Glycerolipids from a Sarcotragus Species Sponge
Abstract
:Introduction
Results and Discussion
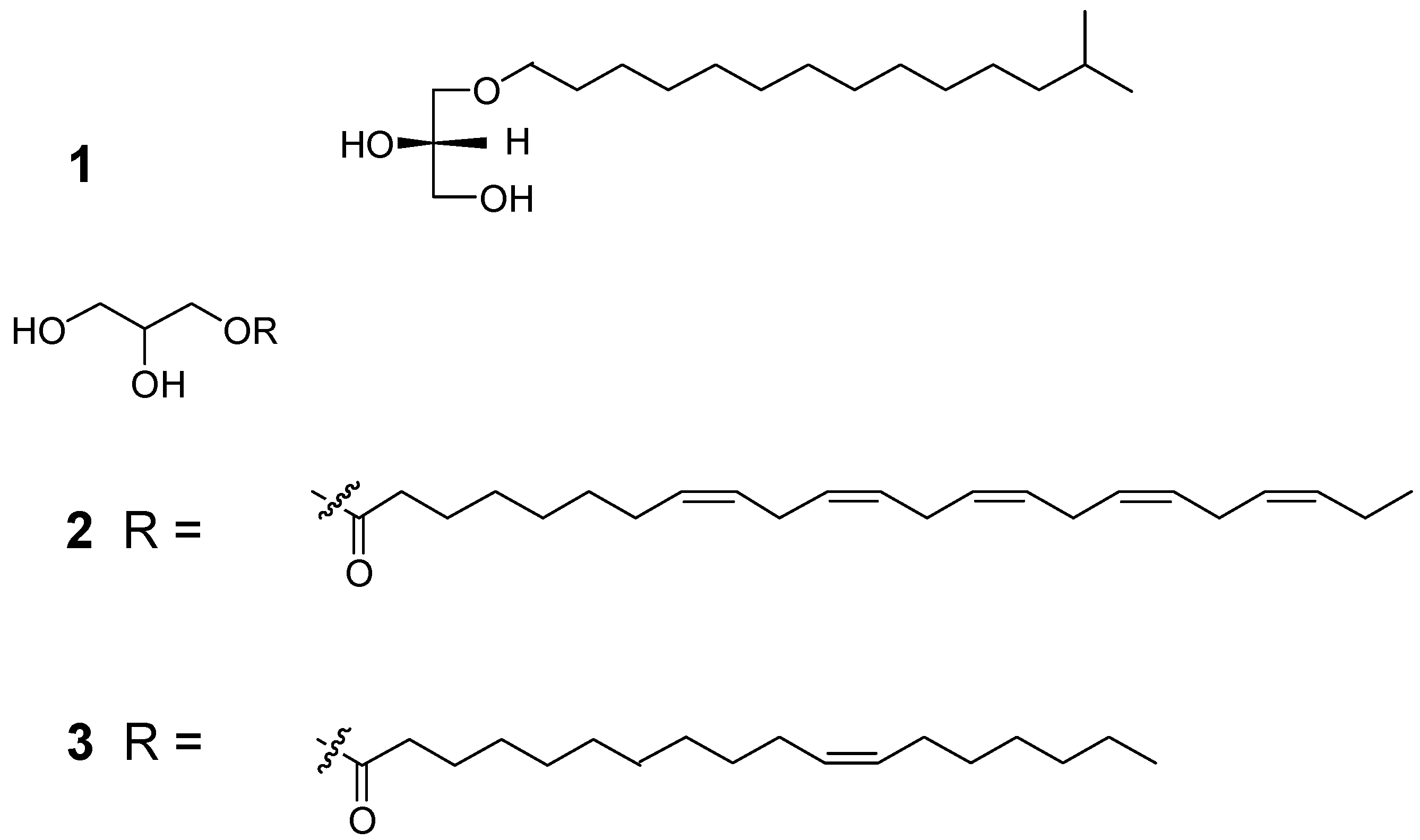
| Position | δH (mult, J) | δC |
| 1 | 4.14 (dd, 11.5, 4.5) 3.5) | 71.2 |
| 4.05(dd,11.5, 6.5) | ||
| 2 | 3.81 (m) | 71.0 |
| 3 | 3.54 (dd, 5.0, 2.5) | 73.1 |
| 1' | 175.4 | |
| 2' | 2.34 (t, 7.5) | 34.9 |
| 3' | 1.62 (quint, 8.0) | 25.9 |
| 4' | 1.28 - 1.39 (m) | 28.0 |
| 5' | 1.28 - 1.39 (m) | 29.8 |
| 6' | 1.28 - 1.39 (m) | 29.8 |
| 7' | 2.08 (m) | 28.0 |
| 8' | 5.36 (m) | 132.8 |
| 9' | 5.36 (m) | 130.9 |
| 10' | 2.83 (m) | 26.6 |
| 11' | 5.36 (m) | 129.5 |
| 12' | 5.36 (m) | 129.4 |
| 13' | 2.83 (m) | 26.6 |
| 14' | 5.36 (m) | 129.0 |
| 15' | 5.36 (m) | 129.0 |
| 16' | 2.83 (m) | 26.6 |
| 17' | 5.36 (m) | 129.0 |
| 18' | 5.36 (m) | 129.0 |
| 19' | 2.83 (m) | 26.4 |
| 20' | 5.36 (m) | 128.9 |
| 21' | 5.36 (m) | 128.2 |
| 22' | 2.08 (m) | 21.5 |
| 23' | 0.97 (t, 7.5) | 14.6 |
Acknowledgments
Experimental
General
Animal Material
Extraction and Isolation
References and Notes
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2005, 15–41, and earlier reviews cited therein. [Google Scholar]
- Faulkner, D.J. Marine Natural Products. Nat. Prod. Rep. 2002, 1, 1–49, and earlier reviews cited therein. [Google Scholar]
- Liu, Y.; Bae, B.K.; Alam, N.; Hong, J.; Sim, C.J.; Lee, C-O.; Im, K.S.; Jung, J.H. New Cytotoxic Sesterterpenes from the Sponge Sarcotragus Species. J. Nat. Prod. 2001, 64, 1301–1304. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, J.; Lee, C-O.; Im, K.S.; Kim, N.D.; Choi, J.S.; Jung, J.H. Cytotoxic Pyrrolo- and Furanoterpenoids from the Sponge Sarcotragus Species. J. Nat. Prod. 2002, 65, 1307–1314. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, C-O.; Hong, J; Jung, J.H. Cyclitol Derivatives from the Sponge Sarcotragus Species. Bull. Korean Chem. Soc. 2002, 23, 1467–1469. [Google Scholar] [CrossRef]
- Liu, Y.; Mansoor, Tayyab, A; Hong, J.; Lee, C-O.; Sim, C.J.; Im, K. S.; Jung, J.H. New Cytotoxic Sesterterpenoids and Norsesterterpenoids from Two Sponges of the Genus Sarcotragus. J. Nat. Prod. 2003, 11, 1451–1456. [Google Scholar]
- Liu, Y.; Shinde, P.B.; Hong, J.; Lee, C-O.; Im, K.S.; Jung, J.H. Trisoxazole Macrolide from a Marine Sponge Sarcotragus Species. Nat. Prod. Sci. 2005, 11, 50–53. [Google Scholar]
- Harvey, D.J. Identification and Quantification of Lipids from Rabbit Harderian Glands by Gas Chromatography/Mass Spectrometry. Biomed. Chromatogr. 1991, 5, 143–147. [Google Scholar] [CrossRef]
- Quijano, L.; Cruz, F.; Navarrete, I.; Gomez, P.; Rios, T. Alkyl Glycerol Monoethers in the Marine Sponge Desmapsamma anchorata. Lipids 1994, 29, 731–734. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Aknin, M.; Fall, A.; Samb, A.; Miralls, J. An Unusaual Ether Glycolipid from the Senegalese Sponge Trikentrion loeve Carter. Tetrahedron 1993, 49, 2711–2716. [Google Scholar] [CrossRef]
- Hirai, N.; Kingston, D.G. Structure Elucidation of a Potent Mutagen from Human Feces. J. Am. Chem. Soc. 1982, 104, 6149–6150. [Google Scholar] [CrossRef]
- Watanabe, N.; Watanabe, S.; Ide, J.; Watanabe, Y.; Sakata, K.; Okamoto, K. Chemical Signals involved in Larval Metamorphosis in Hydroides ezoensia (Serpulidae; Polychaeta). Part II: Isolation and Identification of a New Monacyl Glycerol from Adult Tube Clumps as a Metamorphosis – Inducing Substance. J. Mar. Biotechnol. 1998, 6, 11–15. [Google Scholar]
- Sample availability: Compounds 1-3 are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liu, Y.; Jung, J.H.; Ji, H.; Zhang, S. Glycerolipids from a Sarcotragus Species Sponge. Molecules 2006, 11, 714-719. https://doi.org/10.3390/11090714
Liu Y, Jung JH, Ji H, Zhang S. Glycerolipids from a Sarcotragus Species Sponge. Molecules. 2006; 11(9):714-719. https://doi.org/10.3390/11090714
Chicago/Turabian StyleLiu, Yonghong, Jee H. Jung, Hong Ji, and Si Zhang. 2006. "Glycerolipids from a Sarcotragus Species Sponge" Molecules 11, no. 9: 714-719. https://doi.org/10.3390/11090714




