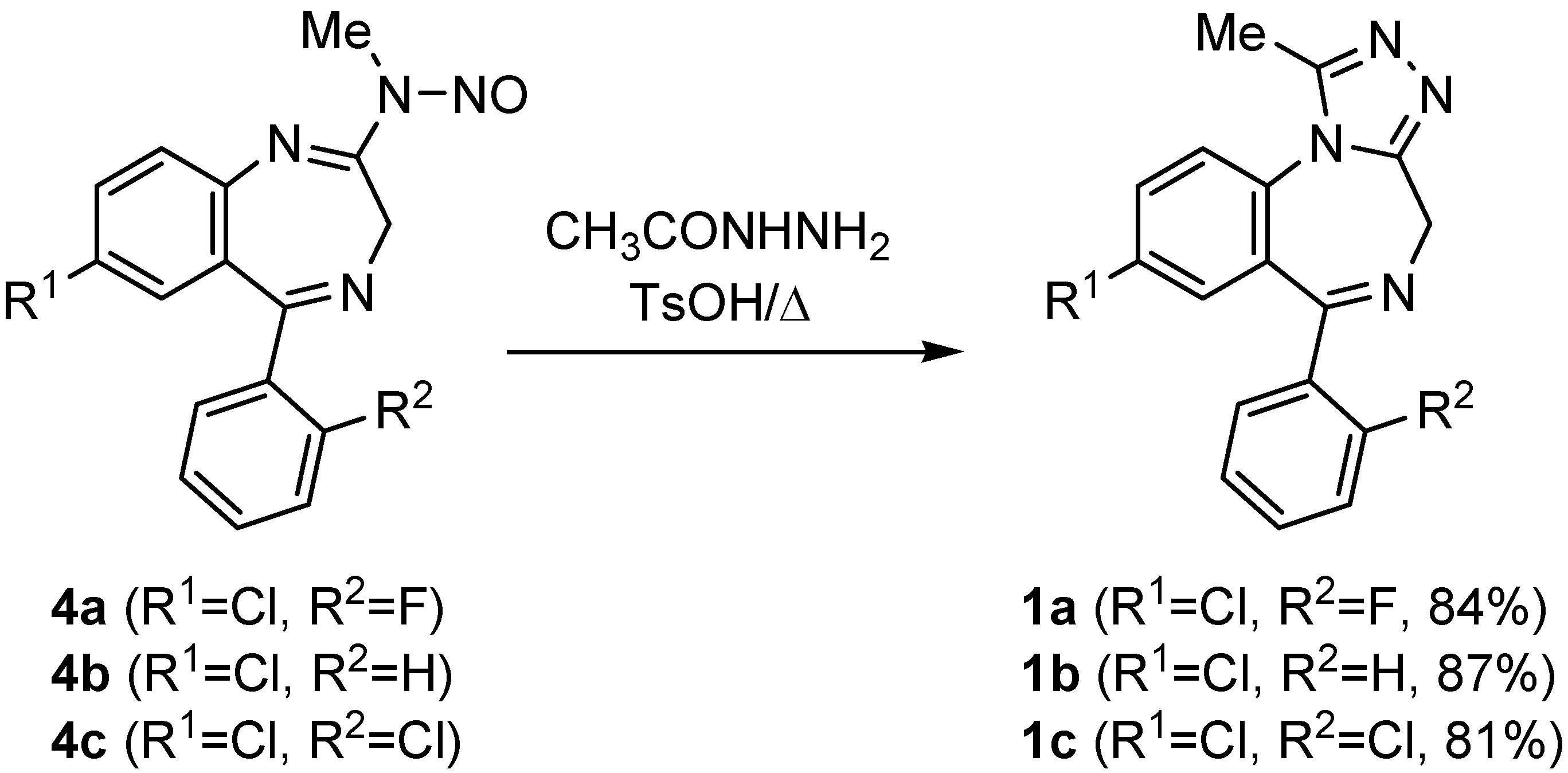
Results and Discussion
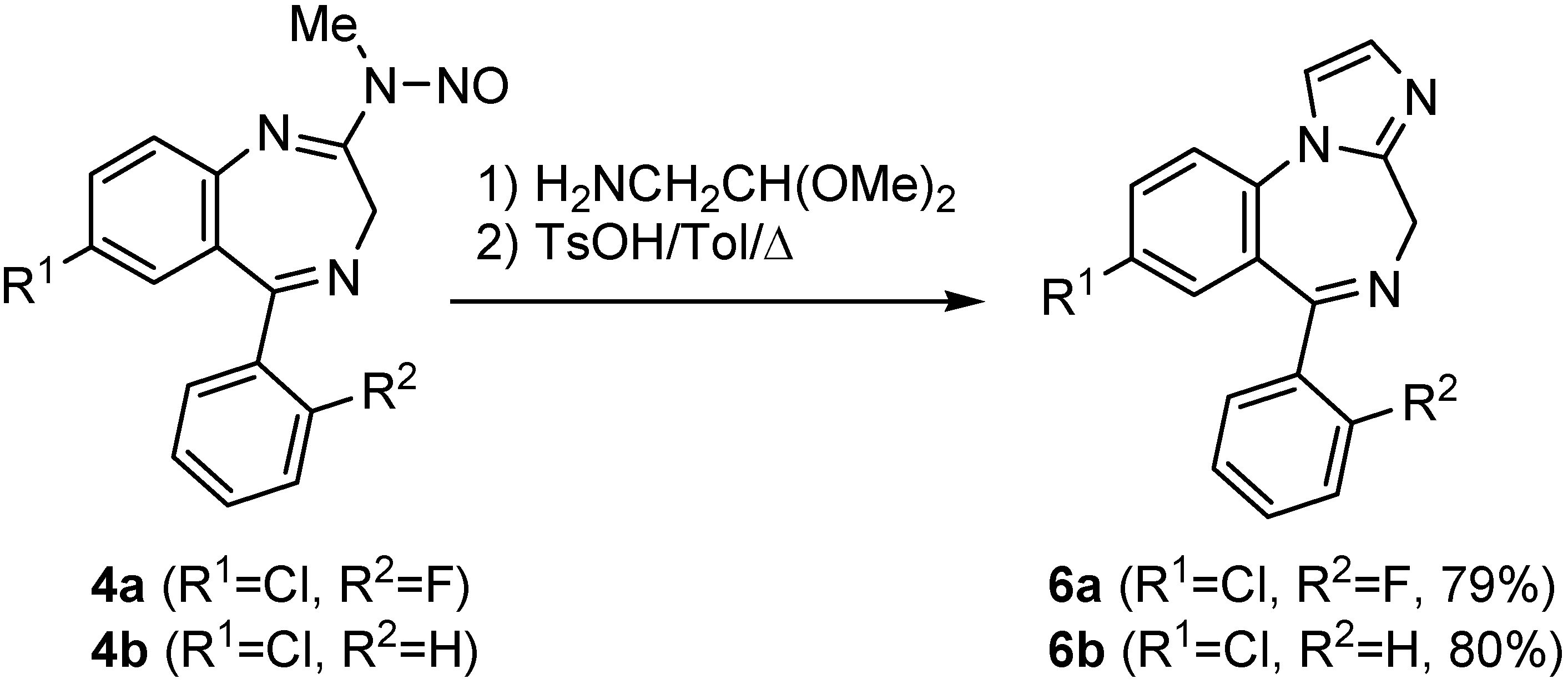
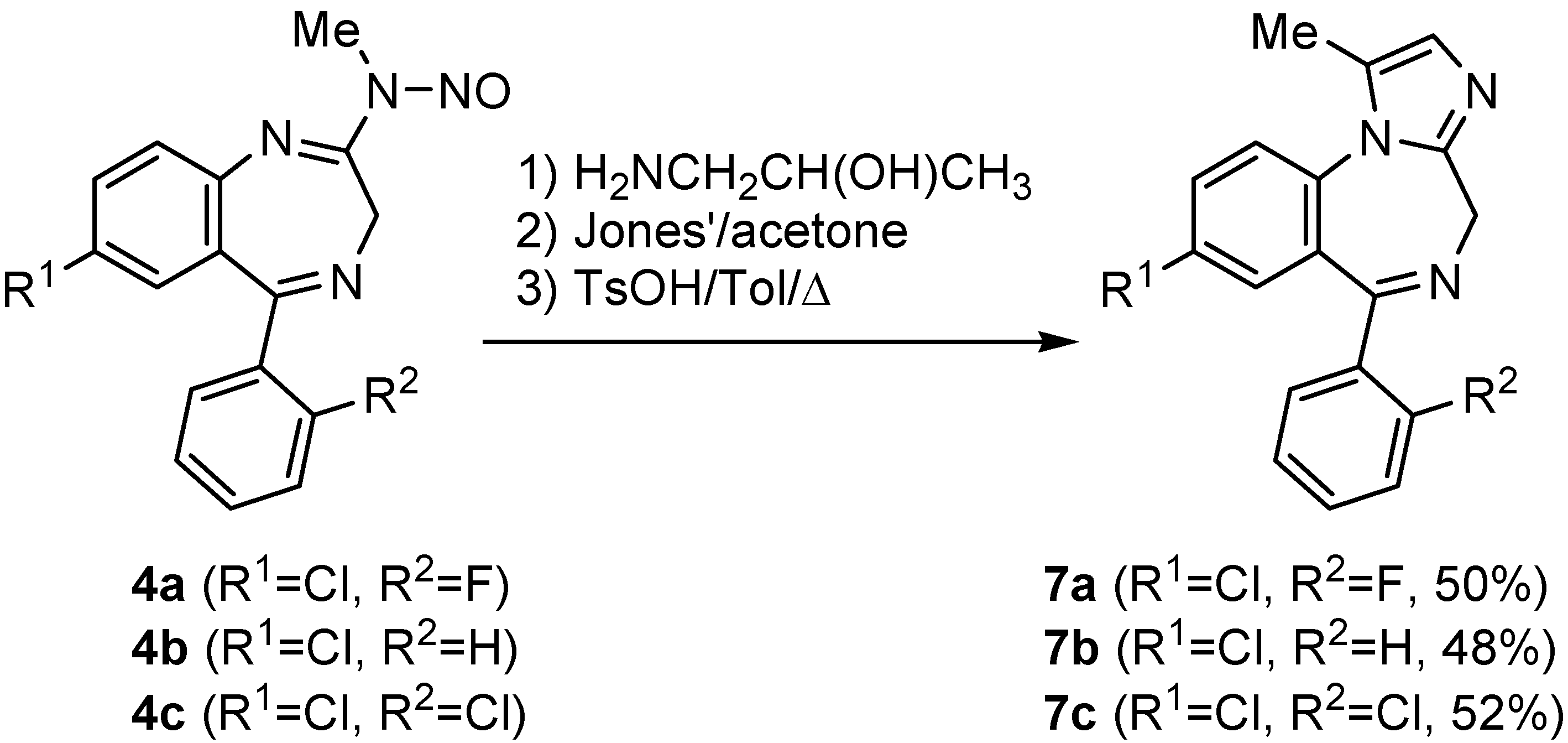
Conclusions
Experimental
General
General procedure for the preparation of triazolobenzodiazepines 1
General procedure for the preparation of imidazobenzodiazepines 6
General procedure for the preparation of imidazobenzodiazepines 7
Acknowledgements
References and Notes
- Reviews: Fielding, S.; LaI, H. Anxiolytics; Futurea: New York, 1979. [Google Scholar] Möhler, H.; Okada, T. Science 1977, 198, 849. [CrossRef]
- Vida, J. A. Medicinal Chemistry; Wolf, M. W., Burger, A., Eds.; Wiley: New York, 1981; p. 787. [Google Scholar] Landquist, J. K. Comprehensive Heterocyclic Chemistry; Katritzky, A. R., Rees, C. W., Eds.; Pergamon Press: Oxford, 1984; Vol 1, Ch. 1/06. [Google Scholar] Sharp, J. T. Comprehensive Heterocyclic Chemistry; Katritzky, A. R., Rees, C. W., Eds.; Pergamon Press: Oxford, 1984; Vol 7, Ch. 5/18. [Google Scholar] Hester, J. B., Jr. Antianxiety Agents; Berger, J. G., Ed.; Wiley: New York, 1986; p. 51. [Google Scholar] Fryer, R. I. Comprehensive Medicinal Chemistry; Hansch, C., Ed.; Pergamon Press: New York, 1990; Vol 3, p. 539. [Google Scholar]
- Hester, J. B., Jr. J. Heterocycl. Chem. 1980, 17, 575. [CrossRef] Hester, H. B., Jr.; Rudzik, A. D.; Voigtlander, P. F. V. J. Med. Chem. 1980, 23, 392. [CrossRef] Hester, H. B., Jr.; Rudzik, A. D.; Voigtlander, P. F. V. J. Med. Chem. 1980, 23, 402. [CrossRef] Hester, H. B., Jr.; Rudzik, A. D.; Voigtlander, P. F. V. J. Med. Chem. 1980, 23, 643. [CrossRef] Gall, M.; Kamdar, B. V. J. Med. Chem. 1981, 24, 1575. Walser, A.; Fryer, R. I. J. Heterocycl. Chem. 1983, 20, 551. [CrossRef] Gall, M.; Kamdar, B. V. J. Heterocycl. Chem. 1988, 25, 1649. [CrossRef] Banks, W. L.; Digenis, G. A. Tetrahedron Lett. 1989, 6473. [CrossRef] Hester, J. B.; Ludens, J. H.; Emmert, D. E.; West, B. E. J. Med. Chem. 1989, 32, 1157. [CrossRef] Walser, A.; Flynn, T.; Mason, C.; Crowley, H.; Maresca, C.; Yaremko, B.; O´Donnel, M. J. Med. Chem. 1991, 34, 1209. [CrossRef]
- Walser, A.; Fryer, R. I.; Sternbach, L. H. J. Heterocycl. Chem. 1974, 11, 619. [CrossRef] Walser, A.; Fryer, R. I. J. Org. Chem. 1975, 40, 153. [CrossRef] Walser, A.; Benjamin, L. E., Sr.; Flynn, T.; Mason, C.; Schwartz, R.; Fryer, R. I. J. Org. Chem. 1978, 43, 936. [CrossRef]
- del Pozo, C.; Macías, A.; Alonso, E.; González, J. Synthesis 2004, 2697.
- Amides 4 and 1,4-benzodiazepine N-nitrosoamidines 5 were prepared according to the procedure described in the literature: a) ref. 4c Bock, M. G.; Di Pardo, R. M.; Evans, B. E.; Rittle, K. E.; Jobey, D. F.; Freidinger, R. M.; Hirshfield, J.; Springer, J. P. J. Org. Chem. 1987, 52, 3232. [CrossRef]
- Although the synthesis of 5a starting from the corresponding thioamide of 3 via cyclization with acetic acid has already been described, the use of this modified protocol substantially increases the final yields of the overall process: Maffrand, J. P.; Eloy, F. F. Tetrahedron Lett. 1973, 36, 3449. [CrossRef]
- Granier, T.; Panday, N.; Vasella, A. Helv. Chim. Acta 1997, 80, 979. [CrossRef] Panday, N.; Granier, T.; Vasella, A. Helv. Chim. Acta 1998, 81, 475. [CrossRef] Panday, N.; Vasella, N. Synthesis 1999, 1459. [CrossRef]
- The synthesis of these analogs of midazolam 2 through condensation of the corresponding thioamide with propargylamine and further cyclization in acidic media has already been described. The current protocol can be viewed as an alternative for preparing these systems
- Hester, J. B., Jr.; Upjohn Co. US Pat. 3903103, 1975. Hester, J. B., Jr.; Upjohn Co. US Pat. 4000153, 1976. Hester, J., Jr.; Upjohn Co. US Pat. 3993660, 1976.
- Hester, J. B., Jr.; Hanze, A. R.; Upjohn Co. US Pat. 3927016, 1975. Hester, J. B., Jr.; Hanze, A. R.; Upjohn Co. US Pat. 3933794, 1976. Hester, J. B., Jr.; Hanze, A. R.; Upjohn Co. US Pat. 1975. Gall, M.; Upjohn Co. US Pat. 1976.
- Sample Availability: Not available.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fustero, S.; González, J.; Del Pozo, C. 1,4-Benzodiazepine N-Nitrosoamidines: Useful Intermediates in the Synthesis of Tricyclic Benzodiazepines. Molecules 2006, 11, 583-588. https://doi.org/10.3390/11080583
Fustero S, González J, Del Pozo C. 1,4-Benzodiazepine N-Nitrosoamidines: Useful Intermediates in the Synthesis of Tricyclic Benzodiazepines. Molecules. 2006; 11(8):583-588. https://doi.org/10.3390/11080583
Chicago/Turabian StyleFustero, Santos, Javier González, and Carlos Del Pozo. 2006. "1,4-Benzodiazepine N-Nitrosoamidines: Useful Intermediates in the Synthesis of Tricyclic Benzodiazepines" Molecules 11, no. 8: 583-588. https://doi.org/10.3390/11080583



