Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2H,6H)-dione
Abstract
:Introduction
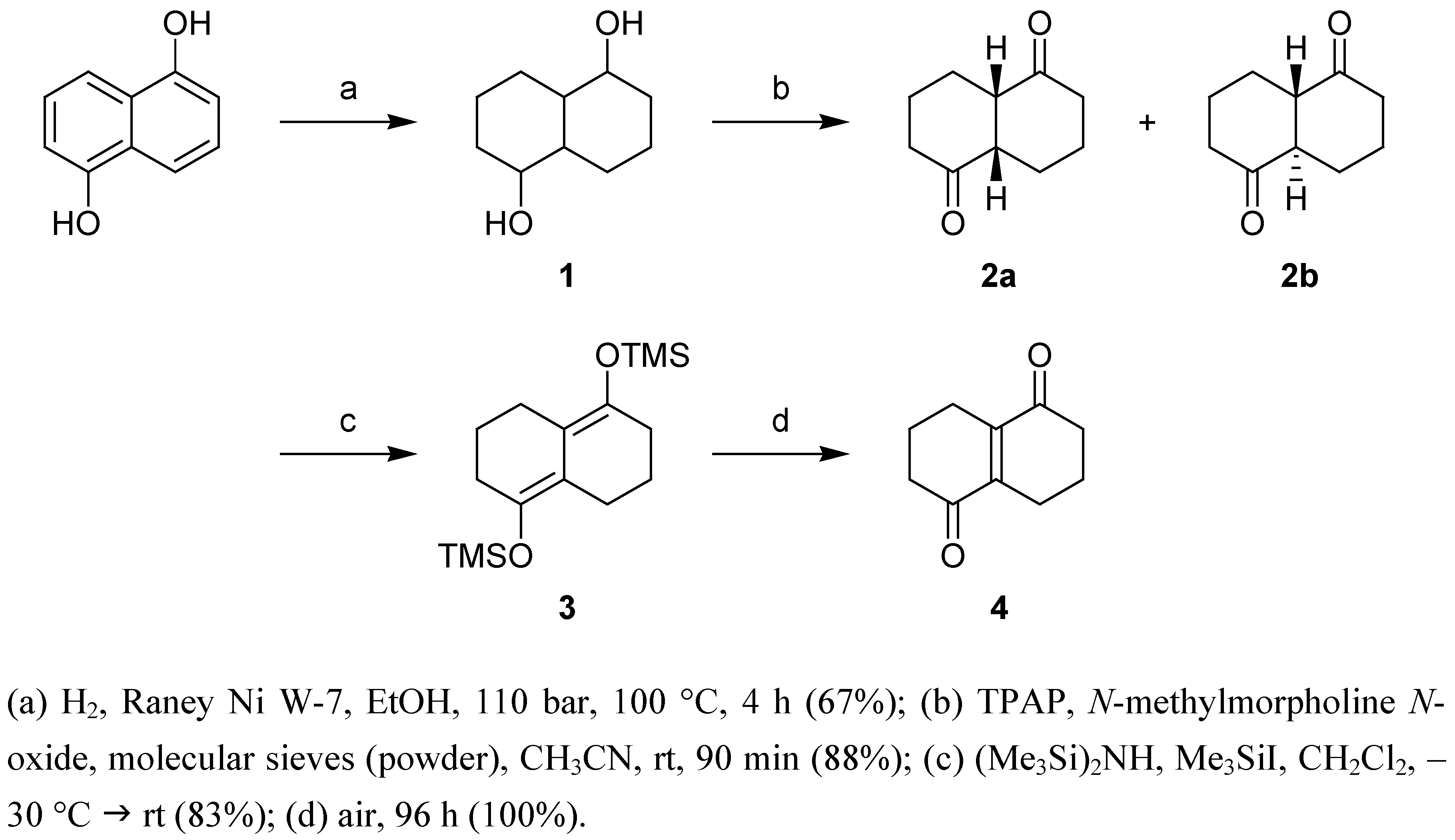
Results and Discussion
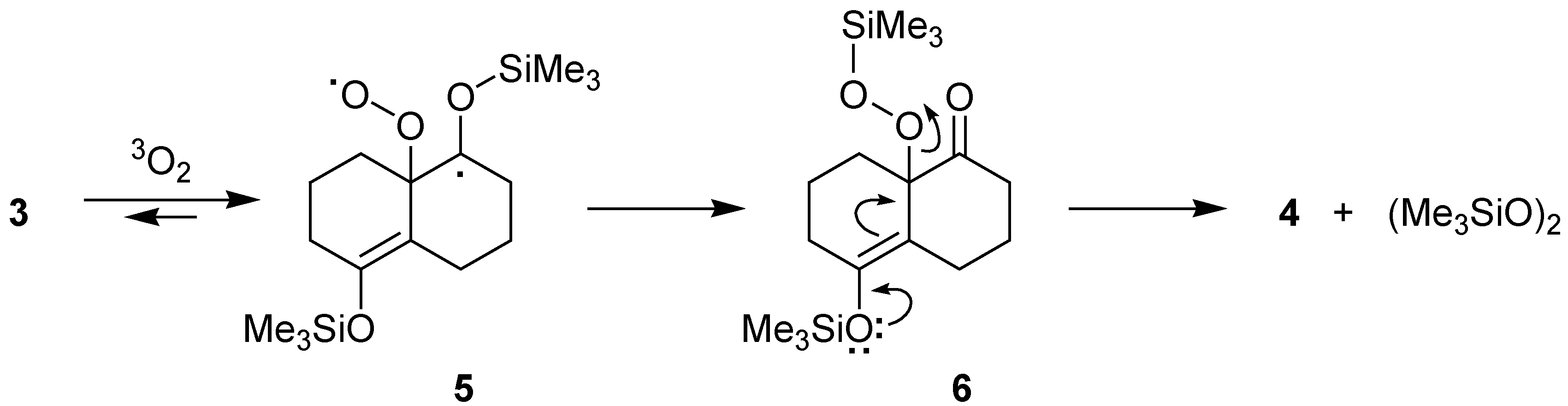
Conclusions
Experimental
General
(4aS,8aS)-Octahydronaphthalene-1,5-dione (2a) and (4aR,8aS)-octahydronaphthalene-1,5-dione (2b)
1,2,3,5,6,7-Hexahydro-4,8-di(trimethylsilyloxy)naphthalene (3)
3,4,7,8-Tetrahydronaphthalene-1,5(2H,6H)-dione (4)
Acknowledgments
References and Notes
- Bouillon, R.; Norman, A. W.; Pasqualini, J. R. (Eds.) Proceedings of the 12th Workshop on Vitamin D.J. Steroid Biochem. Mol. Biol. 2004, 89–90, 1–633, and the previous 11 volumes in this series.(b)Feldman, D.; Glorieux, F. H.; Pike, J. W. (Eds.) Vitamin D; Academic Press: San Diego, 1997.
- Peet, N. P.; Cargill, R. L. J. Org. Chem. 1973, 38, 4281–4285.
- McChesney, J. D.; Kabra, P. M.; Fraher, P. J. Pharm. Sci. 1979, 68, 1116–1119.
- Hamon, D. P. G.; Richards, K. R. Aust. J. Chem. 1983, 36, 2243–2259.
- Jeffrey, D. A.; Cogen, J. M.; Maier, W. F. J. Org. Chem. 1986, 51, 3206–3209.
- Hill, R. K.; Giberson, D. L.; Pendalwar, S. L.; Newton, M. G. Synthetic Commun. 1996, 26, 991–997.
- Nicolaou, K. C.; Montagnon, T.; Baran, P. S.; Zhong, Y.-L. J. Am. Chem. Soc. 2002, 124, 2245–2258. (b) When applying this procedure, however, a moderate 20% yield was obtained.
- Sheikh, S. E.; Kausch, N.; Lex, J.; Neudörfl, J.-M.; Schmalz, H.-G. Synlett 2006, 10, 1527–1530.El Blidi, L.; Crestia, D.; Gallienne, E.; Demuynck, C.; Bolte, J.; Lemaire, M. Tetrahedron: Asymmetry 2004, 15, 2951–2954.
- For the conversion of a similar bis(trialkylsilyl)enol ether into the corresponding enedione with NBS, see: Loerzer, T.; Gerke, R.; Lüttke, W. Angew. Chem. Int. Ed. 1986, 25, 578–579.
- Kato, N.; Nakanishi, K.; Takeshita, H. Bull. Chem. Soc. Jpn. 1986, 59, 1109–1123.Einaga, H.; Nojima, M.; Abe, M. J. Chem. Soc., Perkin Trans. I 1999, 2507–2512.
- Maekawa, H.; Sakai, M.; Uchida, T.; Kita, Y.; Nishiguchi, I. Tetrahedron Lett. 2004, 45, 607–609.
- Johnson, W. S.; Gutsche, C. D.; Banerjee, D. K. J. Am. Chem. Soc. 1951, 73, 5464–5465.
- Griffith, W. P.; Ley, S. V. Aldrichim. Acta 1990, 23, 13–19.
- The structural assignment follows from the equilibrium composition (gaseous HCl) in which the more stable trans-diketone 2b predominated: ratio 9:91 for 2a:2b, respectively.
- Sample Availability: No samples available.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Minne, G.B.; De Clercq, P.J. Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2H,6H)-dione. Molecules 2007, 12, 183-187. https://doi.org/10.3390/12020183
Minne GB, De Clercq PJ. Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2H,6H)-dione. Molecules. 2007; 12(2):183-187. https://doi.org/10.3390/12020183
Chicago/Turabian StyleMinne, Garrett B., and Pierre J. De Clercq. 2007. "Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2H,6H)-dione" Molecules 12, no. 2: 183-187. https://doi.org/10.3390/12020183



