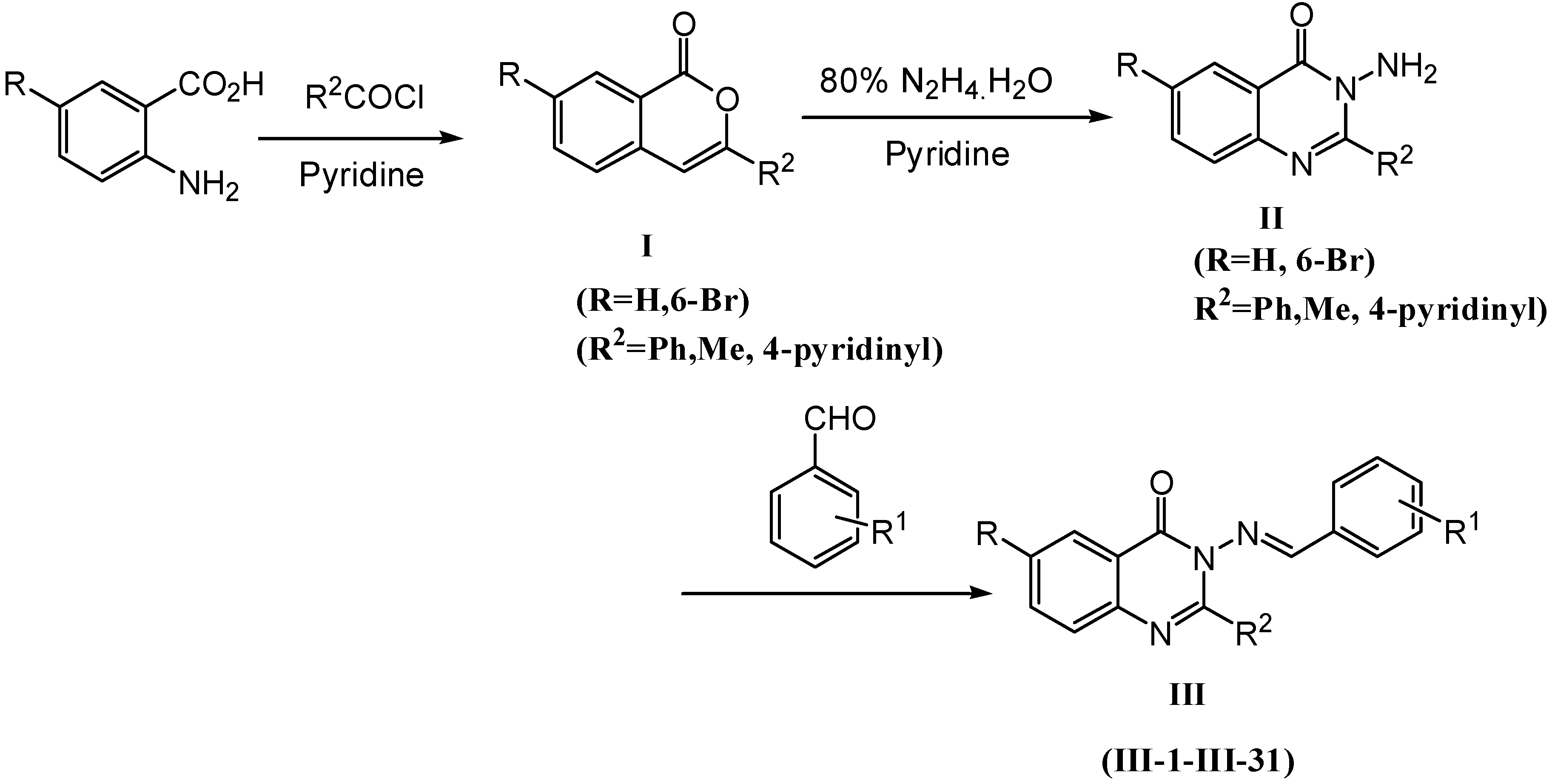
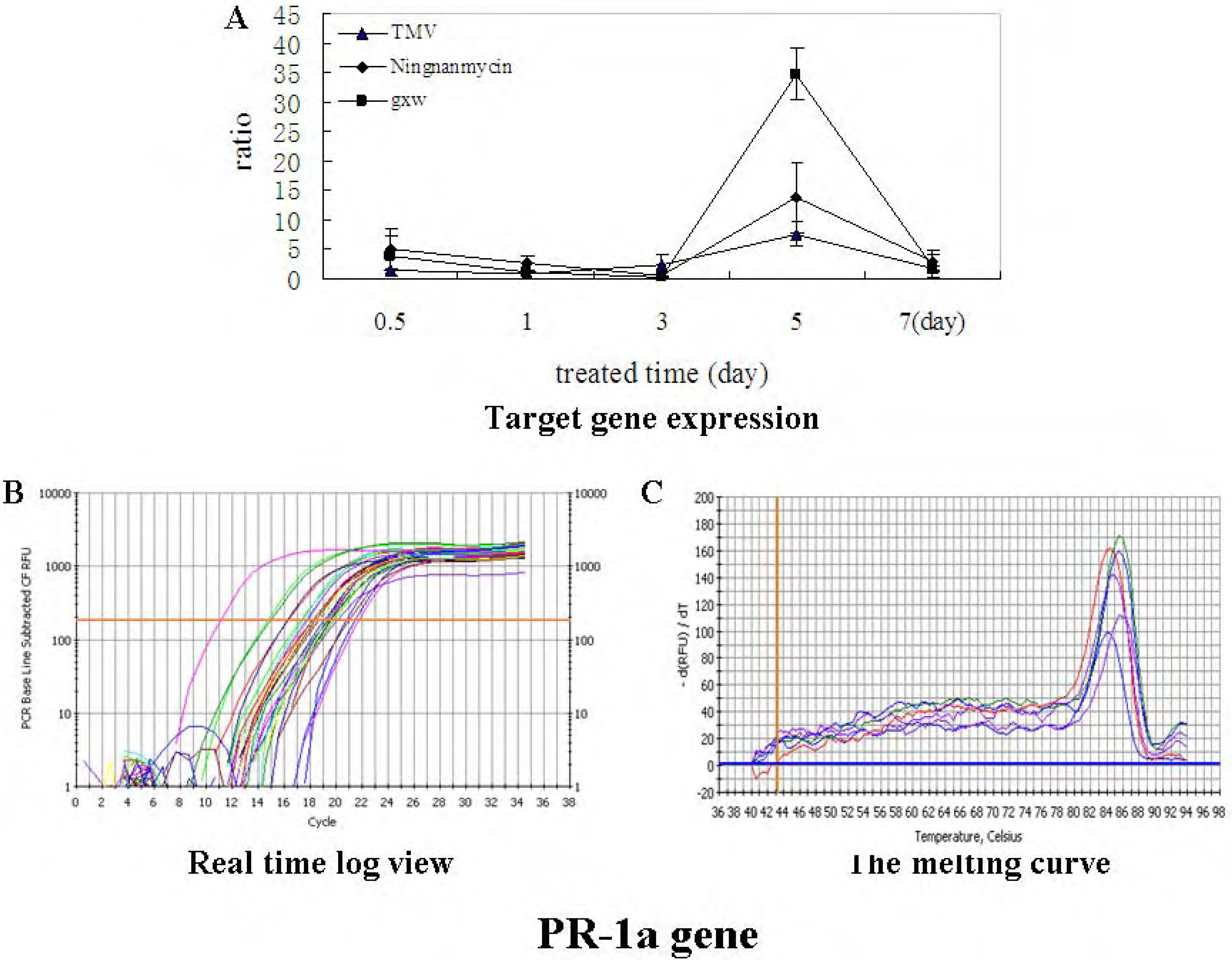
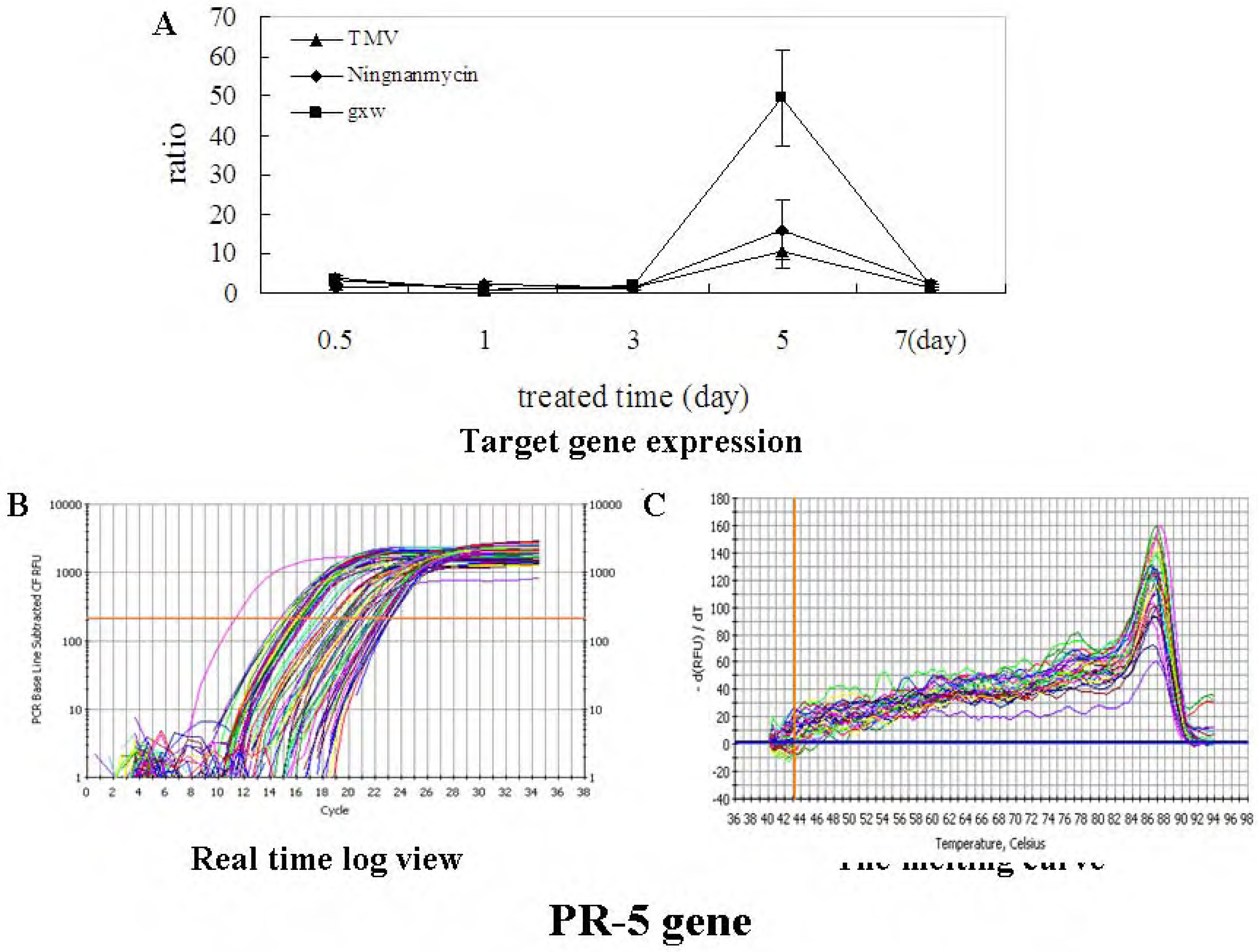
Preparation of Schiff base derivatives III-1~III-31
To a solution of the appropriate substituted benzaldehyde (1.0 mmol) in ethanol (15 mL), were added the 3-amino-2-aryl-4(3H)-quinazolinone (1.0 mmol) and a few drops of acetic acid (0.05 mmol). The reaction mixture was refluxed for 3-24h and the course of the reaction was monitored by TLC [petroleum ether/ethyl acetate (V/V=1:2)] to its completion. The reaction mixture was cooled. The crude product was recrystallized from 95% ethanol to give title compounds III-1~III-31.
2-Phenyl-3-(3,5-di-chlorobenzalamino-4(3H)-quinazolinone (III-1). White solid, yield 79%; m.p. 218~220 °C; IR (cm-1): ν 3088 (quinazolinone-H, Ar-H), 1678 (C=O), 1622, 1590 (C=N), 1551-1489 (C=C, benzene and quinazolinone rings), 875, 775, 688 (1,3,5-substituted benzene), 748, 700 (mono substituted benzene); 1H-NMR: δ 7.45-8.25 (m, 12H, quinazolinone-H, Ar-H), 9.35 (s, 1H, N=CH); 13C-NMR: δ 170.0, 158.3, 153.6, 146.9, 135.2, 135.1, 133.2, 132.8, 130.4, 130.3, 129.6, 129.1, 128.2, 128.1, 127.7, 127.3, 121.6; Anal. calcd. for C21H13Cl2N3O (394.26): C, 63.98%; H, 3.32%; N, 10.66%. Found: C, 63.86%;, H, 3.22%; N, 10.58%.
2-Phenyl-3-(3-nitro-4-chlorobenzalamino)-4(3H)-quinazolinone (III-2). White solid, yield 74%; m.p. 209~211 °C; IR (cm-1): ν 3057 (quinazolinone-H, Ar-H), 1681 (C=O), 1605, 1589 (C=N), 1568-1445 (C=C, benzene and quinazolinone rings), 889, 830 (1,2,4-trisubstituted benzene), 768, 692 (monosubstituted benzene); 1H-NMR: δ 7.28-8.26 (m, 12H, quinazolinone-H and Ar-H), 9.31 (s, 1H, N=CH); 13C-NMR: δ 163.3, 162.7, 161.2, 158.4, 146.8, 135.5 (J3 = 8.8 Hz), 135.2 (J3 = 6.5 Hz), 134.8, 130.3 (J2 = 12.5 Hz), 130.1, 130.0, 128.2 (J3 = 11.3 Hz), 127.9, 127.8, 127.7, 127.4, 127.3, 125.8, 121.6, 120.6 (J2 = 10.0 Hz), 117.0 (J2 = 8.8 Hz); Anal. calcd. for C21H13CIN4O3 (404.85): C, 42.25%; H, 3.21%; N, 13.83%. Found: C, 42.30%; H, 3.15%; N,13.75%.
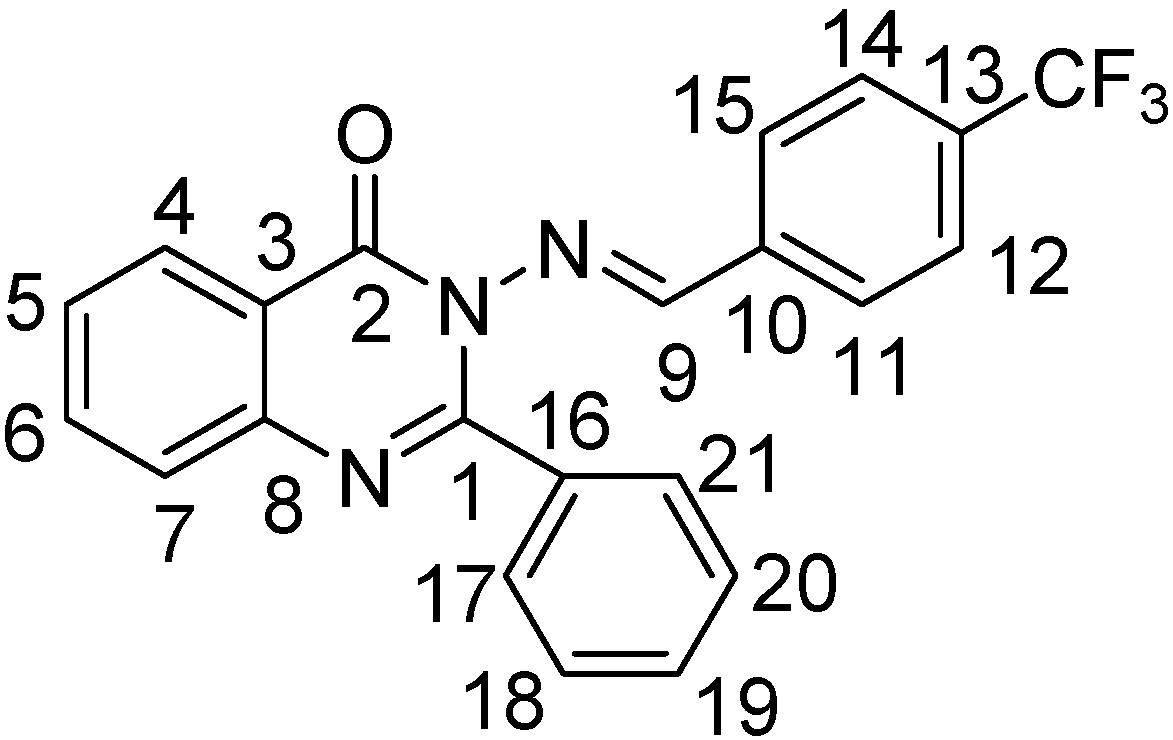
2-Phenyl-3-(4-trifluoromethylbenzalamino)-4(3H)-quinazolinone (III-3). White solid, yield 72%; m.p. 179.4~181.0 °C; IR (cm-1): ν 3061 (quinazolinone-H, Ar-H), 1672 (C=O), 1605, 1591 (C=N), 1564-1445 (C=C, benzene and quinazolinone rings), 843 (1,4-disubstituted benzene), 766, 696 (monosubstituted benzene); 1H-NMR: δ 5.68 (s, 2H), 7.42-8.28 (m, 13H, quinazolinone-H and Ar-H), 9.48 (s, 1H, N=CH); 13C-NMR: δ 164.4, 157.8, 153.0, 146.1, 134.9, 134.6 (J3 = 3.8 Hz), 133.9, 130.2, 129.9, 129.7 (J2 = 12.5 Hz), 127.8, 127.6 (J2 = 12.5 Hz), 127.1, 126.8, 121.0; Anal. Calcd. for C22H14F3N3O (393.37): C, 67.17%; H, 3.59%; N, 10.68%. Found: C, 67.41%; H, 4.09%; N, 10.86%.
2-Phenyl-3-(3-chlorobenzalamino)-4(3H)-quinazolinone (III-4). White solid, yield 71%; m.p. 190.0~192.1 °C; IR (cm-1): ν 3059 (quinazolinone-H, Ar-H), 1680 (C=O), 1605, 1584 (C=N), 1559,1474 (C=C, benzene and quinazolinone rings), 903, 775, 690 (1,3-disubstituted benzene), 765, 692 (monosubstituted benzene); 1H-NMR: δ 7.45-8.26 (m, 13H, quinazolinone-H and Ar-H), 9.12 (s, 1H, N=CH); 13C-NMR: δ 167.2, 157.7, 153.0, 146.1, 134.7, 134.4 (J2 = 7.5 Hz), 133.7, 132.1, 131.0, 129.8, 129.6, 127.6 (J2 = 7.2 Hz), 127.5, , 127.1 (J2 = 5.0 Hz), 126.7, 120.8; Anal. Calcd. for C21H14ClN3O (359.82): C, 70.10%; H, 3.92%; N, 11.68%. Found: C, 69.99%; H, 4.19%; N, 11.78%.
2-Phenyl-3-(2,3-dichlorobenzalamino)-4(3H)-quinazolinone (III-5). White solid, yield 70%; m.p. 207~208 °C; IR (cm-1): ν 3061 (quinazolinone-H, Ar-H), 1684 (C=O), 1603, 1587 (C=N), 1551-1445 (C=C, benzene and quinazolinone rings), 764, 696 (1,2,3-trisubstituted benzene), 768.0, 690.5 (monosubstituted benzene); 1H-NMR: δ 7.42-8.28 (m, 13H, quinazolinone-H and Ar-H), 9.48 (s, 1H, N=CH); 13C-NMR: δ 164.4, 157.8, 153.0, 146.1, 134.9, 134.6 (J3 = 3.8 Hz), 133.9, 130.2, 129.9, 129.7 (J2 = 12.5 Hz), 127.8, 127.6 (J2 = 12.5 Hz), 127.1, 126.8, 121.0; Anal. Calcd. for C21H13Cl2N3O (394.26): C, 63.98%; H, 3.32%; N, 10.66%. Found: C, 63.94%; H, 3.18%; N, 10.83%.
2-Phenyl-3-(2,6-dichlorobenzalamino)-4(3H)-quinazolinone (III-6). White solid, yield 78%; m.p. 179-180.3 °C; IR (cm-1): ν 3055 (quinazolinone-H, Ar-H), 1680 (C=O), 1607, 1587 (C=N), 1566-1472 (C=C, benzene and quinazolinone rings), 770, 690 (1,2,3-trisubstituted benzene), 766, 690 (monosubstituted benzene); 1H-NMR: δ 7.43-8.28 (m, 12H, quinazolinone-H and Ar-H), 9.37 (s, 1H, N=CH); 13C-NMR: δ 166.1, 158.1, 153.8, 146.7, 135.3, 135.0, 133.4, 130.2, 130.1, 130.0, 129.3, 128.3, 128.1, 127.8, 127.5, 121.8; Anal. Calcd. for C21H13Cl2N3O (394.26): C, 63.98%; H, 3.32%; N, 10.66%. Found: C, 63.66%;, H, 3.62%; N, 10.58%.
2-Phenyl-3-(3,4-difluorobenzalamino)-4(3H)-quinazolinone (III-7). White needles, yield 69%; m.p. 137.6~139.6 °C; IR (cm-1): ν 3057 (quinazolinone-H, Ar-H), 1678 (C=O), 1603, 1591 (C=N), 1566-1445 (C=C, benzene and quinazolinone rings), 881, 830 (1,2,4-trisubstituted benzene), 770, 692 (monosubstituted benzene); 1H-NMR δ: 7.45-8.25 (m, 13H, quinazolinone-H and Ar-H), 9.11 (s, 1H, N=CH); 13C-NMR δ: 170.0, 161.7, 158.3, 156.3, 153.6, 151.4, 147.2, 146.7, 135.4 (J2 = 13.8 Hz), 134.9 (J2 = 28.9 Hz), 130.6-130.0 (m), 128.2-127.8 (m), 127.3 (J2 = 10.0 Hz), 126.9 (J3 = 7.5 Hz), 126.6, 121.5, 120.6, 119.1 (J2 = 13.8 Hz), 117.3 (J2 = 17.6 Hz); Anal. Calcd. for C21H13F2N3O (361.35): C, 69.80%; H, 3.63%; N, 11.63%. Found: C, 69.38%; H, 3.28%; N, 11.09%.
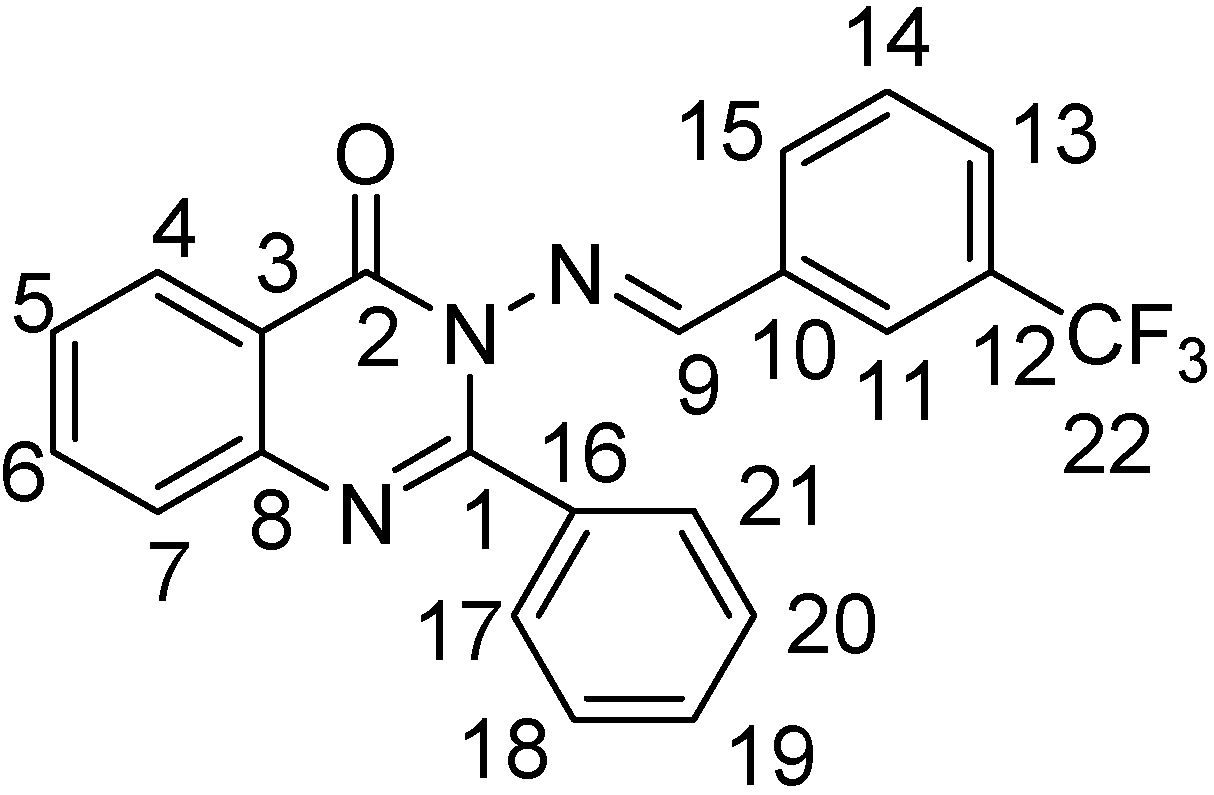
2-Phenyl-3-(3-trifluoromethylbenzalamino)-4(3H)-quinazolinone (III-8). White solid, yield 74%; m.p. 184~186 °C; IR (cm-1): ν 3060 (quinazolinone-H, Ar-H), 1670 (C=O), 1603, 1590 (C=N), 1564, 1445 (C=C, benzene and quinazolinone rings), 903 (1,3-disubstituted benzene), 766, 696 (mono- substituted benzene); 1H-NMR: δ 5.60 (s, 2H, ), 7.38-8.19 (m, 13H, quinazolinone-H and Ar-H), 9.40 (s, 1H, N=CH); 13C-NMR: δ 158.4, 157.8, 153.0, 146.1, 134.9, 134.6 (J3 = 3.8 Hz), 133.9, 130.2, 129.9, 129.7 (J2 = 12.5 Hz), 127.8, 127.6 (J2 = 12.5 Hz), 127.1, 126.8, 121.0; Anal. calcd. for C22H14F3N3O (393.37): C, 67.17%; H, 3.59%; N, 10.68%. Found: C, 67.22%; H, 3.60%; N, 10.80%.
2-(4-Pyridinyl)-3-(benzalamino)-4(3H)-quinazolinone (III-9). White solid, yield 67%; m.p. 225~226 °C; IR (cm-1): ν 3042 (ArH), 1682 (C=O), 1607, 1585 (C=N), 1566-1452 (C=C, benzene, quinazolinone and pyridine rings), 847 (4-substituted pyridine), 770, 691 (monosubstituted benzene); 1H-NMR: δ 7.51-7.73 (t, 9H, Ar-H, Qu-H), 7.82 (d, 2H, 3-Py-H, J = 10 Hz), 7.93 (t, 2H, 2-Py-H, J = 10 Hz), 9.11 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 169.5, 158.2, 150.0, 146.6, 135.3, 133.3, 132.7, 129.7, 129.1 (C-4, C-12, C-14), 128.8, 128.2, 127.4, 124.3, 122.0 (C-3, C-17, C-20); Anal. calcd.for C20H14N4O (326.36): C 73.61%, H 4.32%, N 17.17%. Found: C 73.54%, H 4.48%, N 17.24%.
2-(4-Pyridinyl)-3-(2-chlorobenzalamino)-4(3H)-quinazolinone (III-10). White solid, yield 69%; m.p. 248.7~250.2 °C; IR (cm-1): ν 3046 (ArH), 1682 (C=O), 1611, 1584 (C=N), 1564-1458 (C=C, benzene, quinazolinone and pyridine rings), 835, 725 (4-substituted pyridine), 764 (1,2-disubstituted benzene); 1H-NMR: δ 6.56 (d, 1H, J = 15 Hz), 7.37-7.87 (m, 8H, Ar-H, Qu-H), 7.91 (d, 2H, 3-Py-H, J = 10 Hz), 8.14 (d, 2H, 2-Py-H), 10.22 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 169.2, 163.8, 162.0 (J3 = 10.0 Hz), 158.8, 152.0, 147.8, 135.3, 132.1 (J3 = 8.8 Hz), 130.0 (J2 = 28.0 Hz), 129.4, 127.9, 127.3, 127.0, 125.7, 123.6 (J4 = 10.0 Hz), 121.2, 120.1, 116.7 (J2 = 21.5 Hz); Anal. calcd. for C20H13FN4O (344.35): C 69.76%, H 3.81%, N 16.27%. Found: C 69.59%, H 3.75%, N 16.39%.
2-(4-Pyridinyl)-3-(4-fluorobenzalamino)-4(3H)-quinazolinone (III-11). White solid, yield 89%; m.p. 203~204 °C; IR (cm-1) ν: 3080 (ArH), 1678 (C=O), 1611, 1597 (C=N), 1570-1447 (C=C, benzene, quinazolinone and pyridine rings), 839 (1,4-disubstituted benzene), 825, 726 (4-subsituted pyridine); 1H-NMR: δ 7.36-7.82 (m, 8H, Ar-H, Qu-H), 8.27 (d, 2H, 3-Py-H, J = 5 Hz), 8.70 (d, 2H, 2-Py-H, J = 5 Hz), 9.11 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 168.1, 166.0, 164.0, 158.2, 151.8, 150.0, 146.5, 142.6, 135.4, 131.7 (J3 = 8.8 Hz), 129.4, 128.3 (J4 = 2.5 Hz), 127.4, 124.3, 121.9, 117.1 (J2 = 21.3 Hz); Anal calcd for C20H13FN4O (344.35): C 69.76%, H 3.81%, N 16.27%. Found: C 69.73% H 4.00% N 16.40%.
2-(4-Pyridinyl)-3-(2, 3-dichlorobenzalamino)-4(3H)-quinazolinone (III-12). White solid, yield 89%; m.p. 201~203°C; IR (cm-1): ν 3060 (ArH), 1681 (C=O), 1614 (C=N), 1577-1447 (C=C, benzene, quinazolinone and pyridine rings), 826, 727 (4-subsituted pyridine), 790, 696 (1,2,3-trisubstituted benzene); 1H-NMR: δ 7.48-8.27 (m, 11H, Ar-H, Py-H, Qu-H), 9.56(s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 164.6, 158.4, 153.7, 146.6, 135.4, 135.2, 134.4, 133.4, 133.0, 130.4, 130.3, 129.3, 128.3, 128.1, 127.8, 127.5, 126.7, 121.6.
6-Bromo-2-methyl-3-(2-fluorobenzalamino)-4(3H)-quinazolinone (III-13). White solid, yield 59%; m.p. 179~181 °C; IR (cm-1): ν 3059 (Qu-H, Ar-H), 1680 (C=O), 1614, 1595 (C=N), 1571-1452 (C=C, benzene and quinazolinone rings), 760 (1,2-disubstituted benzene); 675 (Qu-Br), 1H-NMR: δ 2.51 (s, 3H, 2-Qu-CH3), 7.42-8.23 (m, 7H, Qu-H, Ar-H), 9.20 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 163.3 (J = 15 Hz), 161.3, 157.0 154.6, 146.7, 137.8, 135.7 (J = 10 Hz), 129.7, 129.2 (J = 10 Hz), 128.3, 125.9, 123.3, 120.4, 119.2, 117.0 (J = 15 Hz), 22.8; Anal. calcd. for C16H11BrFN3O (360.19): C 53.36% H 3.08% N 11.67%. Found: C 53.51%, H 3.28%, N 11.70%.
6-Bromo-2-methyl-3-(3-fluorobenzalamino)-4(3H)-quinazolinone (III-14). White solid, yield 63%; m.p. 212~214 °C; IR (cm-1): ν 3032 (Qu-H, Ar-H), 1670 (C=O), 1616, 1502 (C=N), 1581-1449 (C=C, benzene and quinazolinone rings), 874, 777, 695 (1,3-disubstituted benzene); 675 (Qu-Br); 1H-NMR: δ 2.53 (s, 3H, 2-Qu-CH3), 7.50-8.23 (m, 7H, Ar-H, Qu-H), 9.04 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 168.6, 163.9, 162.0, 157.0, 154.7, 145.8, 137.9, 135.1 (J = 10.0 Hz), 132.0 (J = 10.0 Hz), 129.8, 126.0, 123.2, 1203 (J = 25.0 Hz), 119.3, 115.0 (J = 25.0 Hz), 22.8; Anal. calcd. for C16H11BrFN3O (360.19): C 53.36%, H 3.08%, N 11.67%. Found: C 53.03%, H 3.49%, N 11.75%.
6-Bromo-2-methyl-3-(4-fluorobenzalamino)-4(3H)-quinazolinone (III-15). White solid, yield 68%; m.p. 228.4~229.0 °C; IR (cm-1): ν 3032 (Qu-H, Ar-H), 1674 (C=O), 1610, 1589 (C=N), 1568-1474 (C=C, benzene and quinazolinone rings), 860, 815 (1,2,4-trsubstituted benzene), 675 (Qu-Br); 1H-NMR: δ 2.57 (s, 3H, 2-Qu-CH3), 7.64-8.24 (m, 5H, Ar-H), 9.42 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 163.2, 158.2 154.1, 146.6, 136.4, 135.1, 130.1. 129.9, 129.8, 129.6, 128.9, 128.8, 127.3, 127.0, 121.6, 22.9; Anal. calcd. for C16H11BrFN3O (360.19): C 53.36%, H 3.08%, N 11.67%. Found: C 53.35%, H 3.29%, N 11.52%
6-Bromo-2-methyl-3-(2-trifluoromethylbenzalamino)-4(3H)-quinazolinone (III-16). White solid, yield 66 %; m.p. 196~198 °C; IR (cm-1): ν 3032 (Qu-H, Ar-H), 2980 (Qu-CH3), 1678 (C=O), 1616, 1602 (C=N), 1568-1469 (C=C, benzene and quinazolinone rings), 772 (1,2-disubstituted benzene); 675 (Qu-Br), 1H-NMR: δ 2.57 (s, 3H, 2-Qu-CH3), 7.64-8.24 (m, 5H, Ar-H), 9.42 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 164.2, 161.1, 158.2 146.5, 136.4, 135.1, 130.1. 131.3, 129.8, 129.6, 128.9, 128.8, 127.3, 117.0, 115.6, 22.9; Anal. calcd. for C17H11BrF3N3O (410.20): C 49.78%, H 2.70%, N 10.24%. Found: C 49.27%, H 3.07%, N 10.26%.
6-Bromo-2-methyl-3-(3-chlorobenzalamino)-4(3H)-quinazolinone (III-17). White solid, yield 63%; m.p. 212~214 °C; 1H-NMR: δ 2.57 (s, 3H, 2-Qu-CH3), 7.64-8.24 (m, 5H, Ar-H), 9.42 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 163.2, 158.2 154.1, 146.6, 136.4, 135.1, 130.1. 129.9, 129.8, 129.6, 128.9, 128.8, 127.3, 127.0, 121.6, 22.9; Anal. calcd. for C16H11BrClN3O (376.64): C 51.02%, H 2.94%, N 11.16%; found: C 51.11%, H 2.88%, N 11.25%.
6-Bromo-2-methyl-3-(2,4-dichlorobenzalamino)-4(3H)-quinazolinone (III-18). White solid, yield 72%; m.p. 212.3~214.4 °C; IR (cm-1): ν 3061 (Qu-H, Ar-H), 1688 (C=O), 1605, 1587 (C=N), 1575-1468 (C=C, benzene and quinazolinone rings), 868, 827 (1,2,4-trisubstituted benzene); 675 (Qu-Br), 1H-NMR: δ 2.55 (s, 3H, 2-Qu-CH3), 7.60-8.24 (m, 6H, Ar-H, Qu-H), 9.39 (s, 1H, Qu-N=CH-Ar); 13C- NMR: δ 164.0, 157.3, 154.8, 145.7. 147.0138.6, 137.8, 130.5, 129.8, 129.7, 129.4, 129.3, 129.0, 123.3, 119.3, 22.9; Anal. calcd. for C16H10BrCl2N3O (411.09): C 46.75%, H 2.45%, N 10.22%. Found: C 46.68%, H 2.30%, N 10.22%.
6-Bromo-2-methyl-3-(2,6-dichlorobenzalamino)-4(3H)-quinazolinone (III-19). White solid, yield 70%; m.p. 222~224 °C; IR (cm-1): ν 3066 (Qu-H, Ar-H), 1678 (C=O), 1597 (C=N), 1579-1443 (C=C, benzene and quinazolinone rings), 781, 710 (1,2,3-trisubstituted benzene), 675 (Qu-Br); 1H-NMR: δ 2.50 (s, 3H, 2-Qu-CH3), 7.45-8.95 (m, 6H, Ar-H, Qu-H), 9.37 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 165.9, 157.0, 154.6, 145.7, 138.0, 135.0, 133.7, 130.2, 129.7, 129.4, 129.3, 123.4, 119.4, 23.4; Anal. calcd. for C16H10BrCl2N3O (411.09): C 46.75%, H 2.45%, N 10.22%. Found: C 46.60%, H 2.60%, N 10.29%.
6-Bromo-2-methyl-3-(3,4-difluorobenzalamino)-4(3H)-quinazolinone (III-20). White solid, yield 64%; m.p. 187~189 °C; IR (cm-1): ν 3030 (Qu-H, Ar-H), 1678 (C=O), 1605, 1585 (C=N), 1517, 1470 (C=C, benzene and quinazolinone rings), 881, 829 (1,2,4-trisubstituted benzene), 675 (Qu-Br), 1H-NMR: δ 2.50 (s, 3H, 2-Qu-CH3), 7.61-8.23 (m, 6H, Qu-H, Ar-H), 9.02 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 167.6, 156.9, 154.8, 151.3, 145.8, 142.0, 137.9, 129.8, 129.1, 127.5, 123.1, 119.3, 119.0, 117.5, 22.8.
6-Bromo-2-methyl-3-(2-chloro-5-nitrobenzalamino)-4(3H)-quinazolinone (III-21). White solid, yield 70%; m.p. 277~279 °C; IR (cm-1): ν 3076 (Qu-H, Ar-H), 1682 (C=O), 1610, 1599 (C=N), 1568-1447 (C=C, benzene and quinazolinone rings), 1529, 1348 (Ar-NO2), 880, 833 (1,2,4-trisubstituted benzene), 675 (Qu-Br); 1H-NMR: δ 2.59 (s, 3H, 2-Qu-CH3), 7.62-8.87 (m, 6H, Ar-H, Qu-H), 9.55 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 163.0, 155.7, 154.7, 147.4, 145.4, 141.5, 140.1, 137.9, 132.6, 129.8, 129.4, 123.4, 128.4, 122.9, 119.6, 22.7; Anal. calcd. for C16H10BrClN4O3 (421.64): C 45.58%, H 2.39%, N 13.29%. Found: C 45.75%, H 2.46%, N 13.58%.
6-Bromo-2-methyl-3-(4-chloro-3-nitrobenzalamino)-4(3H)-quinazolinone (III-22). White solid, yield 68%; m.p. 230~232 °C; IR (cm-1): ν 3062 (Qu-H, Ar-H), 1668 (C=O), 1612, 1597 (C=N), 1469 (C=C, benzene and quinazolinone rings), 1537, 1355 (Ar-NO2), 870, 829 (1,2,4-trisubstituted benzene); 673 (Qu-Br); 1H-NMR: δ 2.55 (s, 3H, 2-Qu-CH3), 7.61-8.66 (m, 6H, Ar-H, Qu-H), 9.20 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 166.1, 157.0, 154.8, 148.6, 145.7, 138.0, 133.7, 133.3, 133.2, 129.8, 129.3, 129.2, 125.8, 123.1, 119.4, 22.9; Anal. calcd. for C16H10BrClN4O3 (421.64): C 45.58%, H 2.39%, N 13.29%; found: C 45.62%, H 2.17%, N 13.46%.
6-Bromo-2-methyl-3-(2-fluoro-3-trifluoromethylbenzalamino)-4(3H)-quinazolinone (III-23). White solid, yield 64%; m.p. 230.7~232.0 °C; IR (cm-1) ν: 3012 (Qu-H, Ar-H), 1670 (C=O), 1614, 1605 (C=N), 1566, 1431 (C=C, benzene and quinazolinone rings), 762, 696 (1,2,3-trisubstituted benzene); 675 (Qu-Br), 1H-NMR: δ 2.53 (s, 3H, 2-Qu-CH3), 7.61-8.39 (m, 6H, Ar-H, Qu-H), 9.02 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 168.5, 156.8, 154.7, 145.8, 137.9, 134.9, 134.5, 133.0, 131.7, 129.8, 129.1, 128.5, 128.2, 124.3, 123.1, 119.3, 22.8.
6-Bromo-2-methyl-3-(3,4-dimethoxybenzalamino)-4(3H)-quinazolinone (III-24). White solid, yield 66%; m.p. 216~218 °C; IR (cm-1): ν 3060 (Qu-H, Ar-H), 2934, 2837 (ArO-CH3), 1674 (C=O), 1599 (C=N), 1571-1466 (C=C, benzene and quinazolinone rings), 1271, 1024 (Ar-O-Me), 880, 833 (1,2,4-trisubstituted benzene); 1H-NMR: δ 2.51 (s, 3H, 2-Qu-CH3), 7.14-8.37 (m, 6H, Qu-H, Ar-H), 8.78 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 170.2, 156.9, 154.7, 153.5, 149.7, 145.9, 139.9, 137.6, 129.7, 129.0, 125.2, 124.0, 123.2, 119.1, 112.0, 110.0, 56.2, 22.8; Anal. calcd. for C18H16BrN3O3 (402.25): C 53.75%, H 4.01%, N 10.45%; found: C 53.67%, H 4.17%, N 10.56%.
6-Bromo-2-methyl-3-(2,3-dimethoxybenzalamino)-4(3H)-quinazolinone (III-25). White solid, yield 61%; m.p. 194.5~196.5 °C; IR (cm-1): ν 3060 (Qu-H, Ar-H), 2965, 2935, 2837 (Ar-CH3, ArO-CH3), 1684 (C=O), 1614, 1597 (C=N), 1577-1468 (C=C, benzene and quinazolinone rings), 1275, 1088 (Ar-O-Me), 746, 711 (1,2,3-trisubstituted benzene), 675 (Qu-Br); 1H-NMR: δ 2.51 (s, 3H, 2-Qu-CH3), 7.26-8.24 (m, 6H, Qu-H, Ar-H), 9.13 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 165.8, 157.0, 154.7, 153.3, 150.4, 145.8, 137.7, 129.7, 129.2, 126.2, 125.2, 123.3, 119.1, 118.3, 117.6, 62.4, 56.5, 22.8; Anal. calcd. for C18H16BrN3O3 (402.25): C 53.75%, H 4.01%, N 10.45%; found: C 53.94%, H 4.22%, N 10.35%.
6-Bromo-2-methyl-3-(2,5-dimethoxybenzalamino)-4(3H)-quinazolinone (III-26). White solid, yield 63%; m.p. 224~226 °C; IR (cm-1): ν 3060 (Qu-H, Ar-H), 2943, 2833 (Ar-CH3, ArO-CH3), 1682 (C=O), 1604 (C=N), 1575-1445 (C=C, benzene and quinazolinone rings), 1223, 1049 (Ar-O-Me), 875, 800 (1,2,4-trisubstituted benzene); 675 (Qu-Br); 1H-NMR: δ 2.51 (s, 3H, 2-Qu-CH3), 3.35-3.84 (m, 6H, ArO-CH3), 6.98-8.36 (m, 6H, Ar-H, Qu-H), 9.13 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 166.0, 164.9, 156.8, 154.8, 154.4, 153.7, 145.8, 137.6, 129.7, 129.2, 121.6, 121.0, 119.0, 114.5, 110.4, 56.9, 56.1, 22.8; Anal. calcd. for C18H16BrN3O3 (402.25): C 53.75%, H 4.01%, N 10.45%; found: C 53.67%, H 3.89%, N 10.32%.
6-Bromo-2-methyl-3-(3-nitrobenzalamino)-4(3H)-quinazolinone (III-27). White solid, yield 56%; m.p. 227~230 °C; IR (cm-1): ν 3086 (Qu-H, Ar-H), 1670 (C=O), 1602 (C=N), 1535 1352 (Ar-NO2), 1470 (C=C, benzene and quinazolinone rings), 843, 770, 700 (1,3-disubstituted benzene); 675 (Qu-Br); 1H-NMR: δ 2.56 (s, 3H, 2-Qu-CH3), 7.63-8.77 (m, 7H, Ar-H, Qu-H), 9.23 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 167.8, 156.8, 154.7, 148.9, 145.8, 138.0, 135.2, 134.5, 131.5, 129.8, 129.2, 127.5, 123.5, 123.1, 119.4, 22.9; Anal. calcd. for C16H11BrN4O3 (387.20): C 49.63%, H 2.86%, N 14.47%. Found: C 49.42%, H 2.75%, N 14.32%.
6-Bromo-2-methyl-3-(2-hydroxybenzalamino)-4(3H)-quinazolinone (III-28). White solid, yield 53%; m.p. 212.4~212.9 °C; IR (cm-1): ν 3364 (Ar-OH), 3072 (Qu-H, Ar-H), 1682 (C=O), 160 (C=N), 1464, 1447 (C=C, benzene and quinazolinone rings), 1273 (Ar-O-H), 750 (1,2-disubstituted benzene); 675 (Qu-Br), 1H-NMR: δ 2.50 (s, 3H, 2-Qu-CH3), 6.97-8.22 (m, 7H, Qu-H, Ar-H), 9.10 (s, 1H, Qu-N=CH-Ar), 10.56 (Ar-OH); 13C-NMR: δ 167.6, 159.3, 157.0, 154.6, 145.9, 140.0, 137.6, 135.1, 129.7, 129.1, 128.6, 123.3, 120.2, 118.7, 117.3, 22.8; Anal. calcd. for C16H12BrN3O2 (358.20); C 53.65%, H 3.38%, N 11.73%. Found: C 53.79%, H 3.66%, N 11.72%.
6-Bromo-2-methyl-3-(2,4-dimethoxybenzalamino)-4(3H)-quinazolinone (III-29). White solid, yield 62 %; m.p. 216~218 °C; IR (cm-1): ν 3032 (Qu-H, Ar-H), 2968, 2941, 2833 (Ar-CH3, ArO-CH3), 1674 (C=O), 1599 (C=N), 1570-1468 (C=C, benzene and quinazolinone rings), 1277, 1107 (Ar-O-Me), 876, 813 (1,2,4-trisubstituted benzene); 675 (Qu-Br); 1H-NMR: δ 2.49 (s, 3H, 2-Qu-CH3), 3.88 (Ar-O-Me), 6.58-8.20 (m, 6H, Qu-H, Ar-H), 8.97 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 165.5, 165.0, 161.6, 157.1, 154.8, 145.9, 137.5, 129.7, 129.1, 128.7, 123.3, 119.0, 113.4, 107.6, 98.7, 56.5, 56.3, 22.8; Anal. calcd. for C18H16BrN3O3 (402.25): C 53.75%, H 4.01%, N 10.45%. Found: C 53.99%, H 4.19%, N 10.27%.
6-Bromo-2-methyl-3-(5-chloro-2-hydroxybenzalamino)-4(3H)-quinazolinone (III-30). Yellow solid, yield 64 %; m.p. 273.2~225.0 °C; IR (cm-1): ν 3064 (Qu-H, Ar-H), 1682 (C=O), 1610 (C=N), 1474 (C=C, benzene and quinazolinone rings), 860, 815 (1,2,4-trisubstituted benzene); 665 (Qu-Br); 1H- NMR: δ 2.50 (s, 3H, 2-Qu-CH3), 7.49-8.21 (m, 6H, Qu-H, Ar-H), 9.11 (s, 1H, Qu-N=CH-Ar); 13C- NMR: δ 165.1, 157.9, 157.0, 154.7, 145.8, 137.7, 134.4, 129.7, 129.1, 126.7, 123.9, 123.2, 120.3, 119.2, 119.1, 22.9; Anal. calcd. for C16H11BrClN3O2 (392.64): C 48.94%, H 2.82%, N 10.70%. Found: C 48.80%, H 2.96%, N 10.75%.
2-Methyl-3-(2,3-dichlorobenzalamino)-4(3H)-quinazolinone (III-31). White needles, yield 87%; m.p. 209~210 °C; IR (cm-1) ν: 3034 (Qu-H, Ar-H), 2982 (Ar-CH3), 1680 (C=O), 1612, 1593 (C=N), 1550-1474 (C=C, benzene and quinazolinone rings), 766, 691 (1,2,3-trisubstituted benzene); 1H-NMR: δ 2.51 (s, 3H, 2-Qu-CH3), 7.53-8.19 (m, 7H, Qu-H, Ar-H), 9.42 (s, 1H, Qu-N=CH-Ar); 13C-NMR: δ 164.0, 158.2, 154.1, 146.7, 135.1, 134.6, 133.4, 133.0, 129.4, 127.4, 127.3, 127.2, 127.0, 121.6, 22.9; Anal. calcd. for C16H11Cl2N3O (332.19): C 57.85%, H 3.34%, N 12.65%. Found: C 57.78%, H 3.52%, N 12.53%.