Mistletonone, a Novel Antioxidative Diarylheptanoid from the Branches and Leaves of Viscum coloratum
Abstract
:Introduction
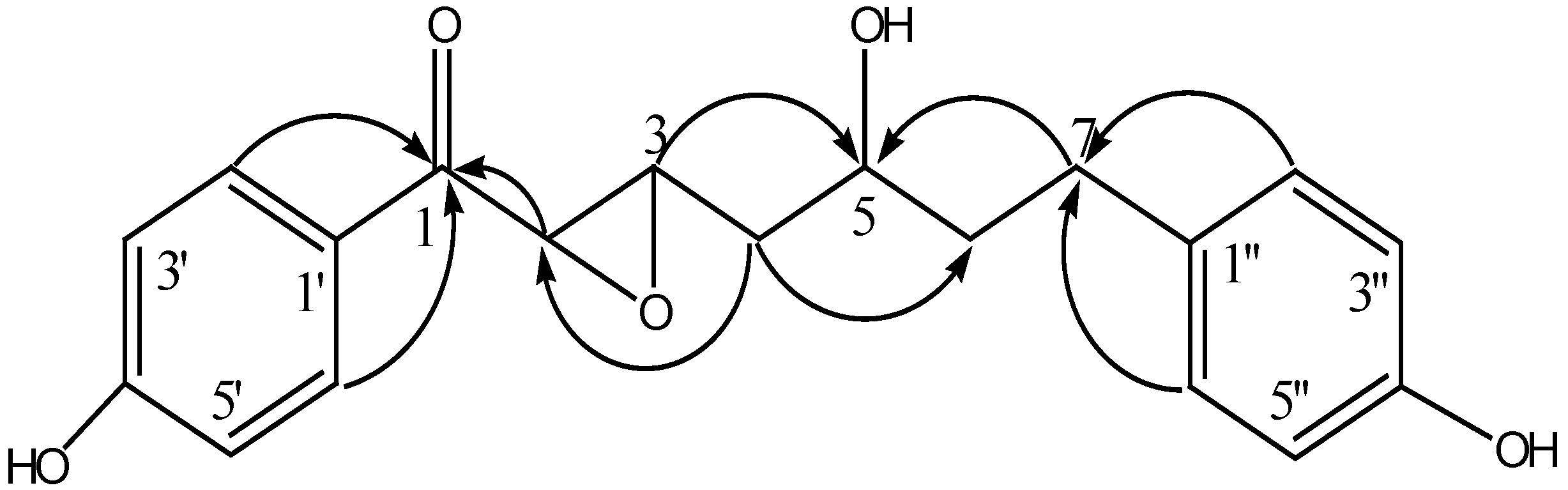
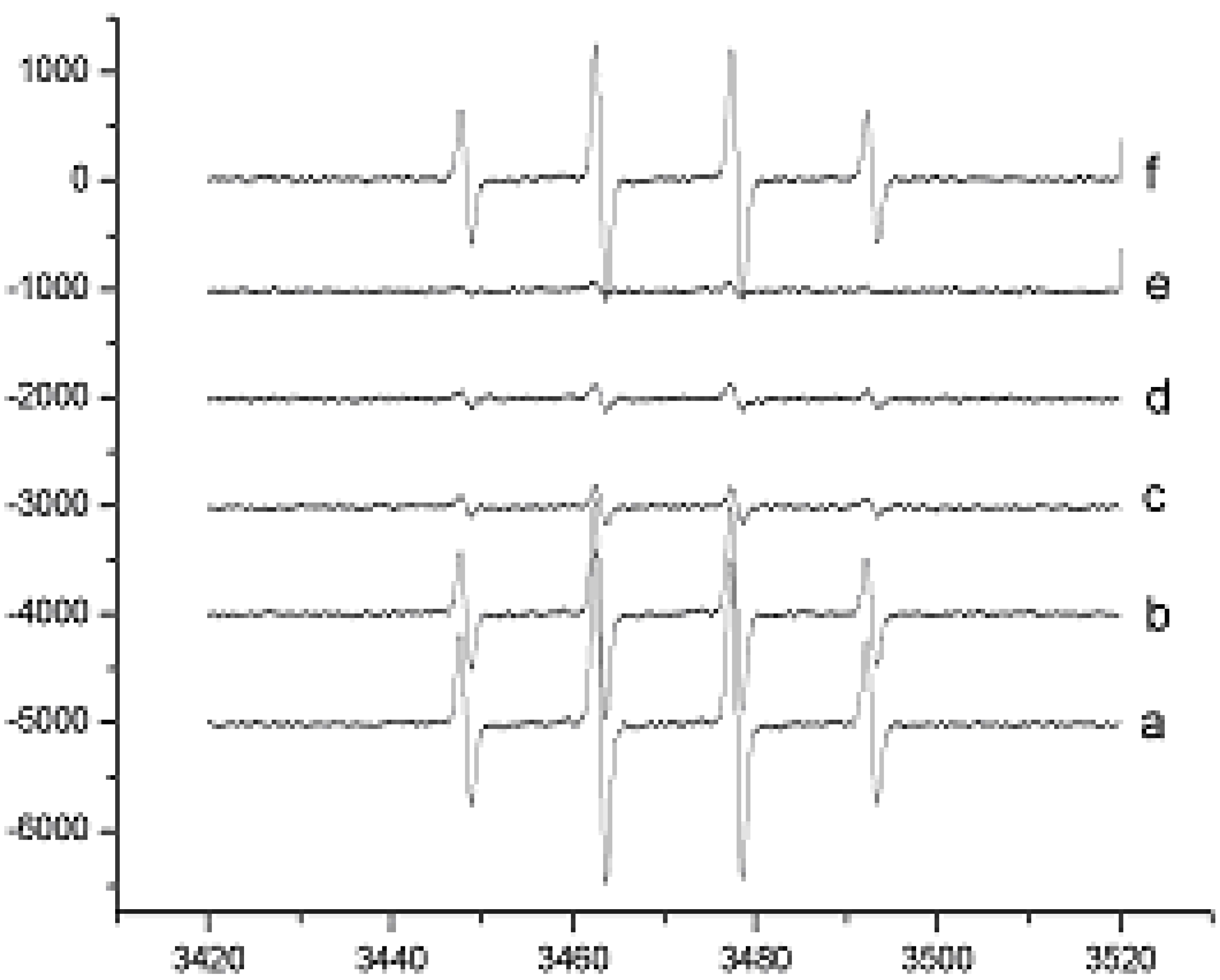
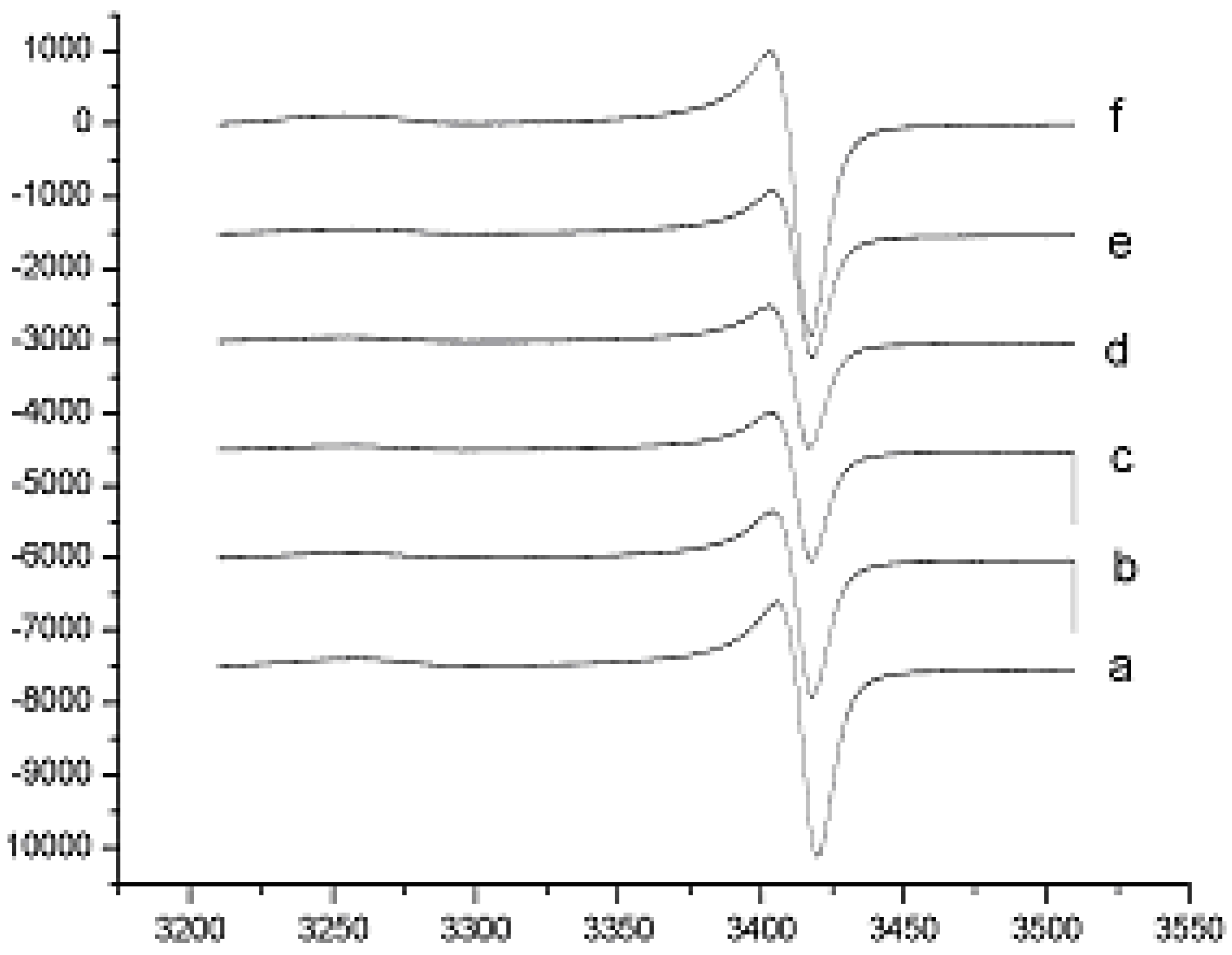
Results and Discussion
| C/H | δ C | δ H | 1H-1H COSY |
|---|---|---|---|
| 1 | 191.8 (s) | ||
| 2 | 56.4 (d) | 4.38 (d, 1H, J = 4.8 Hz) | H-3 |
| 3 | 55.8 (d) | 3.48 (m, 1H) | H-2, H-4 |
| 4 | 35.2 (t) | 1.47 (m, 2H) | H-3, H-5 |
| 5 | 67.7 (d) | 3.48 (m, 1H) | H-4, H-6 |
| 6 | 40.2 (t) | 1.47 (m, 2H) | H-5, H-7 |
| 7 | 30.8 (t) | 2.35 (m, 2H) | H-6 |
| 1' | 127.7 (s) | ||
| 2' | 130.9 (d) | 7.84 (d, 1H, J =8.3 Hz) | H-3’ |
| 3' | 115.8 (d) | 6.76 (d, 1H, J = 8.3 Hz) | H-2’ |
| 4' | 163.3 (s) | ||
| 5' | 115.8 (d) | 6.76 (d, 1H, J = 8.3 Hz) | H-6’ |
| 6' | 130.9 (d) | 7.84 (d, 1H, J = 8.3 Hz) | H-5’ |
| 1'' | 132.5 (s) | ||
| 2'' | 129.2 (d) | 6.84 (d, 1H, J = 7.9 Hz) | H-3” |
| 3'' | 115.4 (d) | 6.60 (d, 1H, J = 7.9 Hz) | H-2” |
| 4'' | 155.5 (s) | ||
| 5'' | 115.4 (d) | 6.60 (d, 1H, J =7.9 Hz) | H-6” |
| 6'' | 129.2 (d) | 6.84 (d, 1H, J =7.9 Hz) | H-5” |
| 4’, 4''(OH) | 3.30 (s, 2H) |
Conclusions
Experimental
General
Plant material
Extraction and Isolation
Hydroxyl Radical Scavenging Assay using ESR
Superoxide Anion Radicals Scavenging Assay using ESR
Acknowledgments
References
- Editorial Board of Zhonghua Bencao. Zhonghua Bencao; Shanghai Scientific and Technical Press: Shanghai, 1999; pp. 328–333. [Google Scholar]
- Kong, D. Y.; Luo, S. Q.; Li, H. T.; Lei, X. H. Studies on chemical components of Viscum coloratum I. Zhongguo Yiyao Gongye Zazhi 1987, 18, 123–127. [Google Scholar]
- Kong, D. Y.; Luo, S. Q.; Li, H. T.; Lei, X. H. Studies on chemical components of Viscum coloratum III. Acta Pharm. Sin. 1988, 23, 593–600. [Google Scholar]
- Kong, D. Y.; Luo, S. Q.; Li, H. T.; Lei, X. H. Studies on chemical components of Viscum coloratum IV. Acta Pharm. Sin. 1988, 23, 707–710. [Google Scholar]
- Kong, D. Y.; Li, H. T.; Luo, S. Q.; Lei, X. H. Studies on chemical components of Viscum coloratum VI, chirality of the acyl group of viscumneoside IV. Acta Pharm. Sin. 1990, 25, 349–352. [Google Scholar]
- Kong, D. Y.; Li, H. T.; Luo, S. Q. Studies on chemical components of Viscum coloratum VII, isolation and structure of viscumneoside VII. Acta Pharm. Sin. 1990, 25, 608–611. [Google Scholar]
- Greatrex, B. W.; Jenkins, N. F.; Taylaor, D. K.; Tiekink, E. R. T. Base- and Co(II)-catalyzed ring-opening reactions of perhydrooxireno [2,3-d] [1,2] dioxines: an efficient route to 4-hydroxy-2,3-epoxy-ketone. J. Org. Chem. 2003, 68, 5205–5210. [Google Scholar] [CrossRef]
- Allen, J. V.; Bergeron, S.; Griffiths, M. J.; Mukherjee, S.; Roberts, S. M.; Williamson, N. M. Juliá-Colona asymmetric epoxidation reactions under non-aqueous conditions: rapid, highly regio- and stereo-selective transformations using a cheap, recyclable catalyst. J. Chem. Soc. Perkin Trans. 1. 1998, 3171–3179. [Google Scholar]
- Keserü, G. M.; Nógrádi, M. The chemistry of natural diarylheptanoids. In Studies in Natural Products Chemistry; Atta ur Rahman, Ed.; Elsevier Science: Amsterdam, 1995; Vol. 17, pp. 357–92. [Google Scholar]
- Yin, J.; Kouda, K.; Tezuka, Y.; Tran, Q. L.; Miyahava, T.; Chen, Y. J. New diarylheptanoids from the rhizomes of Dioscorea spongiosa and their antiosteoporotic activity. Planta Med. 2004, 70, 54–58. [Google Scholar]
- Martin-Cordera, C.; Lopez-Lazaro, M.; Agudo, M. A.; Navarro, E.; Trujillo, J.; Ayuso, M. J. A cytotoxic diarylheptanoid from Viscum cruciatum. Phytochemistry 2001, 58, 567–569. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yao, H.; Zhou, G.-X.; Wu, Q.; Lei, G.-Q.; Chen, D.-F.; Chen, J.-K.; Zhou, T.-S. Mistletonone, a Novel Antioxidative Diarylheptanoid from the Branches and Leaves of Viscum coloratum. Molecules 2007, 12, 312-317. https://doi.org/10.3390/12030312
Yao H, Zhou G-X, Wu Q, Lei G-Q, Chen D-F, Chen J-K, Zhou T-S. Mistletonone, a Novel Antioxidative Diarylheptanoid from the Branches and Leaves of Viscum coloratum. Molecules. 2007; 12(3):312-317. https://doi.org/10.3390/12030312
Chicago/Turabian StyleYao, Hui, Guang-Xiong Zhou, Qiong Wu, Guang-Qing Lei, Dao-Feng Chen, Jia-Kuan Chen, and Tong-Shui Zhou. 2007. "Mistletonone, a Novel Antioxidative Diarylheptanoid from the Branches and Leaves of Viscum coloratum" Molecules 12, no. 3: 312-317. https://doi.org/10.3390/12030312





