Efficient Preparation of Aldoximes from Arylaldehydes, Ethylenediamine and Oxone® in Water
Abstract
:Introduction
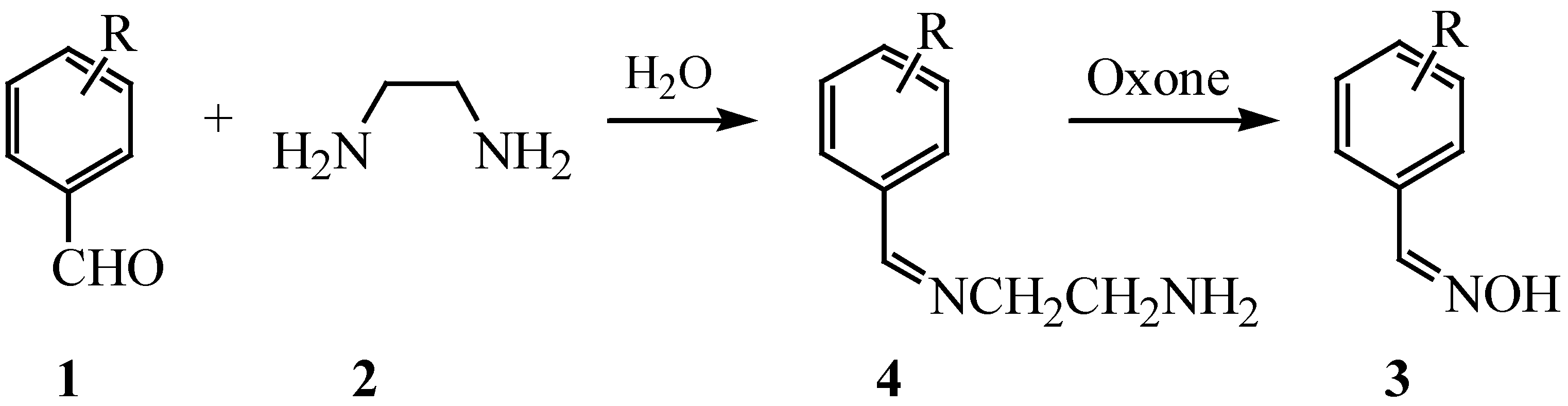
Results and Discussion
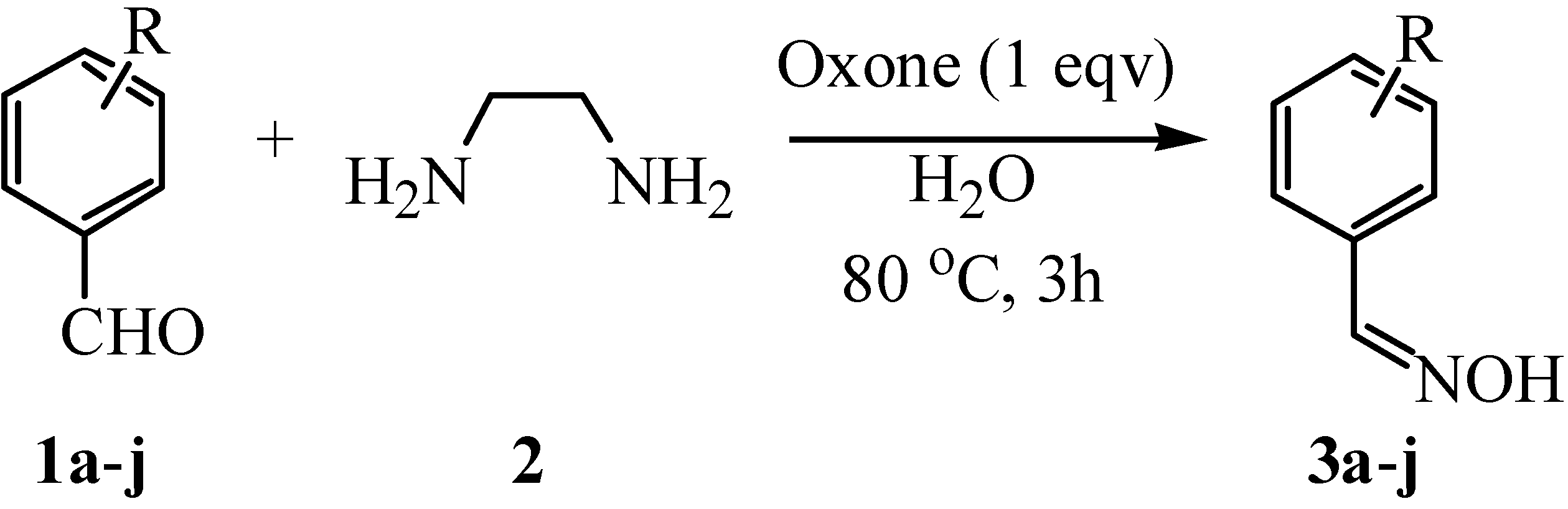
| Entry | R | Product | Yield / %a | Mp (lit) / oC |
|---|---|---|---|---|
| 1 | H | 3a | 92 | 30-32 (33-35 [5] ) |
| 2 | 4-CH3 | 3b | 93 | 73-74 (76-78 [14] ) |
| 3 | 4-CH3O | 3c | 92 | 47-49 (48-49 [15] ) |
| 4 | 3,4-CH3 | 3d | 90 | 67-68 (69 [16] ) |
| 5 | 4-Cl | 3e | 93 | 110-111 (107-109 [14] ) |
| 6 | 2-Cl | 3f | 91 | 74-75 (74-75 [17] ) |
| 7 | 3,4-Cl | 3g | 95 | 120-121 |
| 8 | 4-NO2 | 3h | 88 | 131-132 (132-133 [17] ) |
| 9 | 3-NO2 | 3i | 86 | 123-124 (121-122 [17] ) |
| 10 | 4-CN | 3j | 88 | 180-181 (174-176 [18] ) |
Conclusions
Experimental
General
General procedure for aldoxime synthesis
Acknowledgments
References and Notes
- Park, S.; Choi, Y.; Han, H.; Yang, S. H.; Chang, S. Rh-Catalyzed one-pot and practical transformation of aldoximes to amides. Chem. Commun. 2003, 1936–1937. [Google Scholar]
- Chiang, Y. H. Chlorination of oximes. I. Reaction and mechanism of the chlorination of oximes in commercial chloroform and methylene chloride. J. Org. Chem. 1971, 36, 2146–2155. [Google Scholar] Liu, K. C.; Shelton, B. R.; How, R. K. A particularly convenient preparation of benzo-hydroximinoyl chlorides (nitrile oxide precursors). J. Org. Chem. 1980, 45, 3916–3918. [Google Scholar]
- Schoenewaldt, E. F.; Kinnel, R. B.; Davis, P. Improved synthesis of anti-benzaldoxime, concomitant cleavage and formylation of nitrones. J. Org. Chem. 1968, 33, 4270–4272. [Google Scholar] Smith, P. A. S.; Robertson, J. E. Some factors affecting the site of alkylation of oxime salts. J. Am. Chem. Soc. 1962, 84, 1197–1204. [Google Scholar] Buehler, E. Alkylation of syn- and anti-benzaldoximes. J. Org. Chem. 1967, 32, 261–265. [Google Scholar]
- Sarvari, M. H. ZnO/CH3COCl: A new and highly efficient catalyst for dehydration of aldoximes into nitriles under solvent-free condition. Synthesis 2005, 787–790. [Google Scholar] Sosnovsky, G.; Krogh, J. A. A versatile method for the conversion of aldoximes to nitriles using selenium dioxide. Synthesis 1978, 703–705. [Google Scholar]
- Hajipour, A. R.; Mallakpour, S. E.; Imanzadeh, G. A rapid and convenient synthesis of oximes in dry media under microwave irradiation. J. Chem. Res. (S) 1999, 228–229. [Google Scholar]
- Sharghi, H.; Hosseini, M. Solvent-free and one-step Beckmann rearrangement of ketones and aldehydes by zinc oxide. Synthesis 2002, 1057–1060. [Google Scholar] Sharghi, H.; Sarvari, H. M. Selective synthesis of E and Z isomers of oximes. Synlett 2001, 99–101. [Google Scholar]
- Nicolaou, K. C.; Mathison, C. J. N.; Montagnon, T. o-Iodoxybenzoic acid (IBX) as a viable reagent in the manipulation of nitrogen- and sulfur-containing substrates: scope, generality, and mechanism of IBX-mediated amine oxidations and dithiane deprotections. J. Am. Chem. Soc. 2004, 126, 5192–5201. [Google Scholar] Nicolaou, K. C.; Mathison, C. J. N.; Montagnon, T. New reactions of IBX: oxidation of nitrogenand sulfur-containing substrates to afford useful synthetic intermediates. Angew. Chem. 2003, 115, 4211–4216. [Google Scholar] Joseph, R.; Ravindranathan, T.; Sudalai, A. Selective catalytic oxidation of benzylic and ailylic amines to oximes with H202 over TS-l. Tetrahedron Lett. 1995, 36, 1903–1904. [Google Scholar] Yamada, Y. M. A.; Tabata, H.; Takahashi, H.; Ikegami, S. Oxidation of amines and sulfides catalyzed by an assembled complex of phosphotungstate and non-cross-linked amphiphilic polymer. Synlett 2002, 2031–2034. [Google Scholar]
- Li, C. J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: a decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar]
- Xia, J.-J.; Wang, G.-W. One-pot synthesis and aromatization of 1,4-dihydropyridines in refluxing water. Synthesis 2005, 2379–2383. [Google Scholar] Wang, G.-W.; Xia, J.-J.; Miao, C.-B.; Wu, X.-L. Environmentally friendly and efficient synthesis of various 1,4-dihydropyridines in pure water. Bull. Chem. Soc. Jpn. 2006, 79, 454–459. [Google Scholar] Zhang, Z.; Dong, Y.-W.; Wang, G.-W. Efficient and clean aldol condensation catalyzed by sodium carbonate in water. Chem. Lett. 2003, 32, 966–967. [Google Scholar] Wang, G.-W.; Zhang, Z.; Dong, Y.-W. Environmentally friendly and efficient process for the preparation of â-hydroxyl ketones. Org. Process Res. Dev. 2004, 8, 18–21. [Google Scholar] Wang, G.-W.; Miao, C.-B.; Kang, H. Benign and efficient synthesis of 2-substituted-4(3H)-quinazolinones mediated by ferric chloride hexahydrate in refluxing water. Bull. Chem. Soc. Jpn. 2006, 79, 1426–1430. [Google Scholar] Wang, G.-W.; Jia, C.-S.; Dong, Y.-W. Benign and highly efficient synthesis of quinolines from 2-aminoarylketone or 2-aminoarylaldehyde and carbonyl compounds mediated by hydrochloric acid in water. Tetrahedron Lett. 2006, 47, 1059–1063. [Google Scholar] Wang, G.-W.; Miao, C.-B. Environmentally benign one-pot multi-component approaches to the synthesis of novel unsymmetrical 4-arylacridinediones. Green Chem. 2006, 8, 1080–1085. [Google Scholar]
- Fujioka, H.; Murai, K.; Ohba, Y.; Hiramatsu, A.; Kita, Y. A mild and efficient one-pot synthesis of 2-dihydroimidazoles from aldehydes. Tetrahedron Lett. 2005, 46, 2197–2199. [Google Scholar] [CrossRef]
- Gogoi, P.; Konwar, D. An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of carbon–nitrogen bonds in water. Tetrahedron Lett. 2006, 47, 79–82. [Google Scholar] [CrossRef]
- Sayama, S. A convenient synthesis of oxazolines and imidazolines from aromatic aldehydes with pyridinium hydrobromide perbromide in water. Synlett 2006, 1479–1484. [Google Scholar] [CrossRef]
- For recent examples, see: Shen, Y.-M.; Wang, B.; Shi, Y. Enantioselective synthesis of 2-alkyl-2-aryl cyclopentanones by asymmetric epoxidation of tetrasubstituted cyclobutylidene olefins and epoxide rearrangement. Tetrahedron Lett. 2006, 47, 5455–5458. [Google Scholar] Blatch, A. J.; Chetina, O. V.; Howard, J. A. K.; Patrick, L. G. F.; Smethurst, C. A.; Whiting, A. Synthesis and structure of bifunctional N-alkylbenzimidazole phenylboronate derivatives. Org. Biomol. Chem. 2006, 4, 3297–3302. [Google Scholar] Sawwan, N.; Greer, A. Generation of mono- and bis-dioxiranes from 2,3-butanedione. J. Org. Chem. 2006, 71, 5796–5799. [Google Scholar]
- Wiley, R. H.; Wakefield, B. J. Infrared spectra of the nitrile N-oxides: some new furoxans. J. Org. Chem. 1960, 25, 546–551. [Google Scholar] [CrossRef]
- Quan, C.; Kurth, M. Solid-phase synthesis of 5-isoxazol-4-yl-[1,2,4]oxadiazoles. J. Org. Chem. 2004, 69, 1470–1474. [Google Scholar] [CrossRef]
- Gattermann, L. Syntheses of aromatic aldehydes I. Justus Liebigs Ann. Chem. 1906, 347–386. [Google Scholar] [CrossRef]
- Field, L.; Hughmark, P. B.; Shumaker, S. H.; Marshall, W. S. Isomerization of aldoximes to amides under substantially neutral conditions. J. Am. Chem. Soc. 1961, 83, 1983–1987. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Chung, J. C.; Costello, T. D.; Valvis, I.; Ma, P.; Kauffman, S.; Wad, R. The enantiospecific synthesis of an isoxazoline. A RGD mimic platelet GPIIb/IIIa antagonist. J. Org. Chem. 1997, 62, 2466–2470. [Google Scholar]
- Sample Availability: Samples of the compounds 3a-j are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Xia, J.-J.; Wang, G.-W. Efficient Preparation of Aldoximes from Arylaldehydes, Ethylenediamine and Oxone® in Water. Molecules 2007, 12, 231-236. https://doi.org/10.3390/12020231
Xia J-J, Wang G-W. Efficient Preparation of Aldoximes from Arylaldehydes, Ethylenediamine and Oxone® in Water. Molecules. 2007; 12(2):231-236. https://doi.org/10.3390/12020231
Chicago/Turabian StyleXia, Jing-Jing, and Guan-Wu Wang. 2007. "Efficient Preparation of Aldoximes from Arylaldehydes, Ethylenediamine and Oxone® in Water" Molecules 12, no. 2: 231-236. https://doi.org/10.3390/12020231




