Tungstate Sulfuric Acid (TSA)/ KMnO4 as a Novel Heterogeneous System for Rapid Deoximation
Abstract
:Introduction

Results and Discussion
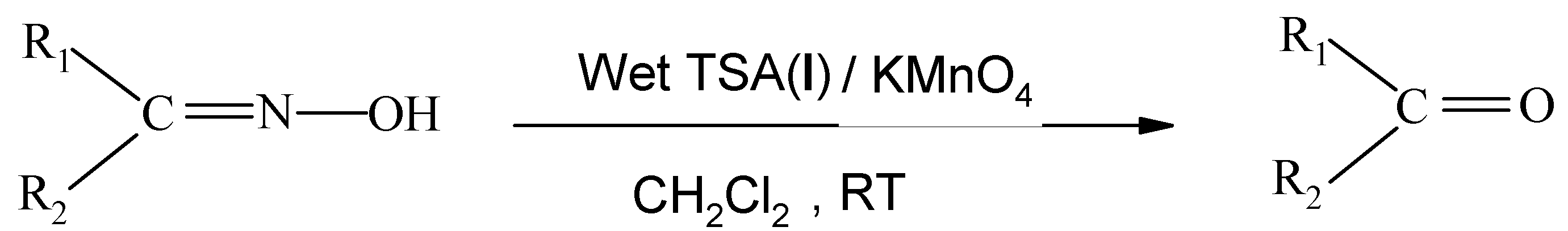
| Entry | Oxime substrate | Product a | Time (min.) | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  | 10 | 91 |
| 2 |  |  | 5 | 90 |
| 3 |  |  | 5 | 92 |
| 4 |  |  | 12 | 90 |
| 5 |  |  | 10 | 93 |
| 6 |  |  | 10 | 90 |
| 7 |  |  | 8 | 92 |
| 8 |  |  | 10 | 94 |
| 9 |  |  | 12 | 90 |
| 10 |  |  | 8 | 90 |
| 11 |  |  | 8 | 94 |
| 12 |  |  | 15 | 91 |
| 13 |  |  | 10 | 92 |
| 14 |  |  | 5 | 90 |
| 15 |  |  | 12 | 90 |
| 16 |  |  | 8 | 92 |
| 17 |  |  | 10 | 90 |
| 18 |  |  | 8 | 90 |
Conclusions
Experimental
General
Preparation of tungstate sulfuric acid (TSA, I).
Typical deoximation procedure: oxidation of 3-nitrobenzaldoxime (Table 1, entry 3).
References
- Zolfigol, M. A. Tetrahedron 2001, 57, 9509–9511.
- Olah, G. A.; Molhotra, R.; Narang, S. C. J. Org. Chem. 1987, 43, 4628.
- Zolfigol, M. A.; Shirin, F.; Ghorbani Choghamarani, A.; Mohammadpoor-Baltork, I. Green Chem. 2002, 4, 562.
- Zolfigol, M. A.; Bamoniri, A. Synlett 2002, 1621.
- Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A. J. Korean Chem. Soc. 2001, 45, 546.Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A. Molecules 2002, 7, 751.
- Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A.; Zarei, A. Bull. Korean Chem. Soc. 2003, 24, 400.Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A.; Zaghaghi, Z. J. Chem. Research (S). 2003, 273.Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A.; Zaghaghi, Z.; Hazar, A. Acta Chem. Slov. 2003, 50, 563.Shirini, F.; Zolfigol, M. A.; Mohammadi, K. Bull. Korean Chem. Soc. 2004, 25, 325.
- Harmer, M. A.; Sun, Q. Appl. Catal. A: General 2001, 221, 45.
- Heydari, A.; Larijani, H.; Emami, J.; Karami, B. Tetrahedron Lett. 2000, 41, 2471.Asgarian Damavandi, J.; Zolfigol, M. A.; Karami, B. Synth. Commun. 2001, 31, 129.Niknam, K.; Karami, B.; Kiasat, A. R. Bull. Korean Chem. Soc. 2005, 26, 975–978.Karami, B.; Montazerozohori, M.; Habibi, M. H. Bull. Korean Chem. Soc. 2005, 26, 1125–1128.
- Hamal, S.; Santosh, K. M.; Chhabilal, G. Ind. J. Chem. 1996, 35B, 1116.
- Mohammadpoor-Baltork, I.; Pouranshirani, S. Synth. Commun. 1996, 26, 1.
- Bandgar, B. P.; Shaikh, S. I.; Iyer, S. Synth. Commun. 1996, 26, 1163.
- Bose, S. D.; Srinivas, P. Synth. Commun. 1997, 27, 3835.
- Barhate, N. B.; Gajare, A. S.; Wakharkar, R. D.; Sadalai, A. Tetrahedron Lett. 1997, 38, 653.
- Verma, R.S.; Meshram, H. M. Tetrahedron Lett. 1997, 38, 5427. [CrossRef]
- Chaudhari, S. S.; Akamanchi, K. G. Tetrahedron Lett. 1998, 39, 3209.
- Hajipour, A. R.; Mahboubghah, N. J. Chem. Res. (S). 1998, 123.
- Verma, R. S.; Dahia, R.; Saini, R. K. Tetrahedron Lett. 1997, 38, 8819.
- House, H. O. “Modern Synthetic Reactions”, 2nd ed.; W. A Benjamin: San Francisco, CA, USA, 1972; p. 275. [Google Scholar]
- Sam, D. J.; Simmons, H. F. J. Am. Chem. Soc. 1972, 94, 4024.
- Hershberg, E. B. J. Org. Chem. 1984, 13, 542.Drabowiez, J. Synthesis 1980, 125.Rao, C. G.; Radhakrishna, A. S.; Singh, R. B.; Bhatnagar, S. P. Synthesis 1983, 808.Bandgar, B. P.; Kunde, M. L. B.; Thote, J. L. Synth. Commun. 1997, 27, 1149.Butler, R. N.; Morris, G. J.; Odonohue, A. M. J. Chem. Res. (S). 1981, 61.Shim, S. B.; Kim, K.; Kim, Y. H. Tetrahedron. Lett. 1987, 28, 645.Salmon, M.; Miranda, R.; Angeles, E. Synth. Commun. 1986, 16, 1827.Moriarty, R. M.; Prakash, O.; Vavilikolanu, R. Synth. Commun. 1986, 16, 1247.Vankar, P.; Rathore, R.; Chandrasekaran, S. J. Org. Chem. 1986, 51, 3063.Chidambaram, N.; Satyanarayana, K.; Chandrasekaran, S. Synth. Commun. 1989, 19, 1724.Mona, D.; Cramman, P.; Spranzo, G.; Tagliapietra, P.; Manam, P. Synth. Commun. 1986, 16, 803.
- Vogel’s Text Book of Practical Organic Chemistry, 4th ed.; Longman Group Limited: London, 1978.
- Dictionary of Organic Compounds, 6th ed.; Chapman and Hall: London, 1982.
- Sample Availability: Available from the authors.
© 2006 by MDPI (http:www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Karami, B.; Montazerozohori, M. Tungstate Sulfuric Acid (TSA)/ KMnO4 as a Novel Heterogeneous System for Rapid Deoximation. Molecules 2006, 11, 720-725. https://doi.org/10.3390/11090720
Karami B, Montazerozohori M. Tungstate Sulfuric Acid (TSA)/ KMnO4 as a Novel Heterogeneous System for Rapid Deoximation. Molecules. 2006; 11(9):720-725. https://doi.org/10.3390/11090720
Chicago/Turabian StyleKarami, Bahador, and Morteza Montazerozohori. 2006. "Tungstate Sulfuric Acid (TSA)/ KMnO4 as a Novel Heterogeneous System for Rapid Deoximation" Molecules 11, no. 9: 720-725. https://doi.org/10.3390/11090720




