Heteropolyacids as Green and Reusable Catalysts for the Synthesis of 3,1,5-Benzoxadiazepines
Abstract
:Introduction
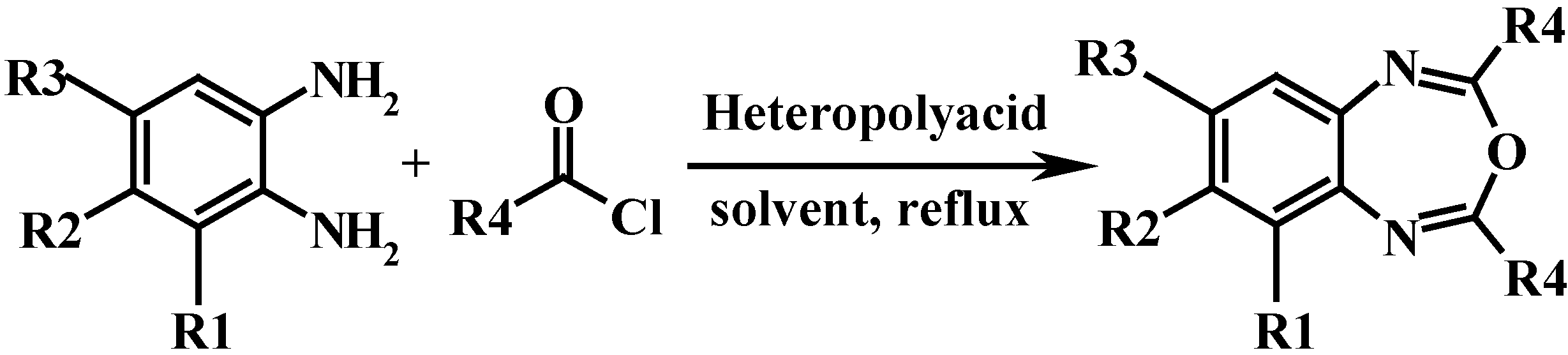
Results and Discussion
| Entry | R1 | R2 | R3 | R4 | Catalyst | Yield %a |
|---|---|---|---|---|---|---|
| 1 | H | H | H | CH3 | H14[NaP5W30O110] | 80 |
| 2 | H | H | H | CH3 | H6[P2W18O62] | 74.8 |
| 3 | H | H | H | CH3 | H5[PMo10V2O40] | 60 |
| 4 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 85.7 |
| 5 | H | CH3 | H | CH3 | H6[P2W18O62] | 78.25 |
| 6 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 61 |

| Entry | R1 | R2 | R3 | R4 | Catalyst | Time (h) | Yield%a | ||
|---|---|---|---|---|---|---|---|---|---|
| 25°C | 50°C | 82°C | |||||||
| 1 | H | H | H | CH3 | H14[NaP5W30O110] | 0.5 | 41 | 44 | 50 |
| 2 | H | H | H | CH3 | H14[NaP5W30O110] | 1 | 50 | 55 | 59 |
| 3 | H | H | H | CH3 | H14[NaP5W30O110] | 2 | 56 | 60 | 65 |
| 4 | H | H | H | CH3 | H14[NaP5W30O110] | 3 | 67 | 69 | 74 |
| 5 | H | H | H | CH3 | H14[NaP5W30O110] | 4 | 73 | 77 | 80 |
| 6 | H | H | H | CH3 | H6[P2W18O62] | 0.5 | 38 | 43 | 48 |
| 7 | H | H | H | CH3 | H6[P2W18O62] | 1 | 47 | 51 | 55 |
| 8 | H | H | H | CH3 | H6[P2W18O62] | 2 | 54 | 59 | 63 |
| 9 | H | H | H | CH3 | H6[P2W18O62] | 3 | 59 | 67 | 68 |
| 10 | H | H | H | CH3 | H6[P2W18O62] | 4 | 65 | 70 | 74.8 |
| 11 | H | H | H | CH3 | H5[PMo10V2O40] | 0.5 | 23 | 27 | 29 |
| 12 | H | H | H | CH3 | H5[PMo10V2O40] | 1 | 29 | 33 | 37 |
| 13 | H | H | H | CH3 | H5[PMo10V2O40] | 2 | 33 | 38 | 42 |
| 14 | H | H | H | CH3 | H5[PMo10V2O40] | 3 | 46 | 49 | 53 |
| 15 | H | H | H | CH3 | H5[PMo10V2O40] | 4 | 53 | 57 | 60 |
| 16 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 0.5 | 44 | 52 | 58 |
| 17 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 1 | 52 | 59 | 63 |
| 18 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 2 | 61 | 67 | 71 |
| 19 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 3 | 60 | 69 | 76 |
| 20 | H | CH3 | H | CH3 | H14[NaP5W30O110] | 4 | 66 | 72 | 85.7 |
| 21 | H | CH3 | H | CH3 | H6[P2W18O62] | 0.5 | 39 | 42 | 48 |
| 22 | H | CH3 | H | CH3 | H6[P2W18O62] | 1 | 48 | 52 | 57 |
| 23 | H | CH3 | H | CH3 | H6[P2W18O62] | 2 | 55 | 59 | 64 |
| 24 | H | CH3 | H | CH3 | H6[P2W18O62] | 3 | 64 | 68 | 70 |
| 25 | H | CH3 | H | CH3 | H6[P2W18O62] | 4 | 70 | 72 | 78.25 |
| 26 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 0.5 | 22 | 30 | 36 |
| 27 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 1 | 29 | 35 | 40 |
| 28 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 2 | 35 | 41 | 48 |
| 29 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 3 | 49 | 51 | 53 |
| 30 | H | CH3 | H | CH3 | H5[PMo10V2O40] | 4 | 57 | 59 | 61 |
| Entry | R1 | R2 | R3 | R4 | Catalyst | Yield %a |
|---|---|---|---|---|---|---|
| 1 | H | NO2 | H | CH3 | H14[NaP5W30O110] | 98.5 |
| 2 | H | NO2 | H | CH3 | H6[P2W18O62] | 97 |
| 3 | H | NO2 | H | CH3 | H5[PMo10V2O40] | 95.7 |
| 4 | H | NO2 | H | C6H5 | H14[NaP5W30O110] | 98 |
| 5 | H | NO2 | H | C6H5 | H6[P2W18O62] | 94 |
| 6 | H | NO2 | H | C6H5 | H5[PMo10V2O40] | 85 |
| 7 | NO2 | H | NO2 | CH3 | H14[NaP5W30O110] | 97 |
| 8 | NO2 | H | NO2 | CH3 | H6[P2W18O62] | 95.7 |
| 9 | NO2 | H | NO2 | CH3 | H5[PMo10V2O40] | 90.4 |
| 10 | NO2 | H | NO2 | C6H5 | H14[NaP5W30O110] | 85.7 |
| 11 | NO2 | H | NO2 | C6H5 | H6[P2W18O62] | 78.2 |
| 12 | NO2 | H | NO2 | C6H5 | H5[PMo10V2O40] | 77.3 |
Experimental
General
General Procedure
Spectral data for selected samples
Catalyst reusability
Acknowledgments
References
- Misono, M.; Ono, L.; Koyano, G.; Aoshima, A. Heteropolyacids. Versatile green catalysts usable in a variety of reaction media. Pure Appl. Chem. 2000, 72, 1305–1311. [Google Scholar]
- Pope, M.T.; Muller, A. Polyoxometalates: from Platonic Solids to Anti-Retroviral Activity; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Heravi, M. M.; Rajabzadeh, G.; Bamoharram, F. F.; Seifi, N. An eco-friendly catalytic route for synthesis of 4-amino-pyrazolo[3,4-d]pyrimidine derivatives by Keggin heteropolyacids under classical heating and microwave irradiation. J. Mol. Catal. A: Chem. 2006, 256, 238–241. [Google Scholar] [CrossRef]
- Heravi, M. M.; Bakhtiari, Kh.; Bamoharram, F. F. An efficient and chemoselective synthesis of acylals from aromatic aldehydes and their regeneration, catalyzed by 12-molybdophosphoric acid. Catal. Commun. 2006, 7, 499–501. [Google Scholar] [CrossRef]
- Heravi, M. M.; Bamoharram, F. F.; Rajabzadeh, G.; Seifi, N.; Khatami, M. Preyssler heteropolyacid [NaP5W30O110]14-, as a new, green and recyclable catalyst for the synthesis of [1,2,4]triazino[4,3-b][1,2,4,5]tetrazines. J. Mol. Catal. A: Chem. 2006, 259, 213–217. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, 1983. [Google Scholar]
- Heravi, M. M.; Bakhtiari, K.; Bamoharram, F. F. 12-Molybdophosphoric acid: A recyclable catalyst for the synthesis of Biginelli-type 3,4-dihydropyrimidine-2(1H)-ones. Catal. Commun. 2006, 7, 373–376. [Google Scholar] [CrossRef]
- Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Gharib, A.; Jahangir, M. A catalytic method for synthesis of γ-butyrolactone, ε-caprolactone and 2-cumaranone in the presence of Preyssler's anion, [NaP5W30O110]14−, as a green and reusable catalyst. J. Mol. Catal. A: Chem. 2006, 252, 90–95. [Google Scholar] [CrossRef]
- Heravi, M. M.; Motamedi, R.; Seifi, N.; Bamoharram, F. F. Catalytic synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacid: H14[NaP5W30O110] and H3PW12O40. J. Mol. Catal. A: Chem. 2006, 249, 1–3. [Google Scholar] [CrossRef]
- Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Akbarpour, M. Catalytic performance of Preyssler heteropolyacid as a green and recyclable catalyst in oxidation of primary aromatic amines. J. Mol. Catal. A: Chem. 2006, 255, 193–198. [Google Scholar] [CrossRef]
- Tandon, V. K.; Kumar, M. BF3Et2O promoted one-pot expeditious and convenient synthesis of 2-substituted benzimidazoles and 3,1,5-benzoxadiazepines. Tetrahedron Lett. 2004, 45, 4185–4187. [Google Scholar] [CrossRef]
- Mazurkiewicz, R. Novel synthesis and rearrangement of 3,1,5-benzoxadiazepines. Monatsh. Chem. 1988, 119, 1279–1287. [Google Scholar] [CrossRef]
- Sulkowski, T. S.; Childress, S.J. The formation and subsequent rearrangement of 7-Chloro-5-phenyl-3,1,4-benzoxadiazepine2(1H)-one. J. Org. Chem. 1962, 27, 4424–4426. [Google Scholar] [CrossRef]
- Sternbach, L.H.; Kaiser, S.; Reeder, E. Quinazoline 3-Oxide Structure of Compounds Previously Described in the Literature as 3,1,4-benzoxadiazepines. J. Am. Chem. Soc. 1960, 82, 475–480. [Google Scholar] [CrossRef]
- Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Tavakoli, N. N-oxidation of pyridine carboxylic acids using hydrogen peroxide catalyzed by a green heteropolyacid catalyst: Preyssler's anion, [NaP5W30O110]14−. J. Mol. Catal. A: Chem. 2006, 252, 219–225. [Google Scholar] [CrossRef]
- Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler catalyst, [NaP5W30O110]14−: A green, efficient and reusable catalyst for esterification of salicylic acid with aliphatic and benzylic alcohols. J. Appl. Catal. A: Gen.l 2006, 302, 42–47. [Google Scholar] [CrossRef]
- Heravi, M. M.; Behbahani, F. K.; Bamoharram, F. F. H14[NaP5W30O110]: A heteropolyacid catalyzed acetylation of alcohols and phenols in acetic anhydride. J. Mol. Catal. A: Chem. 2006, 253, 16–19. [Google Scholar] [CrossRef]
- Heravi, M. M.; Ranjbar, L.; Derikvand, F.; Bamoharram, F. F. H6P2W18O62: An efficient and reusable catalyst for one-pot synthesis of β-acetamido ketone and esters. Catal. Commun. 2007, 8, 289–291. [Google Scholar] [CrossRef]
- Anastas, P. T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University: Oxford, UK, 1998. [Google Scholar]
- Oskooie, H. A.; Heravi, M. M.; Bakhtiari, K.; Zadsirjan, V.; Bamoharram, F. F. H14[NaP5W30O110] as an efficient catalyst for the one-pot synthesis of alpha-amino nitriles. Synlett 2006, 11, 1768–1770. [Google Scholar]
- Heravi, M. M.; Zadsirjan, V.; Bakhtiari, K.; Oskooie, H. A.; Bamoharram, F. F. Green and reusable heteropolyacid catalyzed oxidation of benzylic, allylic and aliphatic alcohols to carbonyl compounds. Catal. Commun. 2007, 8, 315–318. [Google Scholar] [CrossRef]
- Heravi, M. M.; Benmord, T.; Bakhtiari, K.; Bamoharram, F. F.; Oskooie, H. A. H3+xPMo12-xVxO40(heteropolyacids)-catalyzed regioselective nitration of phenol to o-nitrophenol in heterogeneous system. J. Mol. Catal. A: Chem. 2006, 264, 318–321. [Google Scholar]
- Romanelli, G.; Autino, J.C.; Baronetti, G.; Thomas, H. Efficient Deprotection of Phenol Methoxymethyl Ethers Using a Solid Acid Catalyst with Wells-Dawson Structure. Molecules 2001, 6, 1006–1011. [Google Scholar] [CrossRef] [Green Version]
- Sample Availability: Samples of the compounds presented in this paper are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Heravi, M.M.; Sadjadi, S.; Oskooie, H.A.; Shoar, R.H.; Bamoharram, F.F. Heteropolyacids as Green and Reusable Catalysts for the Synthesis of 3,1,5-Benzoxadiazepines. Molecules 2007, 12, 255-262. https://doi.org/10.3390/12020255
Heravi MM, Sadjadi S, Oskooie HA, Shoar RH, Bamoharram FF. Heteropolyacids as Green and Reusable Catalysts for the Synthesis of 3,1,5-Benzoxadiazepines. Molecules. 2007; 12(2):255-262. https://doi.org/10.3390/12020255
Chicago/Turabian StyleHeravi, Majid M., Samaheh Sadjadi, Hossein A. Oskooie, Rahim Hekmat Shoar, and Fatemeh F. Bamoharram. 2007. "Heteropolyacids as Green and Reusable Catalysts for the Synthesis of 3,1,5-Benzoxadiazepines" Molecules 12, no. 2: 255-262. https://doi.org/10.3390/12020255




