Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus
Abstract
:Introduction
Results and Discussion
Growth-inhibitory activity
| Alcohol | Broth dilution method | Broth dilution with shaking | log10Po/w | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | DP* | ||
| 1-Octanol | 256 | 256 | N.D. | N.D. | 0.0 | 3.07a |
| 1-Nonanol | 64 | 128 | N.D. | N.D. | 17.5 | 4.02a |
| 1-Decanol | 32 | 64 | 40 | 80 | ≥ 48.0 | 4.57a |
| 1-Undecanol | 16 | 32 | 40 | 40 | ≥ 48.0 | 4.53b |
| 1-Dodecanol | 8 | 16 | 10 | N.D. | ≥ 48.0 | 5.13a |
| 1-Tridecanol | 4 | 8 | 10 | N.D. | ≥48.0 | 5.59b |
| 1-Tetradecanol | 4 | 8 | N.D. | N.D. | 29.7 | 6.03a |
| 1-Pentadecanol | 4 | 8 | N.D. | N.D. | 27.2 | 6.24c |
| 1-Hexadecanol | 256 | ≥ 512 | N.D. | N.D. | 17.3 | 6.65d |
| 1-Heptadecanol | ≥ 512 | ≥ 512 | N.D. | N.D. | 6.5 | 7.23c |
| 1-Octadecanol | ≥ 512 | ≥ 512 | N.D. | N.D. | 10.0 | 7.19d |
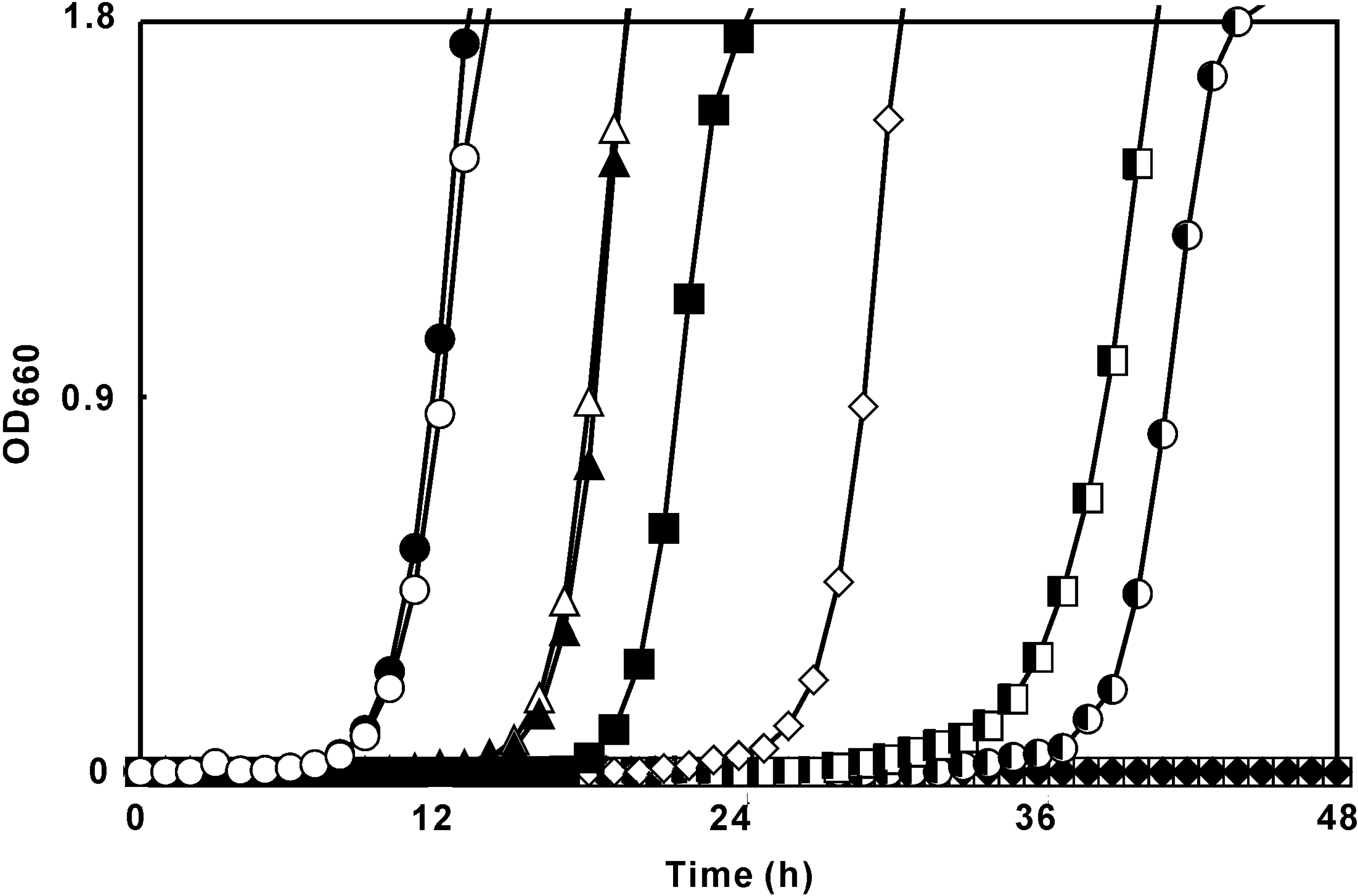
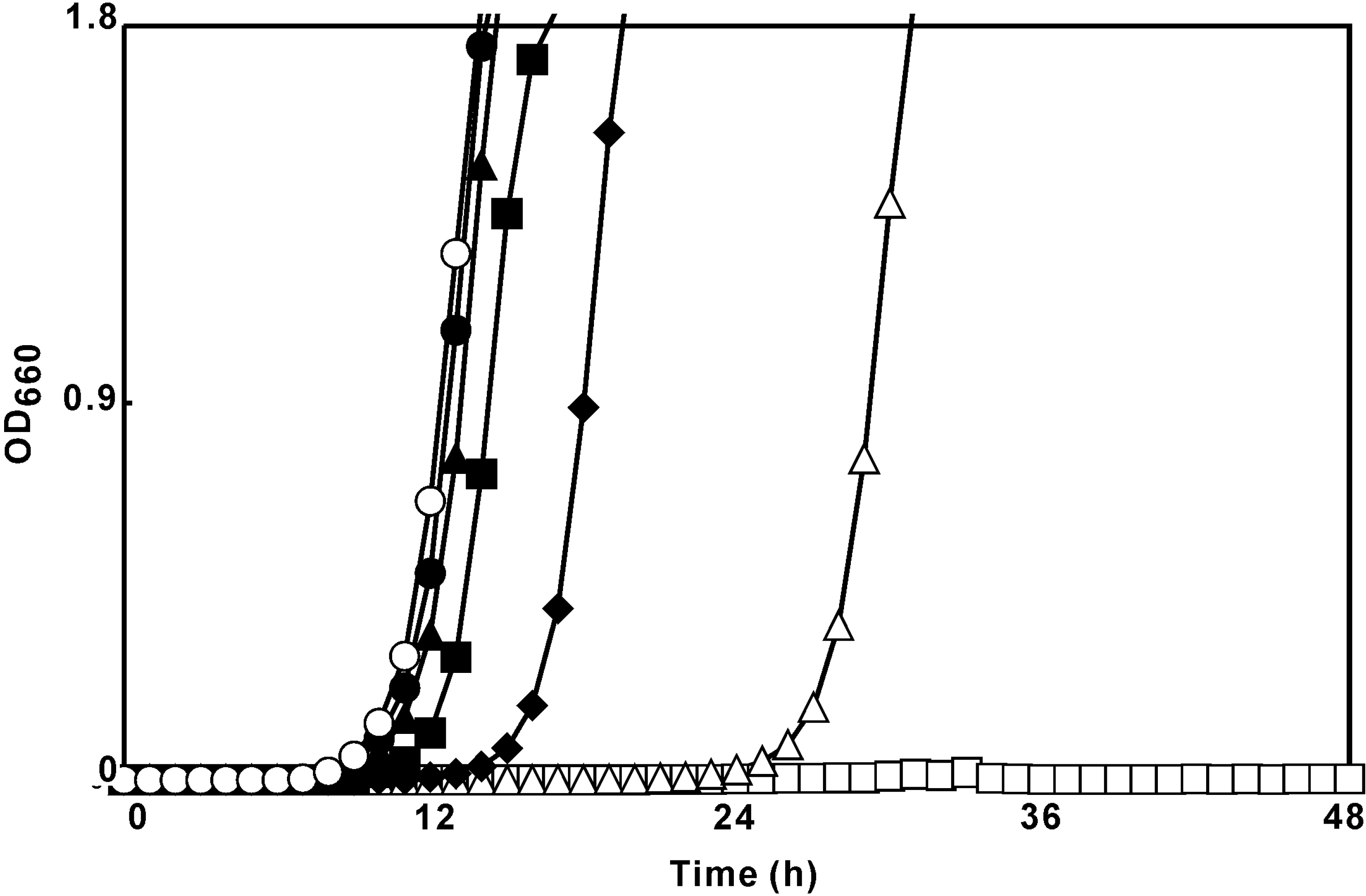
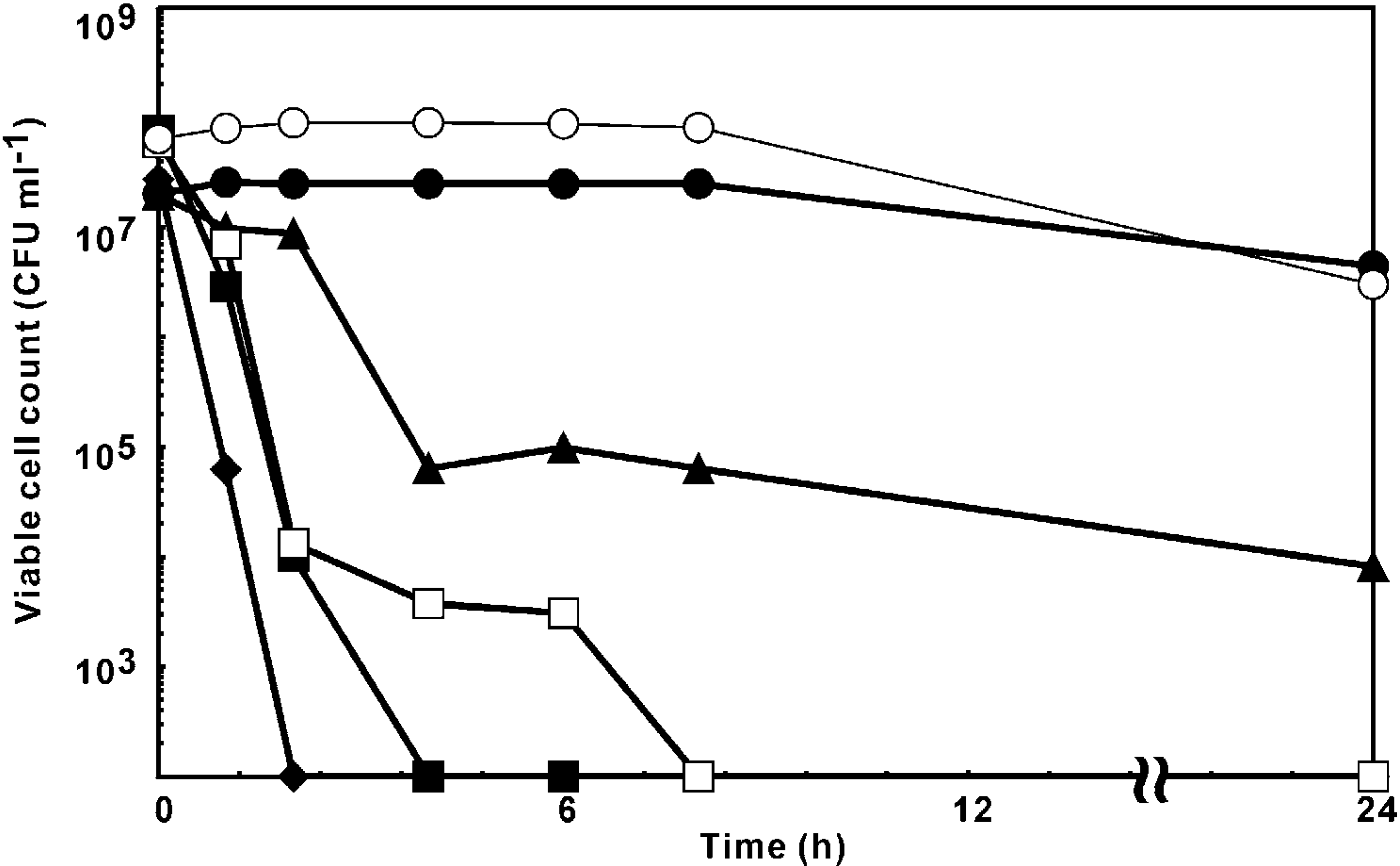
Time-kill assay
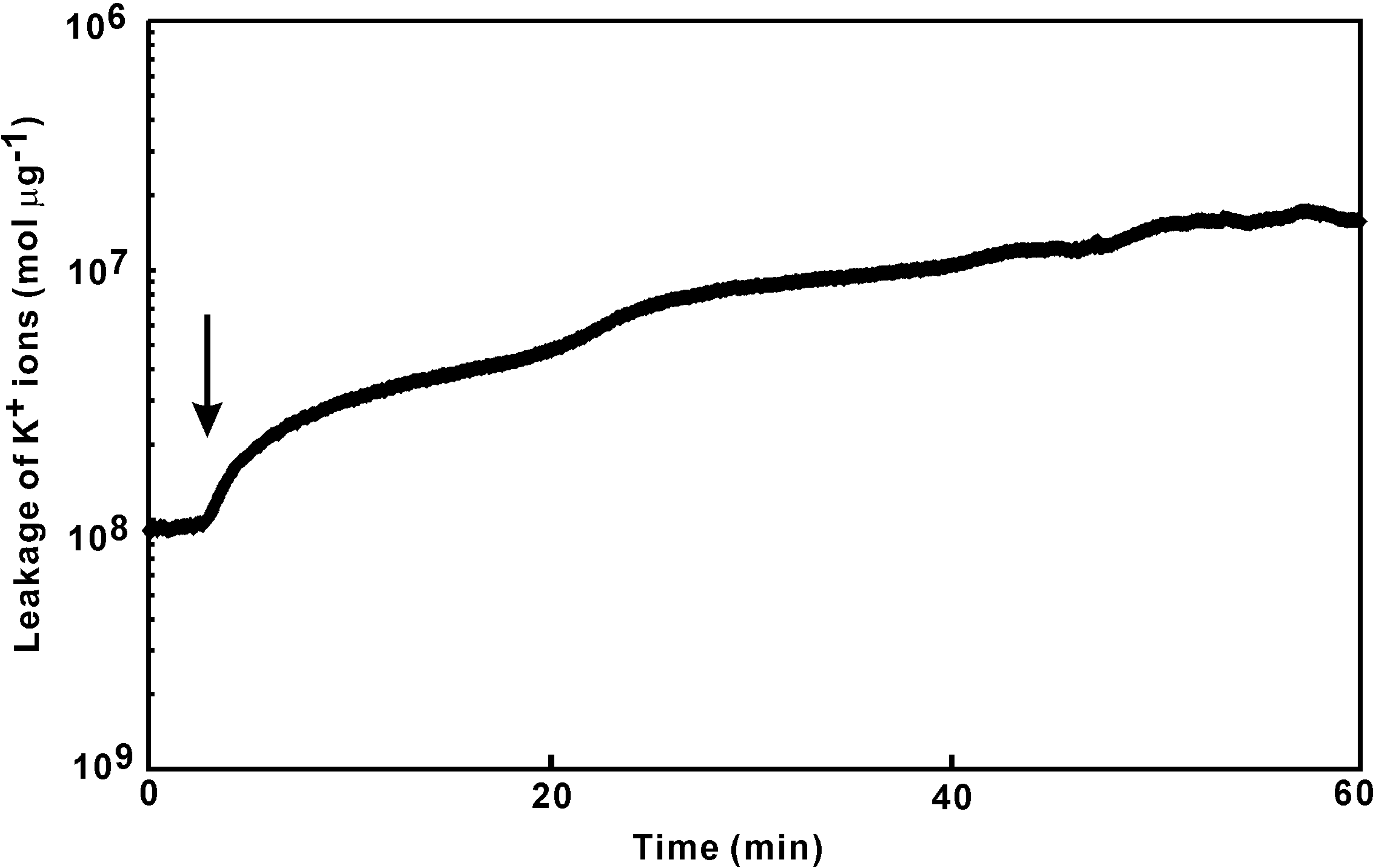
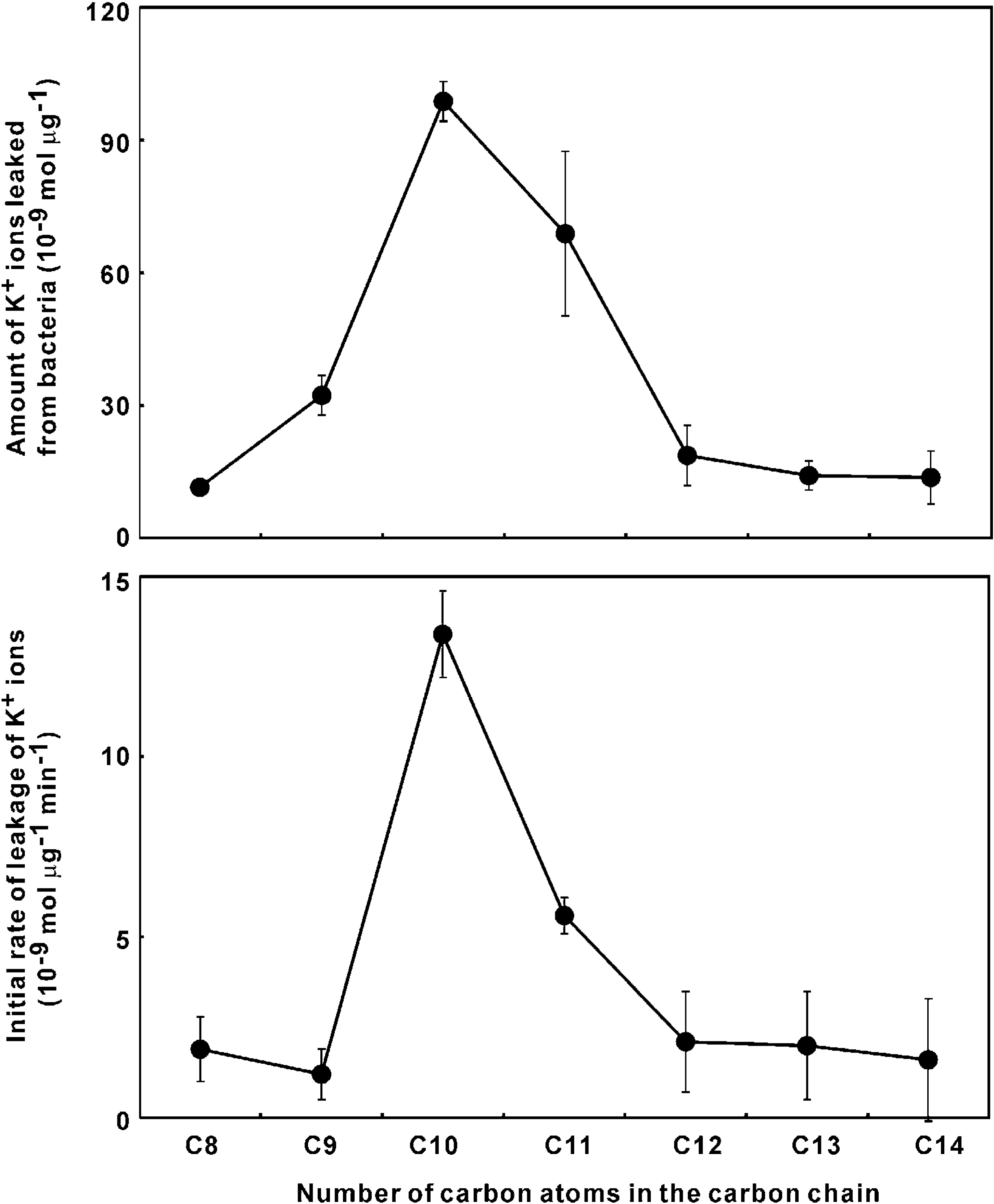
Quantitation of K+ ion leakage
Experimental
General
Broth dilution method
Broth dilution with shaking (BDS) method
Quantitation of K+ ion leakage
Time-kill assay
References
- Arai, T.; Hamashima, H.; Sasatsu, M. Inhibitory effects of fatty acids, purified camellia oil and olive oil on the growth of Staphylococcus aureus. Jpn. J. Chemother. 1996, 44, 786–91. [Google Scholar]
- Hada, T.; Furuse, S.; Matsumoto, Y.; Hamashima, H.; Masuda, K.; Shiojima, K.; Arai, T.; Sasatsu, M. Comparison of the effects in vitro of tea tree oil and plaunotol on methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. Microbios 2001, 106, 133–141. [Google Scholar] [PubMed]
- Hada, T.; Shiraishi, A.; Furuse, S.; Inoue, Y.; Hamashima, H.; Masuda, K.; Shiojima, K.; Shimada, J. Inhibitory effects of terpene on the growth of Staphylococcus aureus. Nat. Med. 2003, 57, 464–467. [Google Scholar]
- Imai, H.; Osawa, K.; Yasuda, H.; Hamashima, H.; Arai, T.; Sasatsu, M. Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios 2001, 106, 31–39. [Google Scholar] [PubMed]
- Osawa, K.; Saeki, T.; Yasuda, H.; Hamashima, H.; Sasatsu, M.; Arai, T. The antibacterial activities of peppermint oil and green tea polyphenols, alone and in combination, against enterohemorrhagic Escherichia coli. Biocontrol Sci. 1999, 4, 1–7. [Google Scholar] [CrossRef]
- Hattori, M.; Miyachi, K.; Hada, S.; Kakiuchi, N.; Kikuchi, F.; Tsuda, Y.; Namba, T. Effects of long-chain fatty acids and fatty alcohols on the growth on Streptococcus mutans. Chem. Pharm. Bull. 1987, 35, 3507–3510. [Google Scholar]
- Kabelitz, N.; Santos, P.M.; Heipieper, H.J. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 2003, 220, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Shibasaki, I. The antimicrobial characteristics of 1-alkanols. J. Antibact. Antifung. Agents 1980, 8, 325–331. [Google Scholar]
- Kato, N.; Yanagida, S.; Okahara, M.; Shibasaki, I. Antibacterial activity of alcohols and oxyethylated alcohols. J. Antibact. Antifung. Agents 1978, 6, 527–531. [Google Scholar]
- Kubo, I.; Muroi, H.; Himejima, H.; Kubo, A. Antibacterial activity of long-chain alcohols: the role of hydrophobic alkyl groups. Bioorg. Med. Chem. Lett. 1993, 3, 1305–1308. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Kubo, A. Antibacterial activity of long-chain alcohol against Streptococcus mutans. J. Agric. Food Chem. 1993, 41, 2447–2450. [Google Scholar] [CrossRef]
- Lansford, E.M., Jr.; Hill, I.D.; Shive, W. Reversal by ribonucleosides of bacterial growth inhibition caused by alcohol. J. Biol. Chem. 1960, 235, 3551–3555. [Google Scholar] [PubMed]
- Mates, A. The effect of alcohols on growth and lipase formation by Staphylococcus aureus. J. Appl. Bacteriol. 1974, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.M.; Felsing, B.; Lansford, E.M., Jr.; Trubey, R.H.; Shive, W. Reversal of alcohol toxicity by glutamine. J. Biol. Chem. 1955, 214, 497–501. [Google Scholar]
- Sheu, C.W.; Freese, E. Lipopolysaccharide layer protection of Gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 1973, 115, 869–875. [Google Scholar]
- Tanaka, Y.; Fukuda, S.; Kikuzaki, H.; Nakatani, N. Antibacterial activity of aliphatic long-chain compounds against upper-airway respiratory tract bacteria. ITE Lett. Batter. New Technol. Med. 2002, 1, 777–780. [Google Scholar]
- Grisham, C.M.; Barnett, R.E. The effects of long-chain alcohols on membrane lipids and the (Na++K+)-ATPase. Biochim. Biophys. Acta 1973, 311, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O. Adaptation of membrane lipids to alcohols. J. Bacteriol. 1976, 125, 670–678. [Google Scholar] [PubMed]
- Hada, T.; Inoue, Y.; Shiraishi, A.; Hamashima, H. Leakage of K+ ions from Staphylococcus aureus in response to tea tree oil. J. Microbiol. Methods 2003, 53, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [PubMed]
- Kajiya, K.; Hojo, H.; Suzuki, M.; Nanjo, F.; Kumazawa, S.; Nakayama, T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem. 2004, 52, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.; Nihei, K. Anti-Salmonella Activity of Alkyl Gallates. J. Agric. Food Chem. 2002, 50, 6692–6696. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Takaishi, Y.; Arakaki, N.; Higuti, T. Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 549–555. [Google Scholar]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.T.; Taylor, P.W. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Fifth Edition: Approved Standard M7-A5; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- Matsumoto, Y.; Hamashima, H.; Masuda, K.; Shiojima, K.; Sasatsu, M.; Arai, T. The antibacterial activity of plaunotol against Staphylococcus aureus isolated from the skin of patients with atopic dermatitis. Microbios 1998, 96, 149–155. [Google Scholar]
- Katsu, T.; Kobayashi, H.; Fujita, Y. Mode of action of gramicidin S on Escherichia coli membrane. Biochim. Biophys. Acta 1986, 860, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus. Molecules 2007, 12, 139-148. https://doi.org/10.3390/12020139
Togashi N, Shiraishi A, Nishizaka M, Matsuoka K, Endo K, Hamashima H, Inoue Y. Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus. Molecules. 2007; 12(2):139-148. https://doi.org/10.3390/12020139
Chicago/Turabian StyleTogashi, Naoko, Akiko Shiraishi, Miki Nishizaka, Keisuke Matsuoka, Kazutoyo Endo, Hajime Hamashima, and Yoshihiro Inoue. 2007. "Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus" Molecules 12, no. 2: 139-148. https://doi.org/10.3390/12020139




