Isoselenocyanates: A Powerful Tool for the Synthesis of Selenium-Containing Heterocycles
Abstract
:Contents:
| 1. Introduction | 505 |
| 2. Synthesis of isoselenocyanates and acylisoselenocyanates | 505 |
| 3. Reactions with amines | 507 |
| 4. Reactions with alcohols and thiols | 513 |
| 5. Reactions with selenolates | 516 |
| 6. Reactions with carbanions | 518 |
| 7. Reactions with azodicaboxylates and diazomethanes | 521 |
| 8. Cycloaddition reactions | 523 |
| 9. Iodo- and acid-catalyzed cyclization reactions | 525 |
| 10. Synthesis of pentalenes | 525 |
| 11. Conclusions | 528 |
1. Introduction
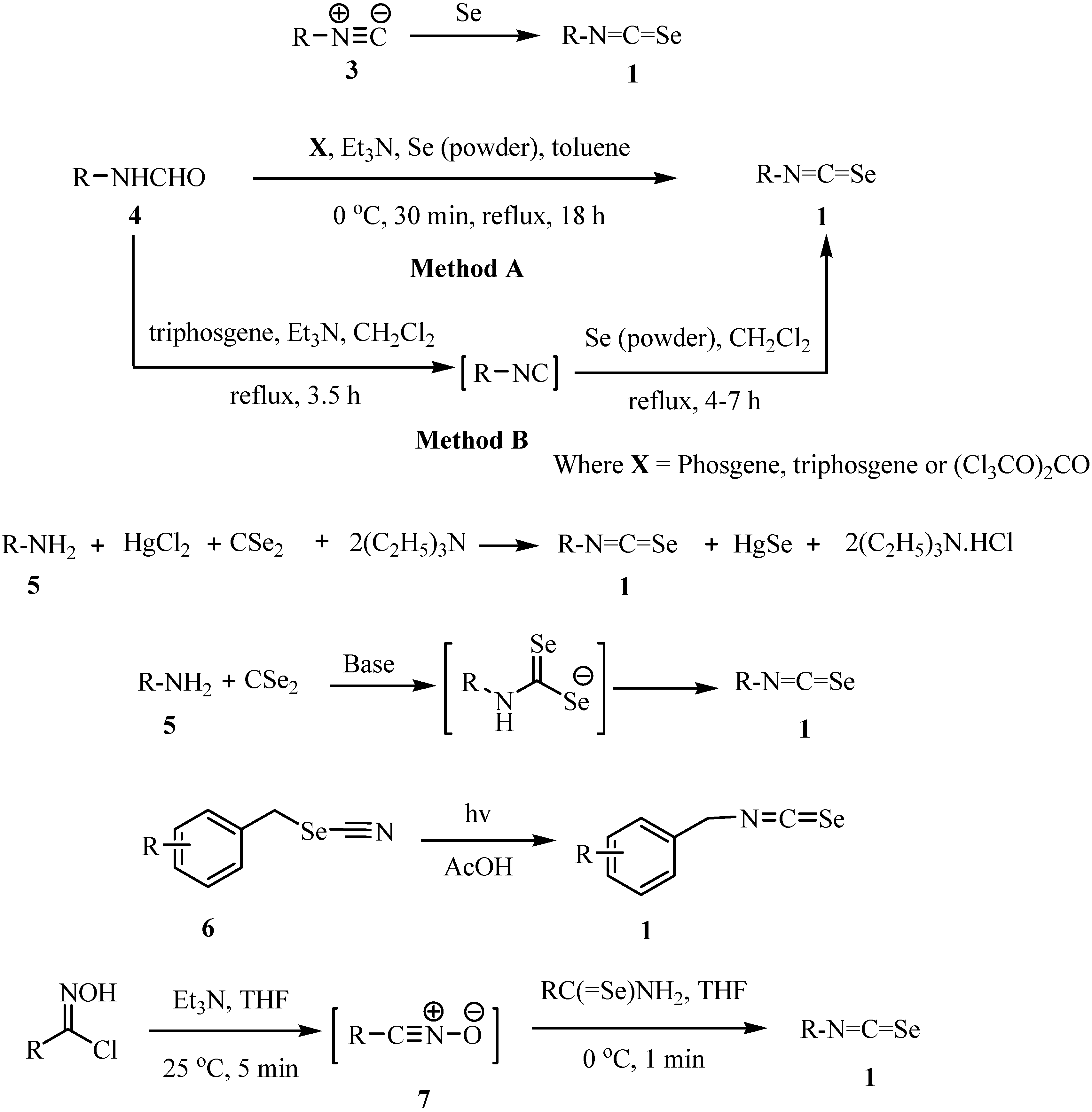
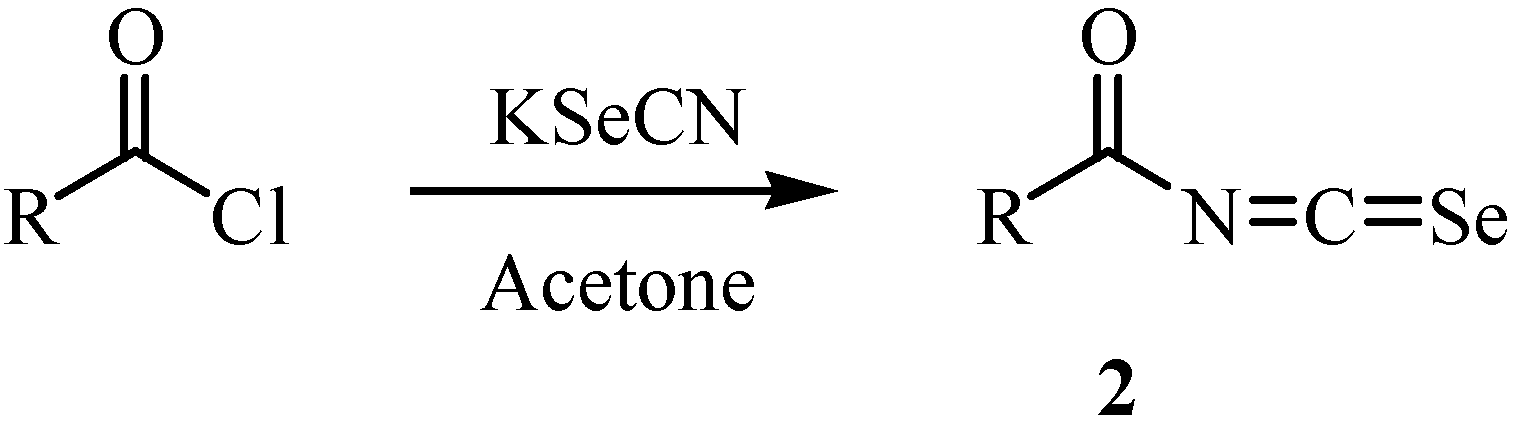
2. Synthesis of isoselenocyanates and acylisoselenocyanates

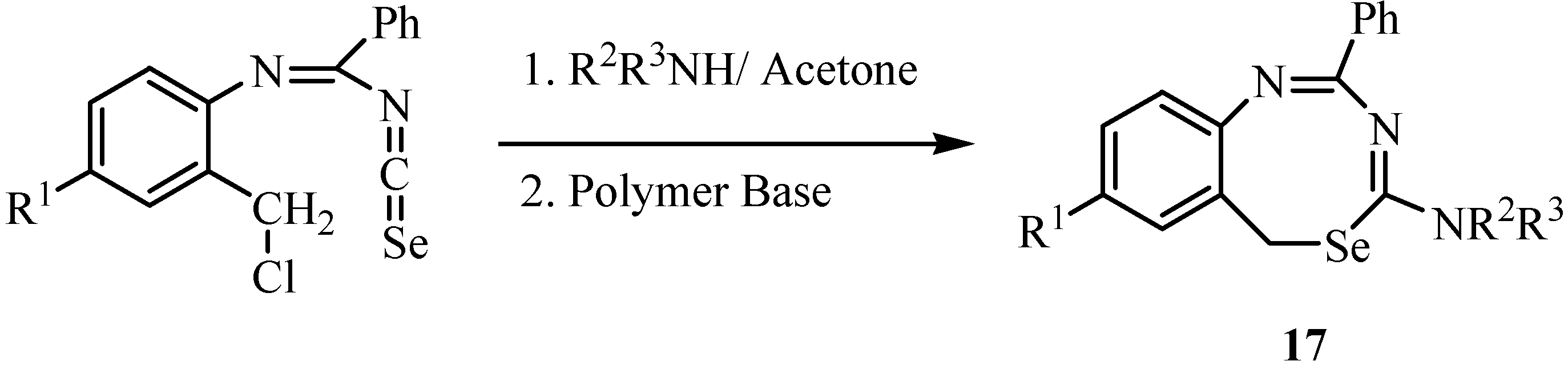
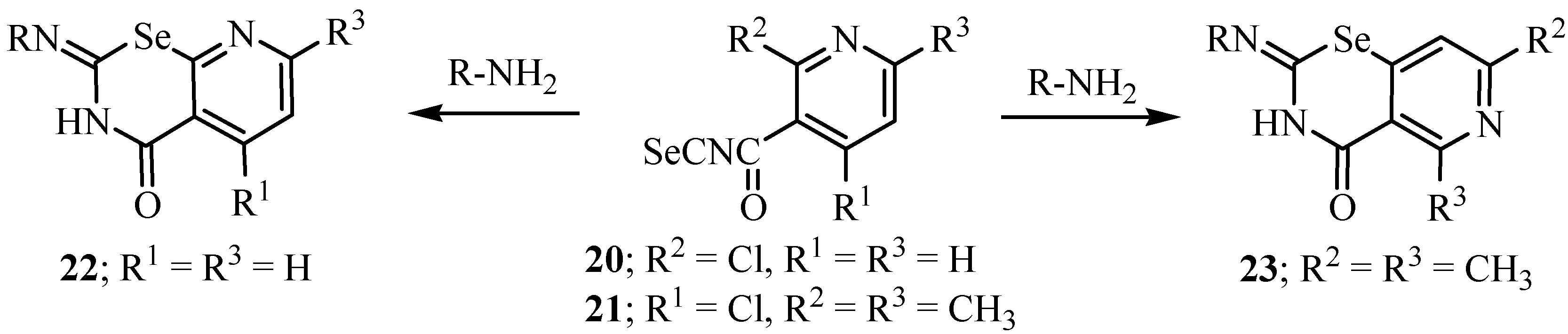
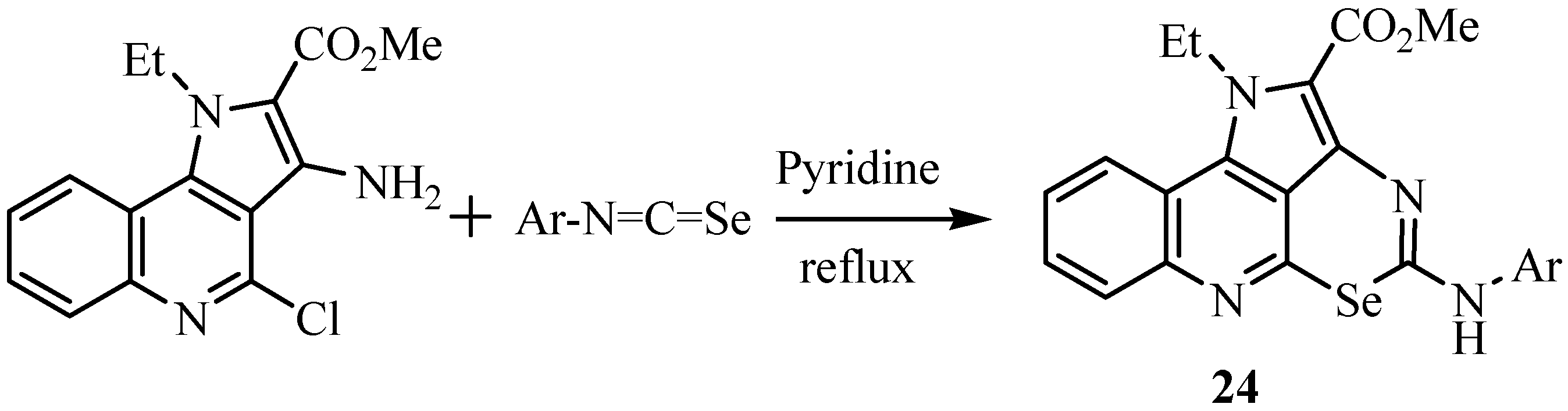
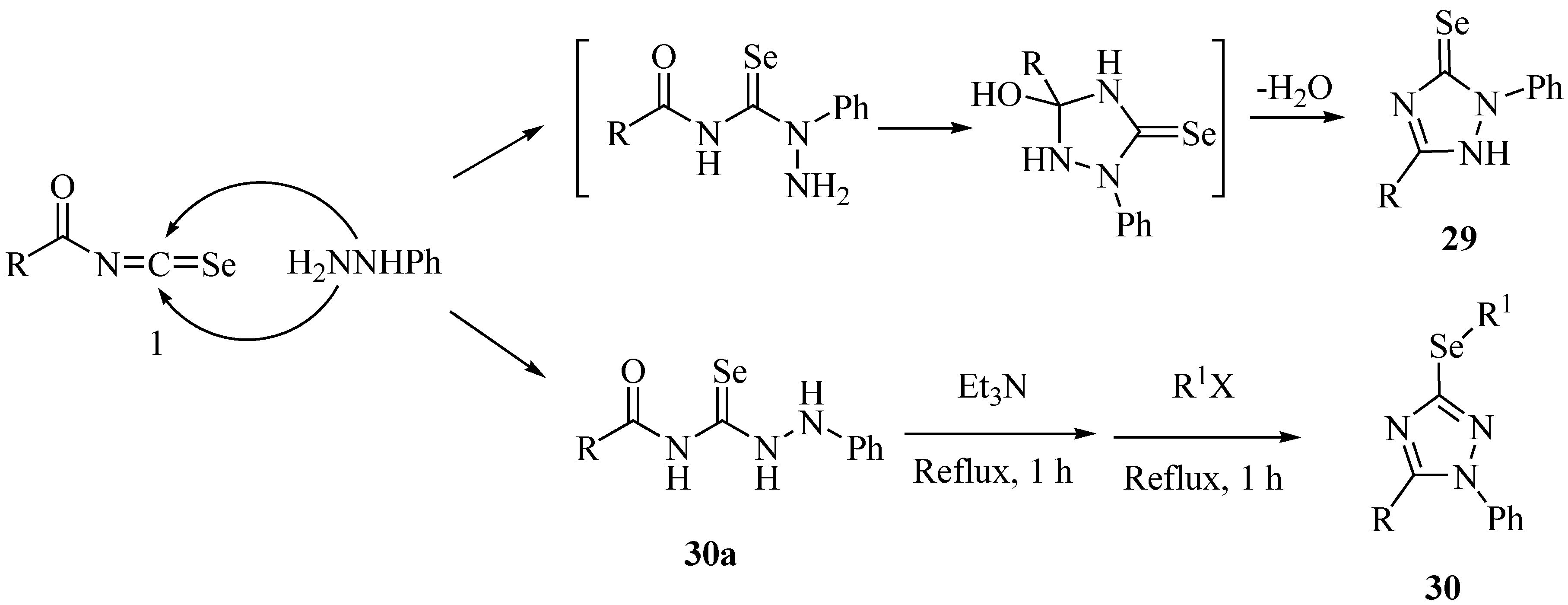
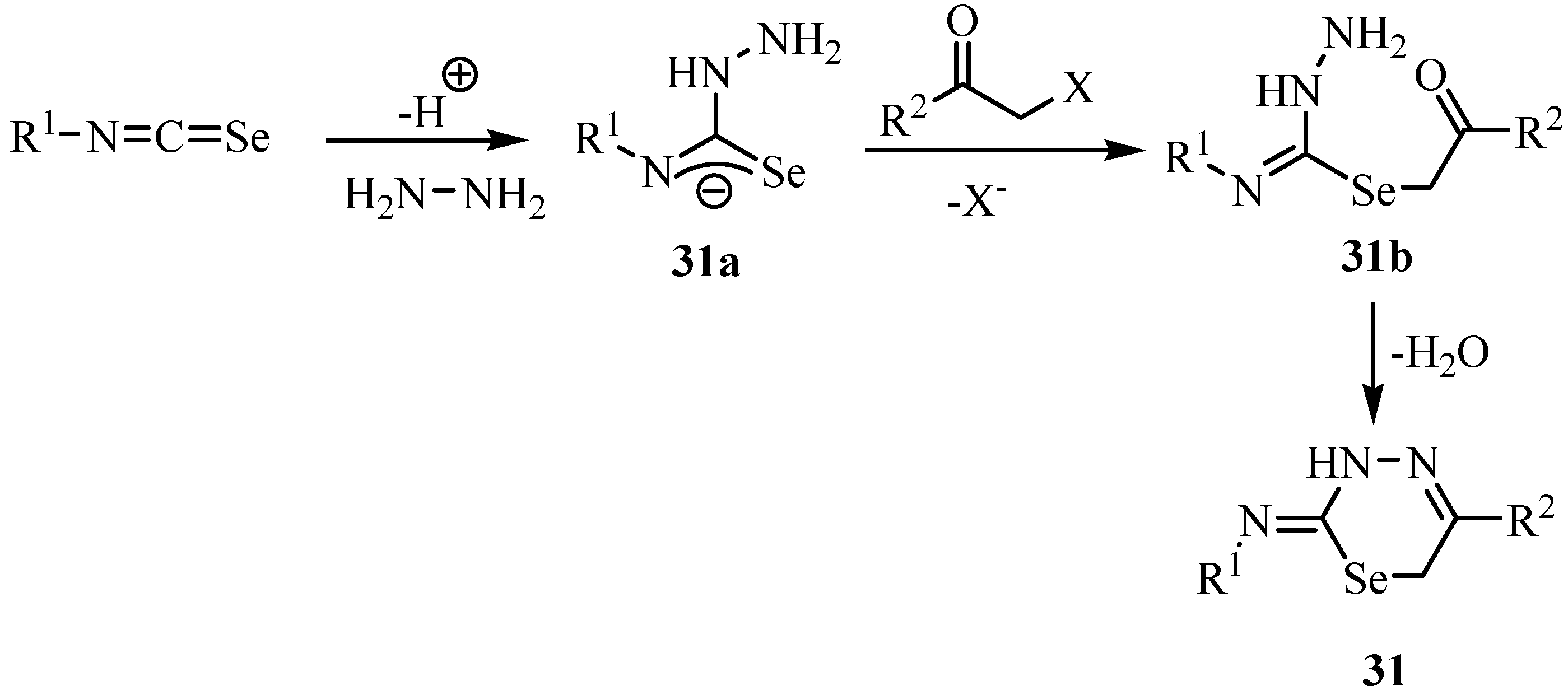
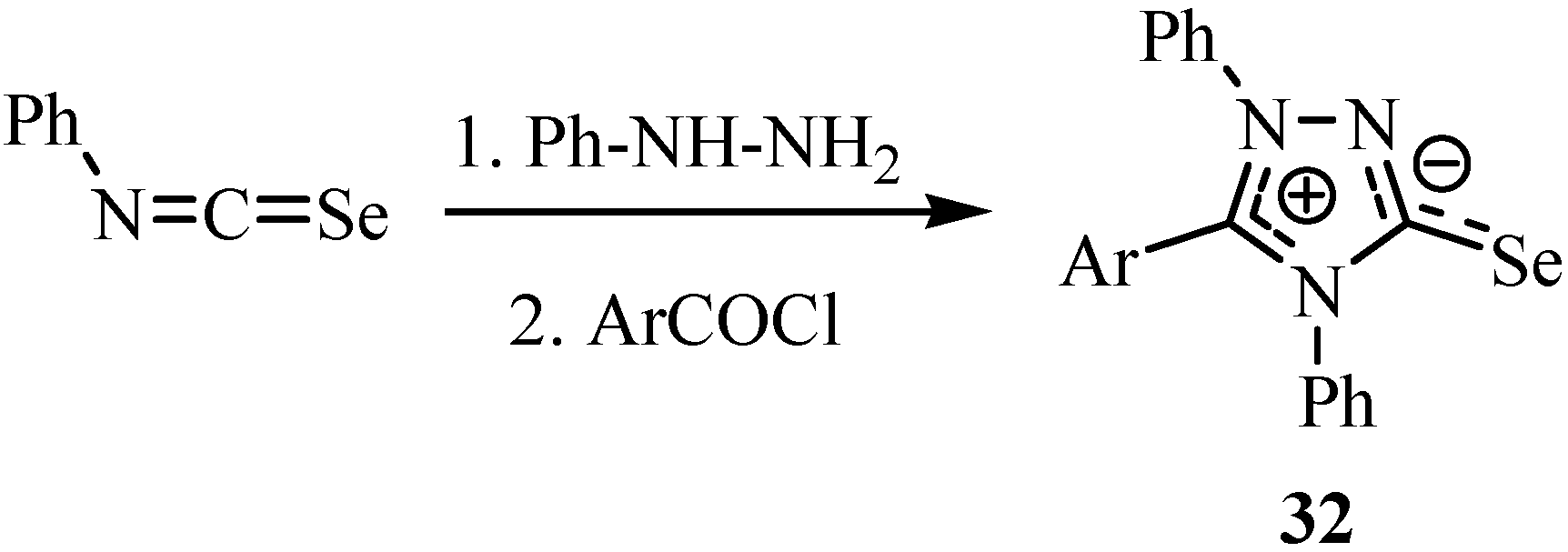
3. Reactions with amines
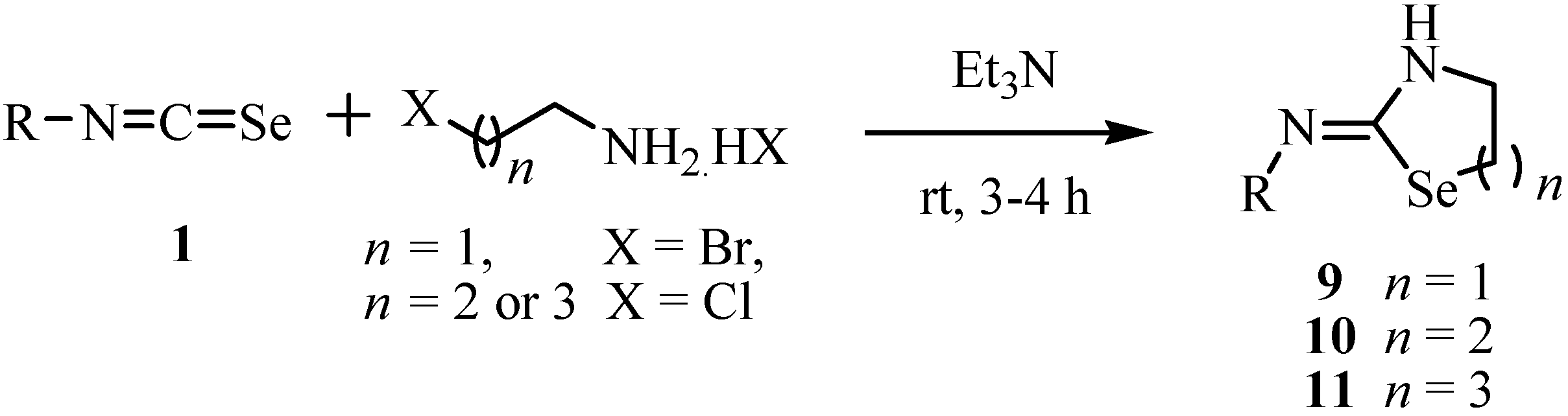

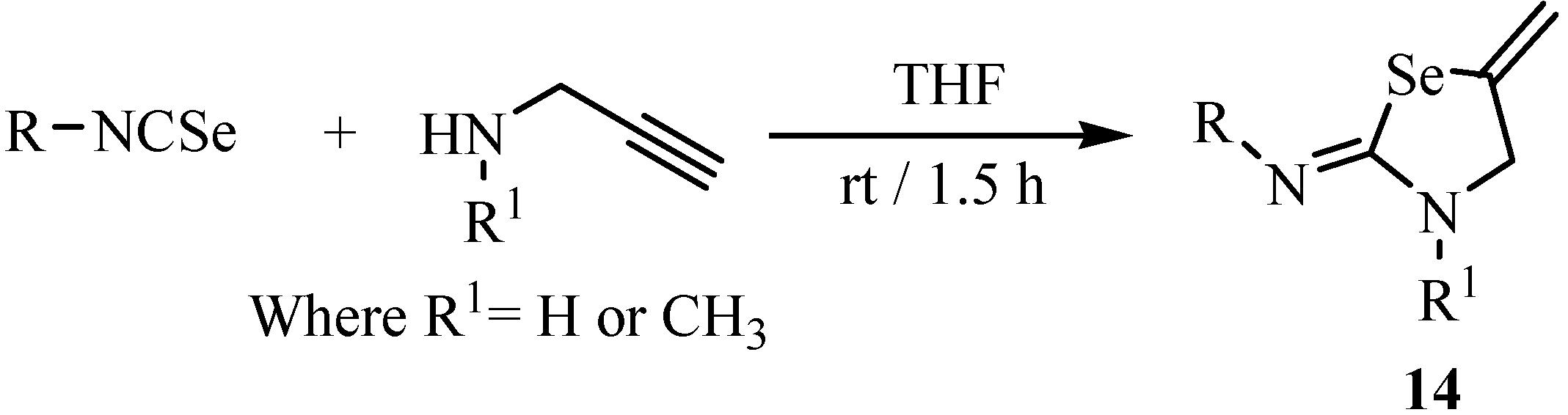
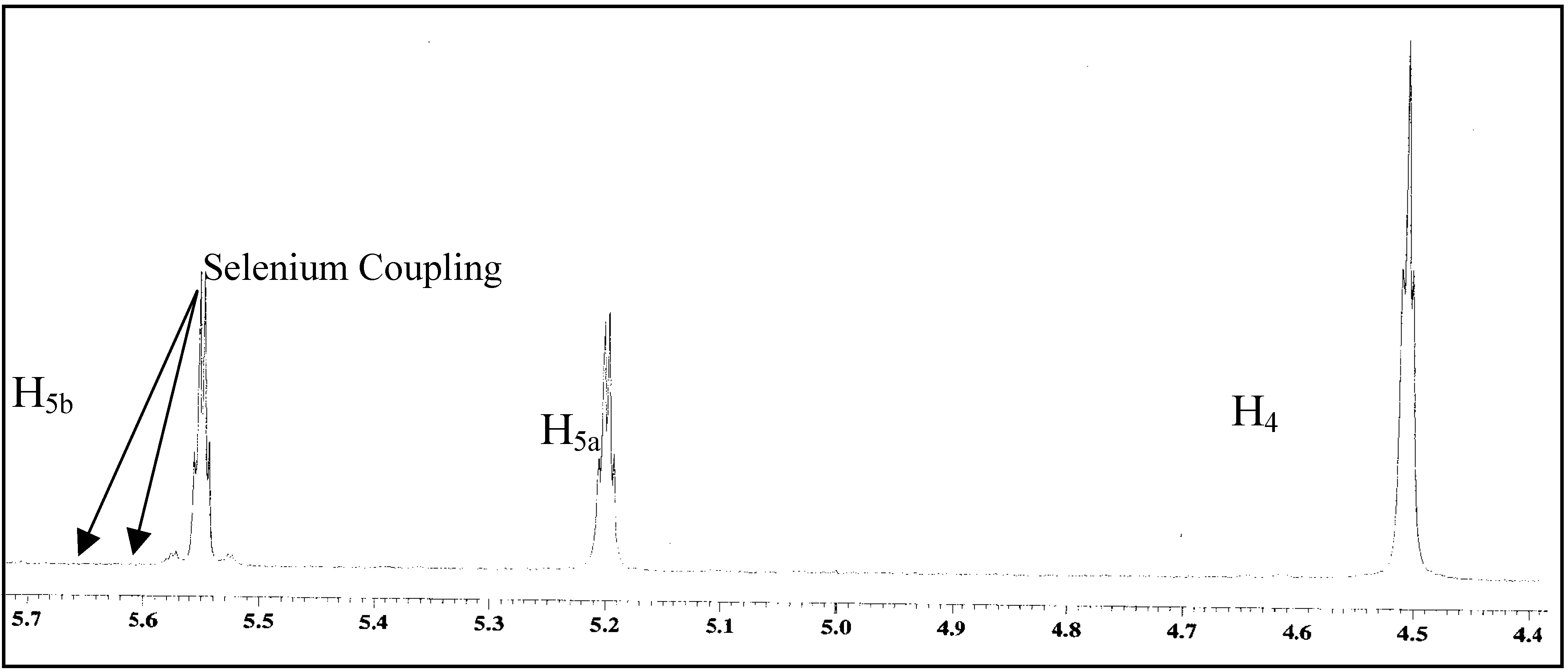
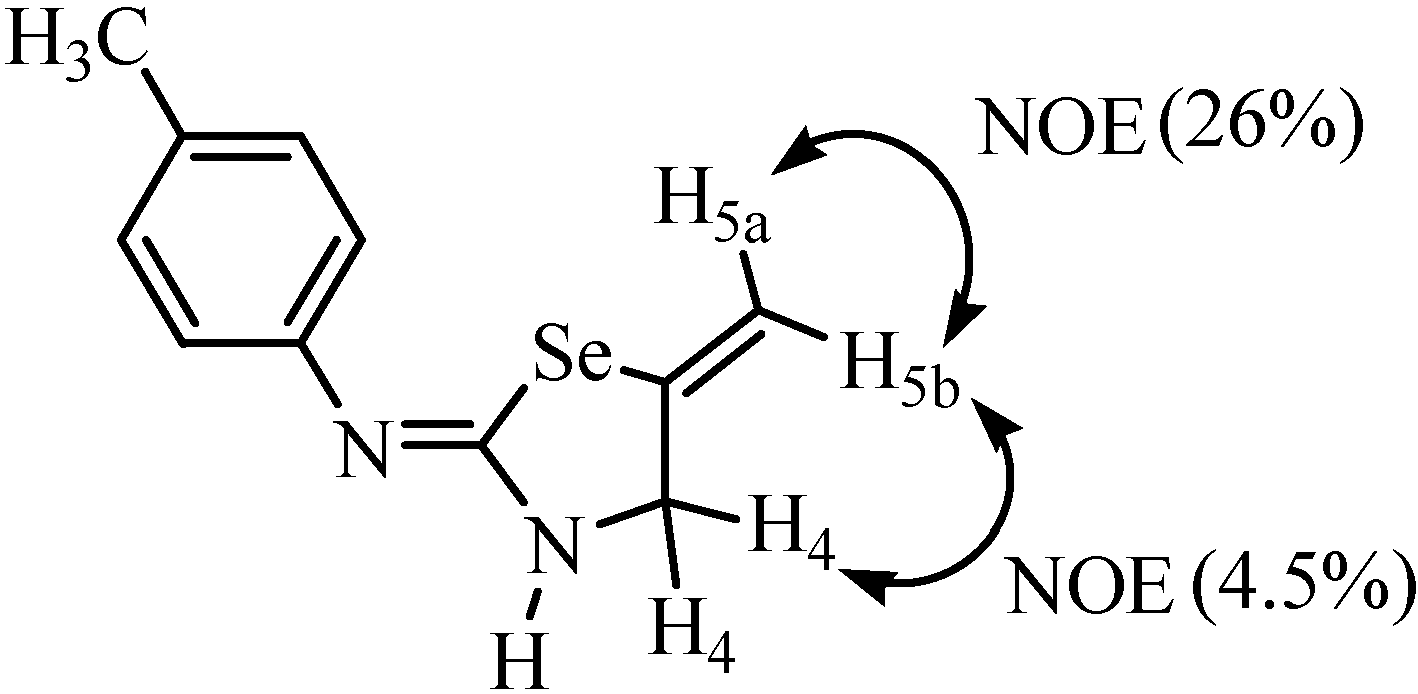
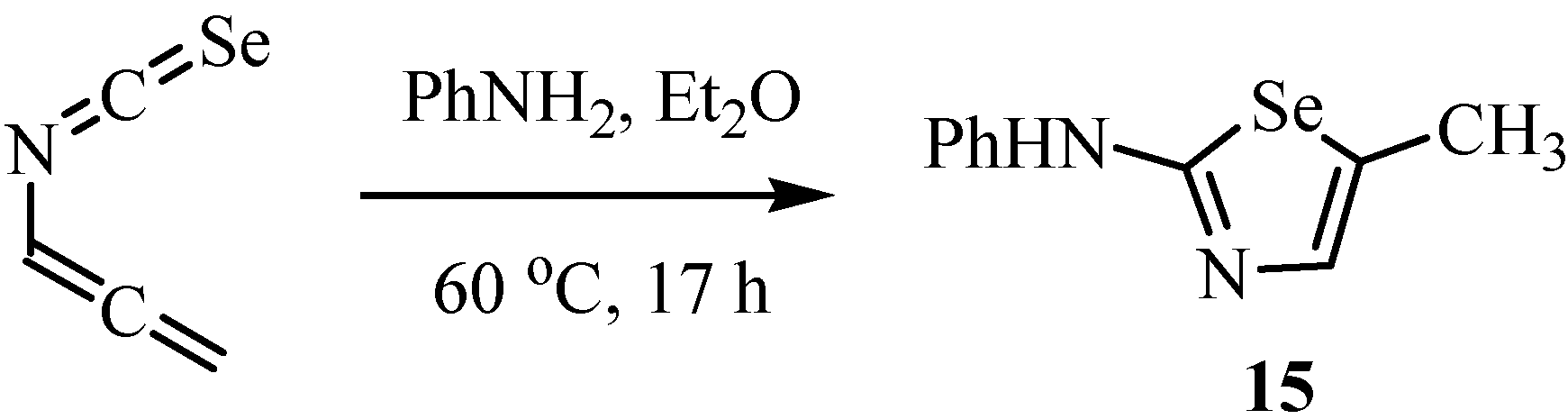
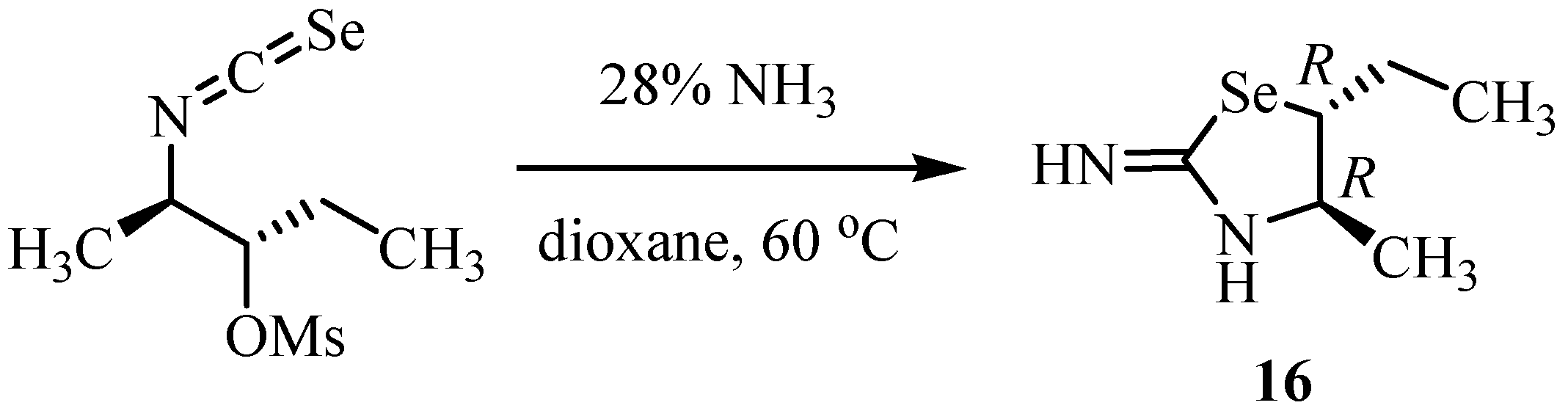
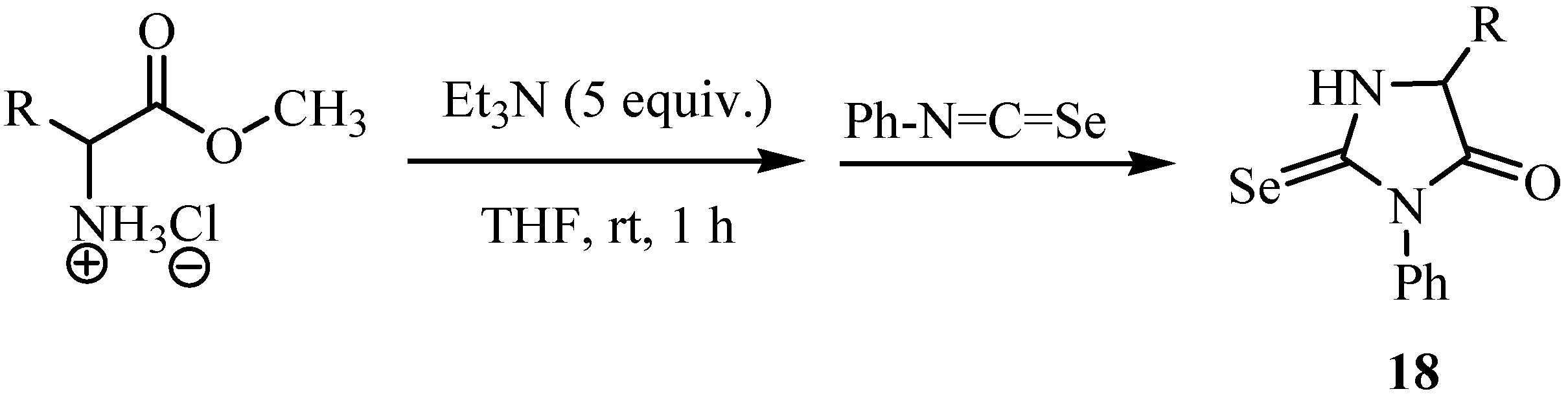

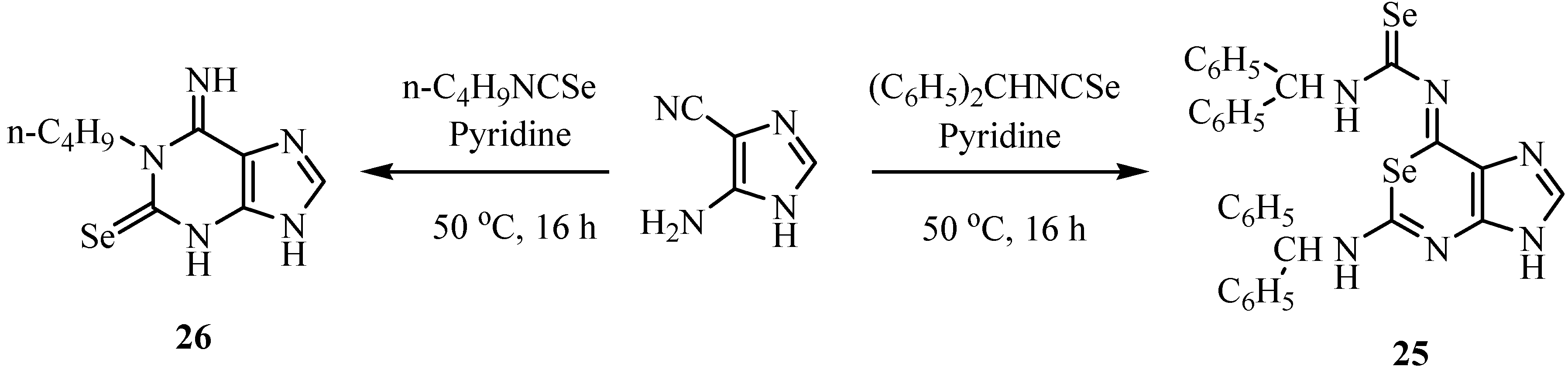
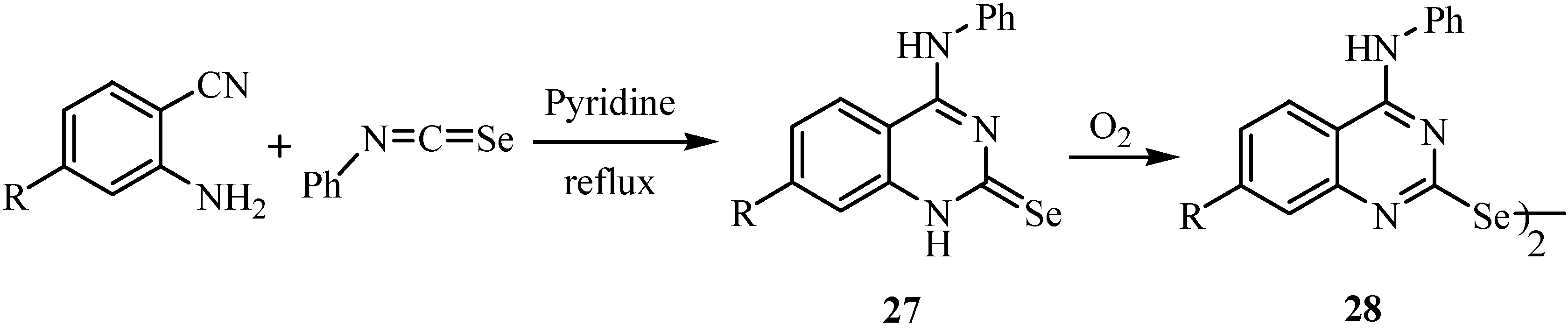
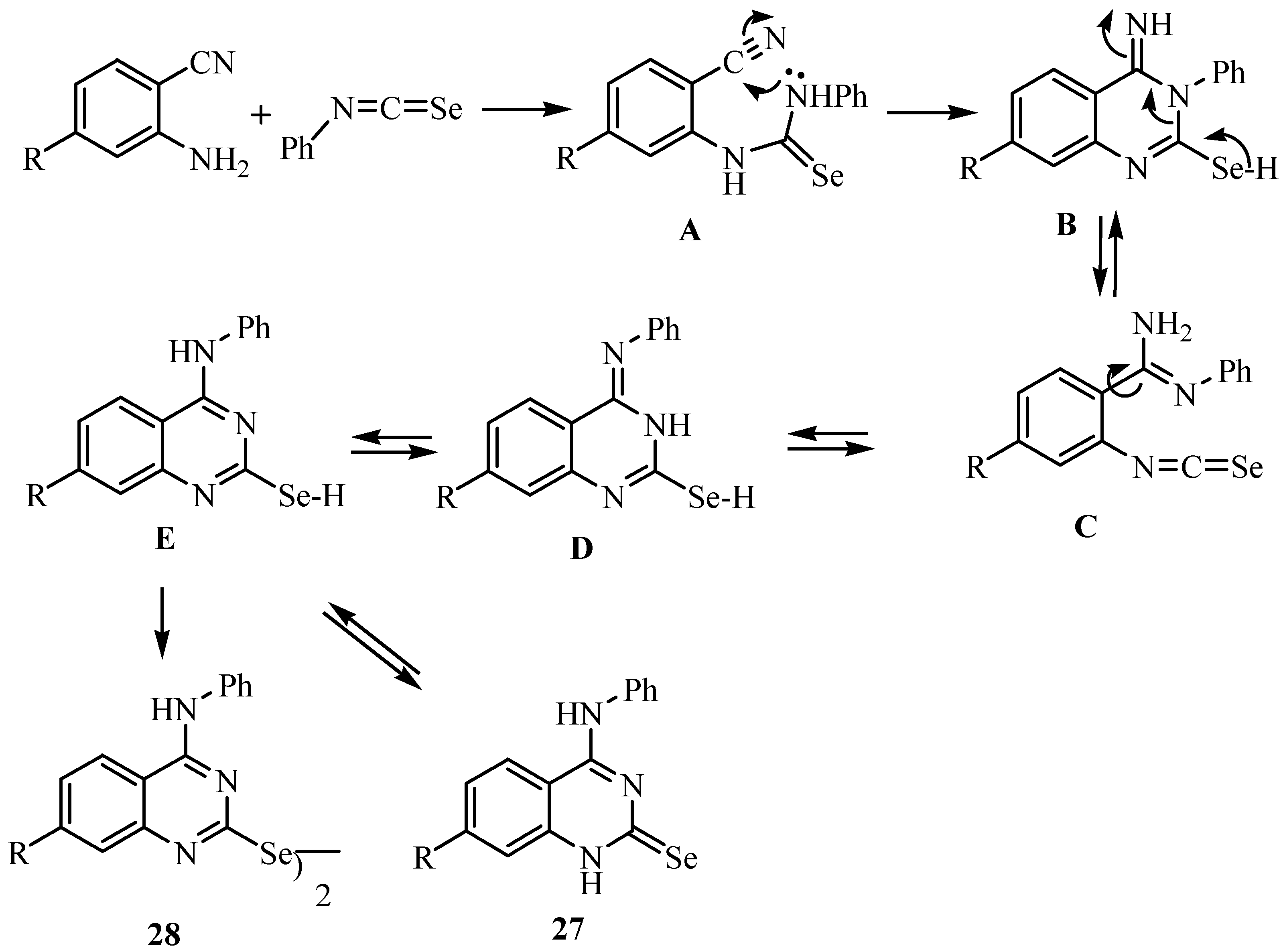
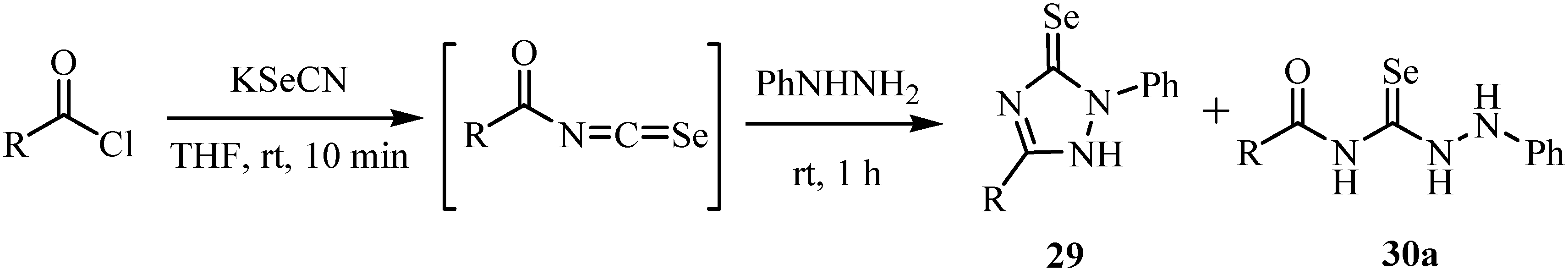

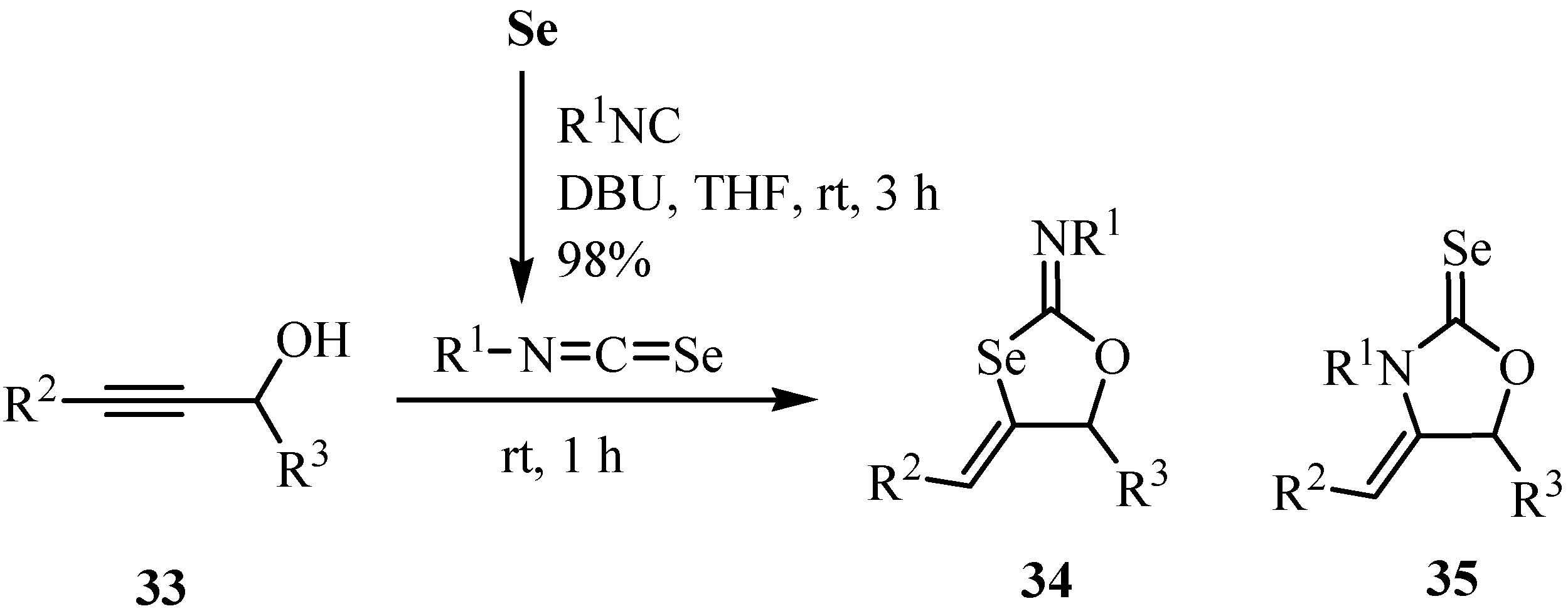
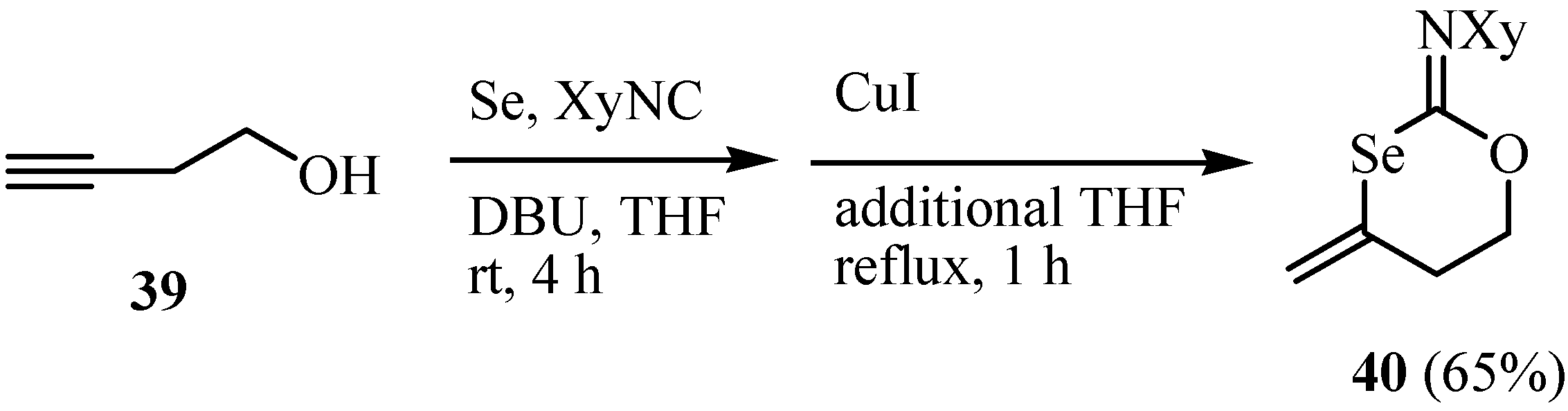
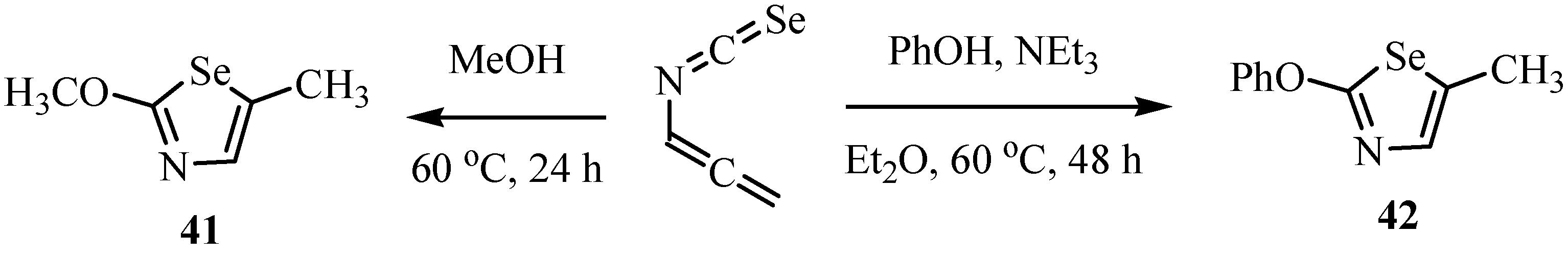
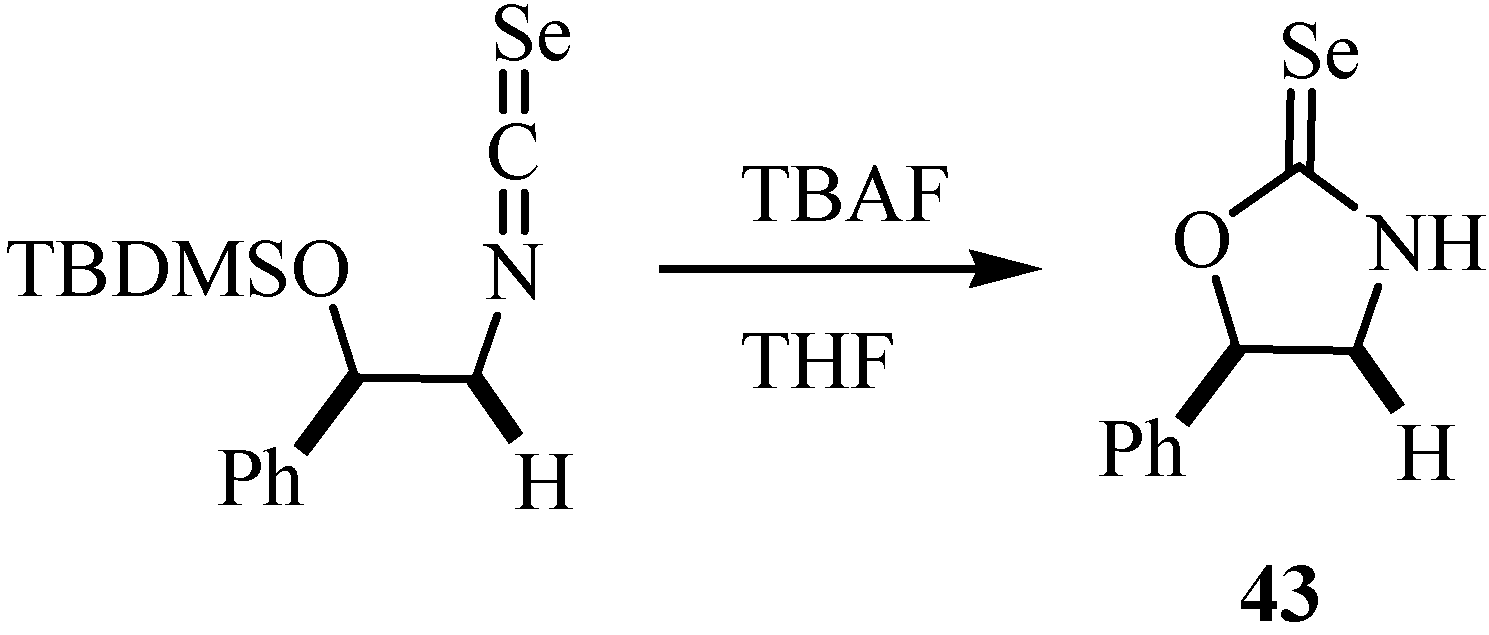
4. Reactions with alcohols and thiols

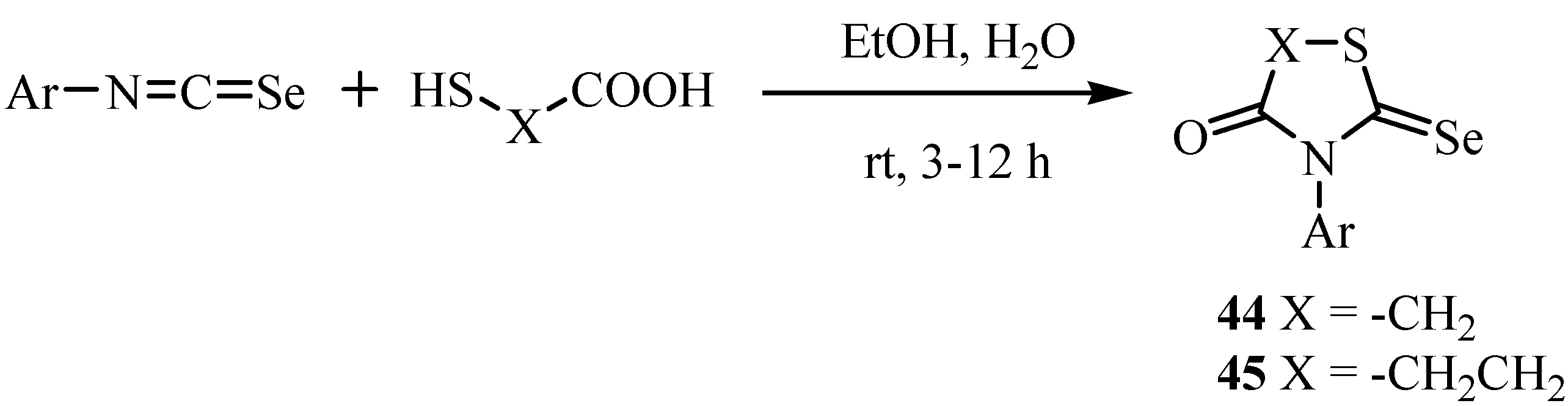

5. Reactions with selenolates
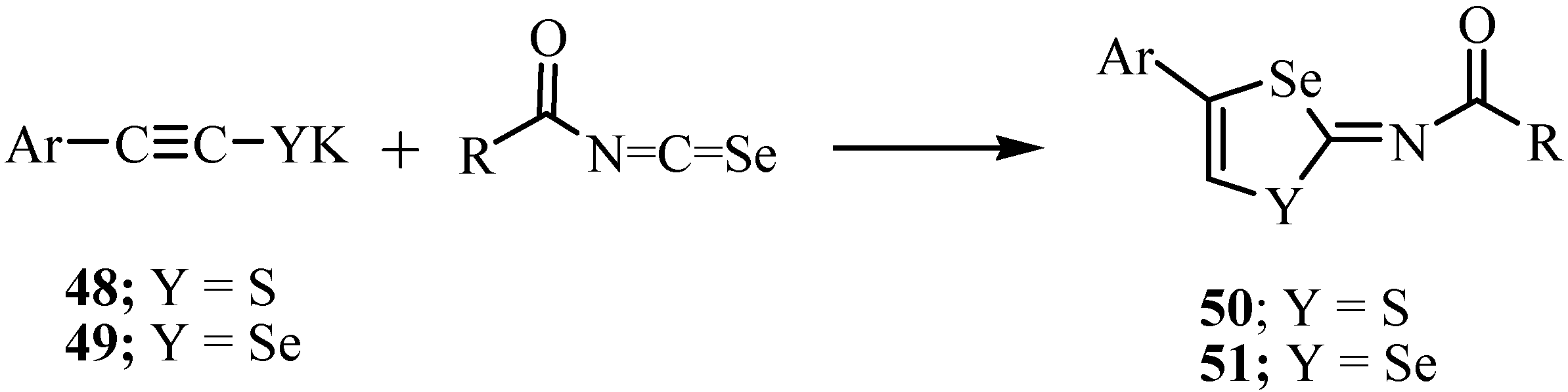
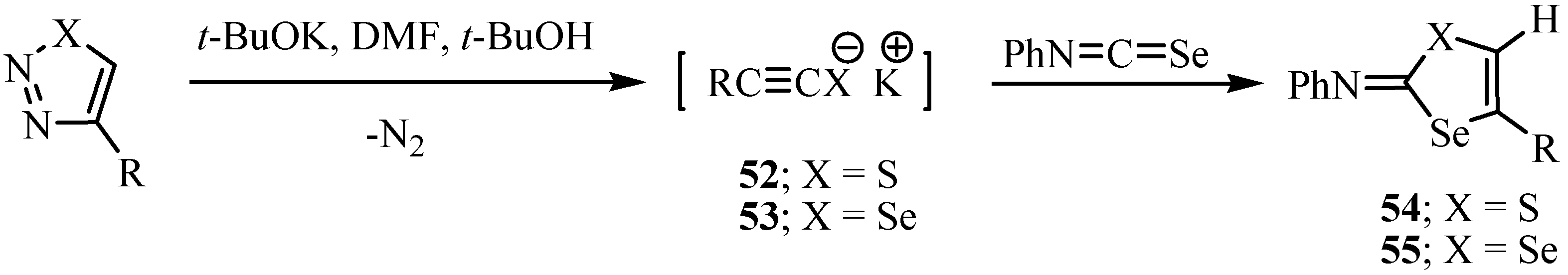
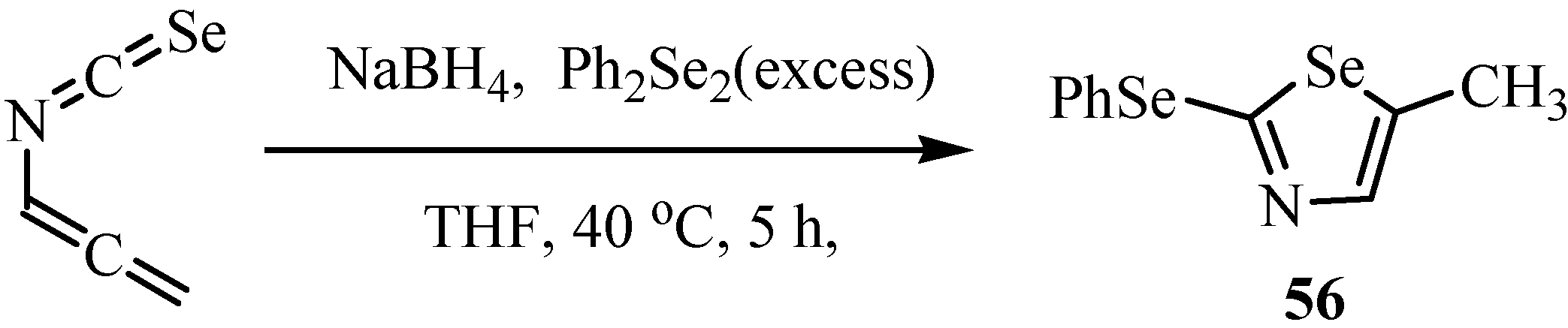
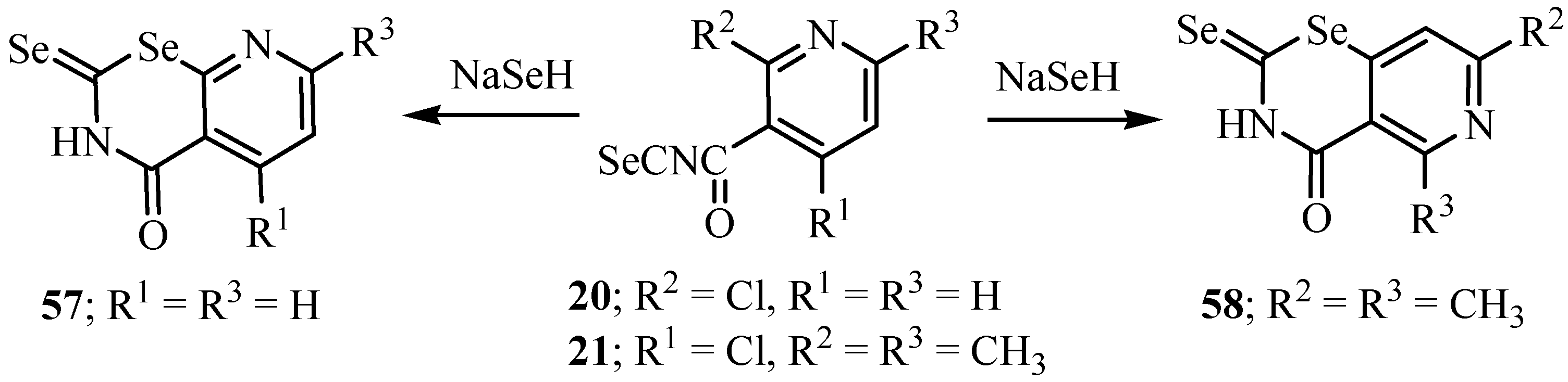
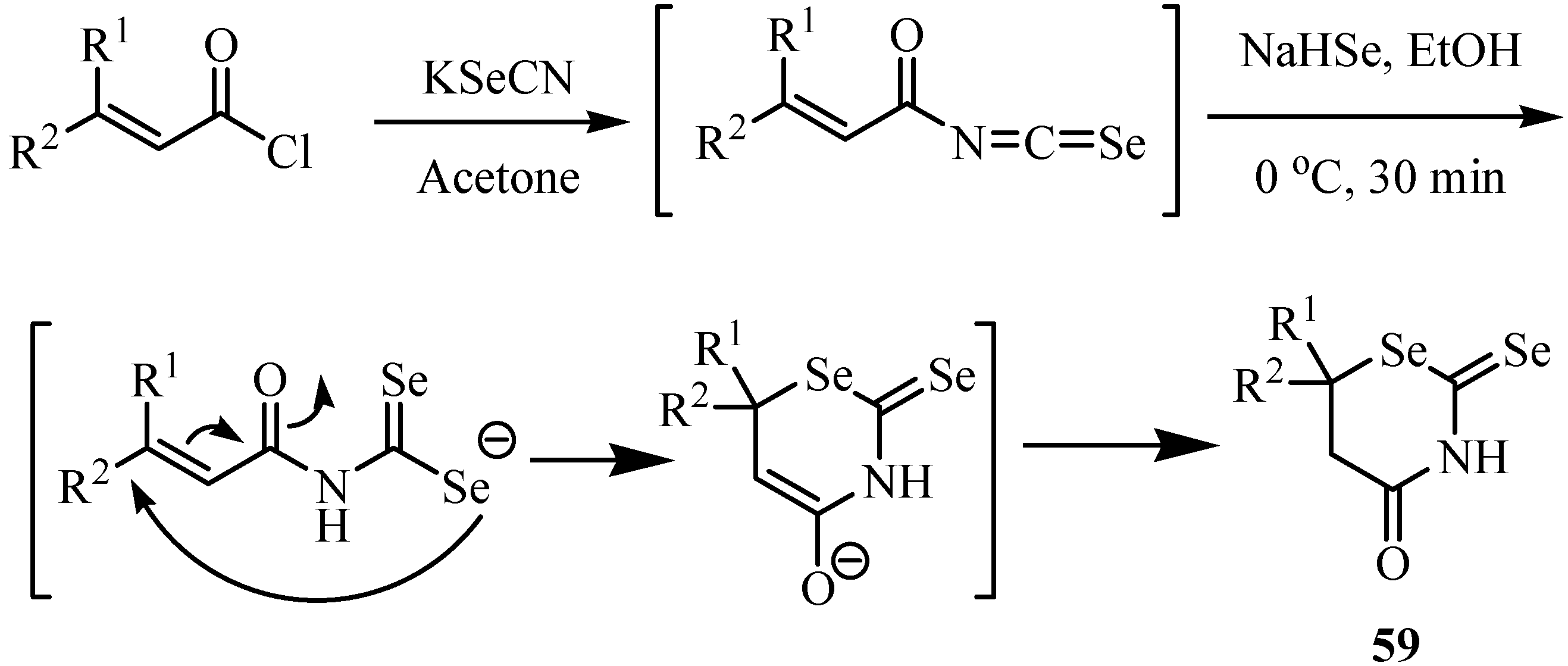
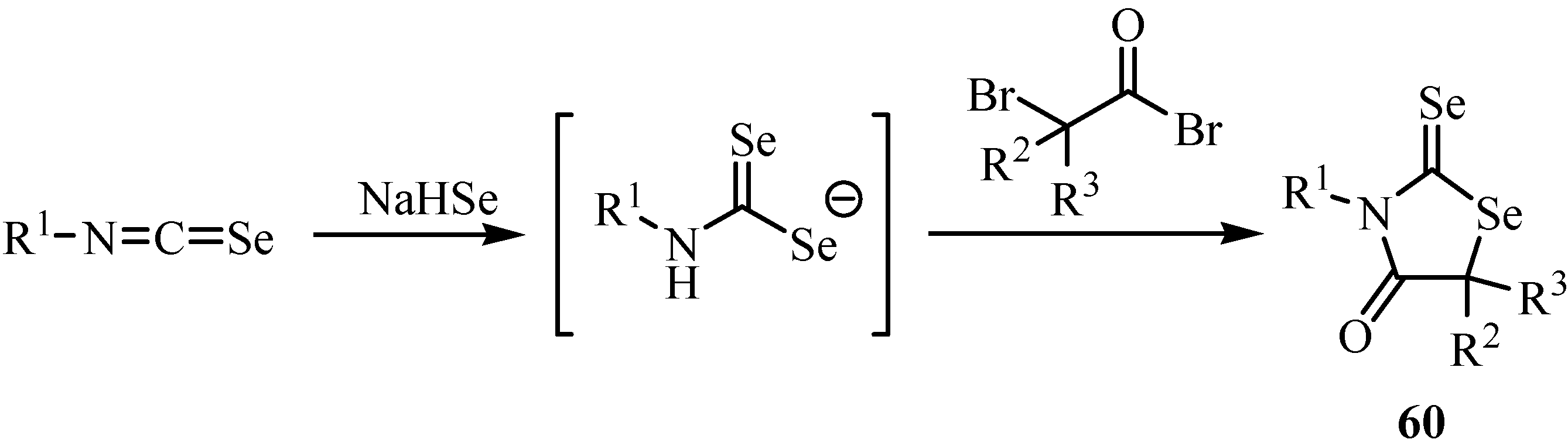
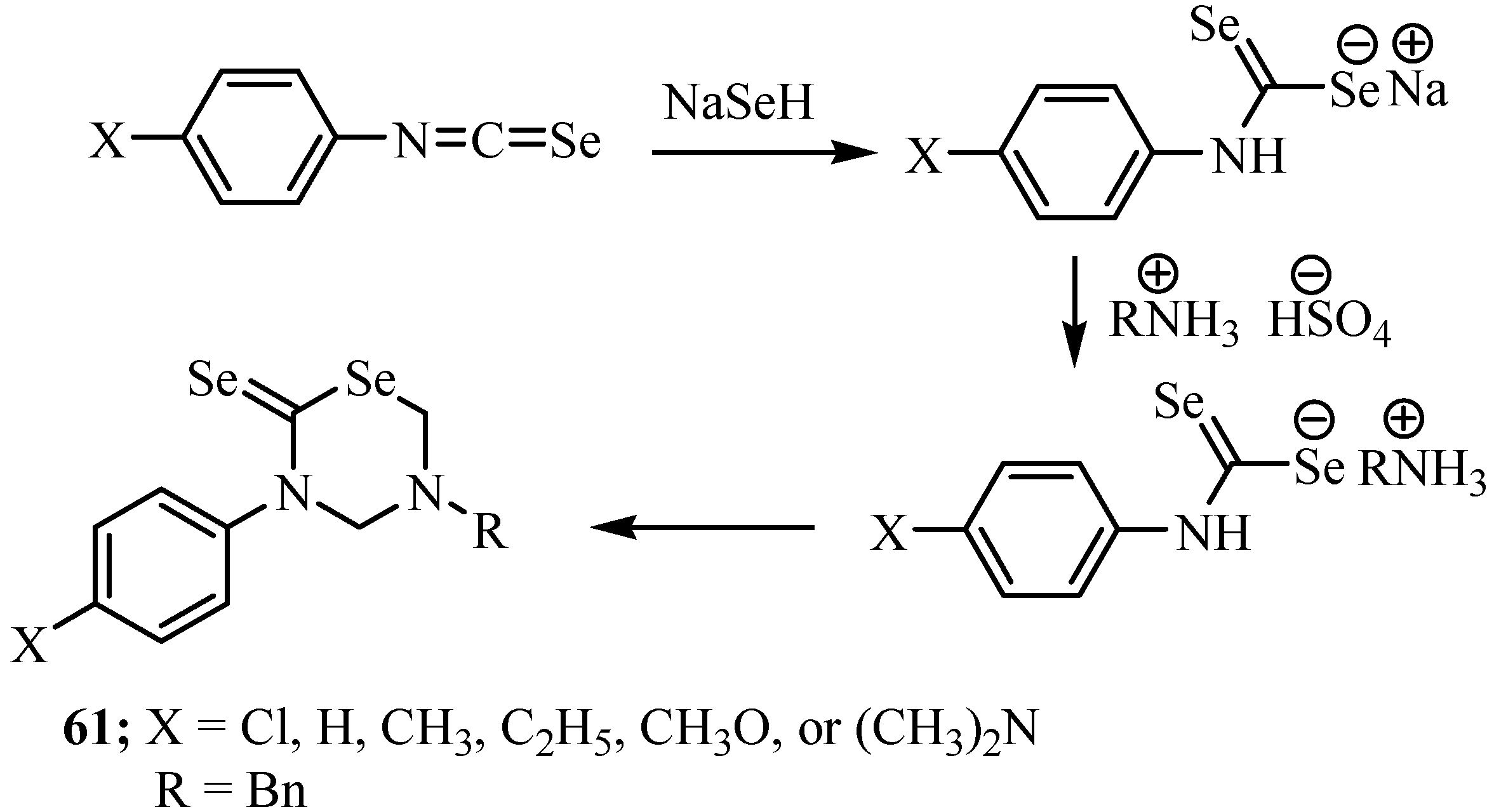
6. Reactions with carbanions
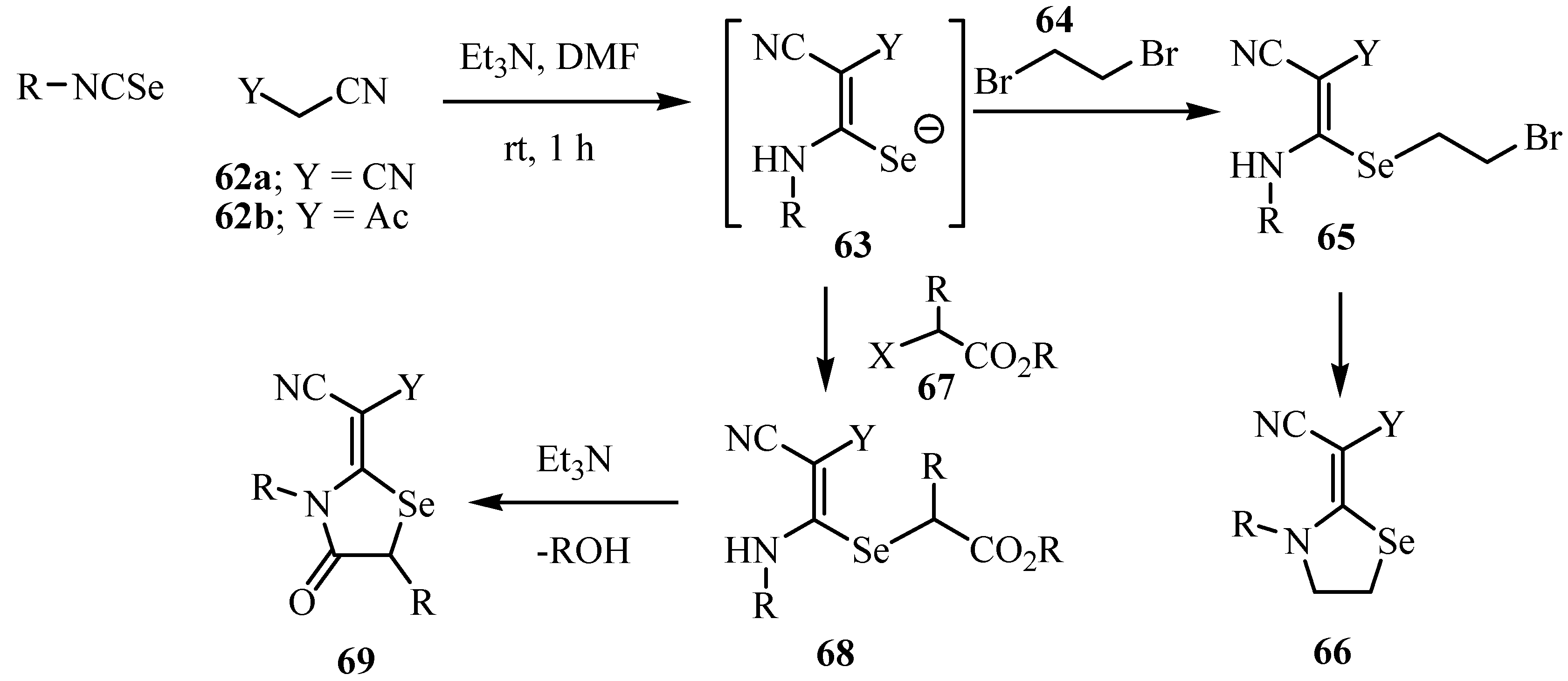
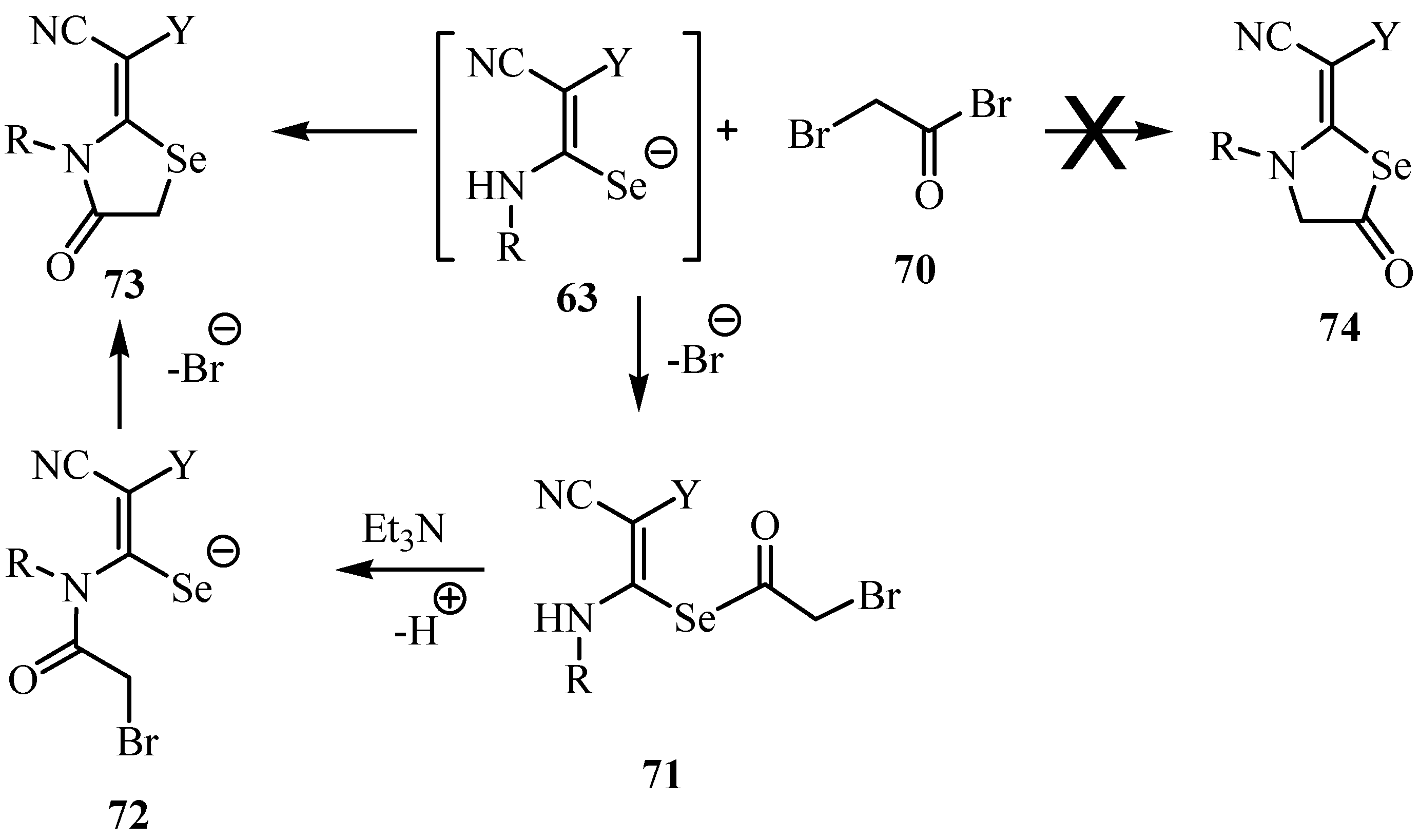
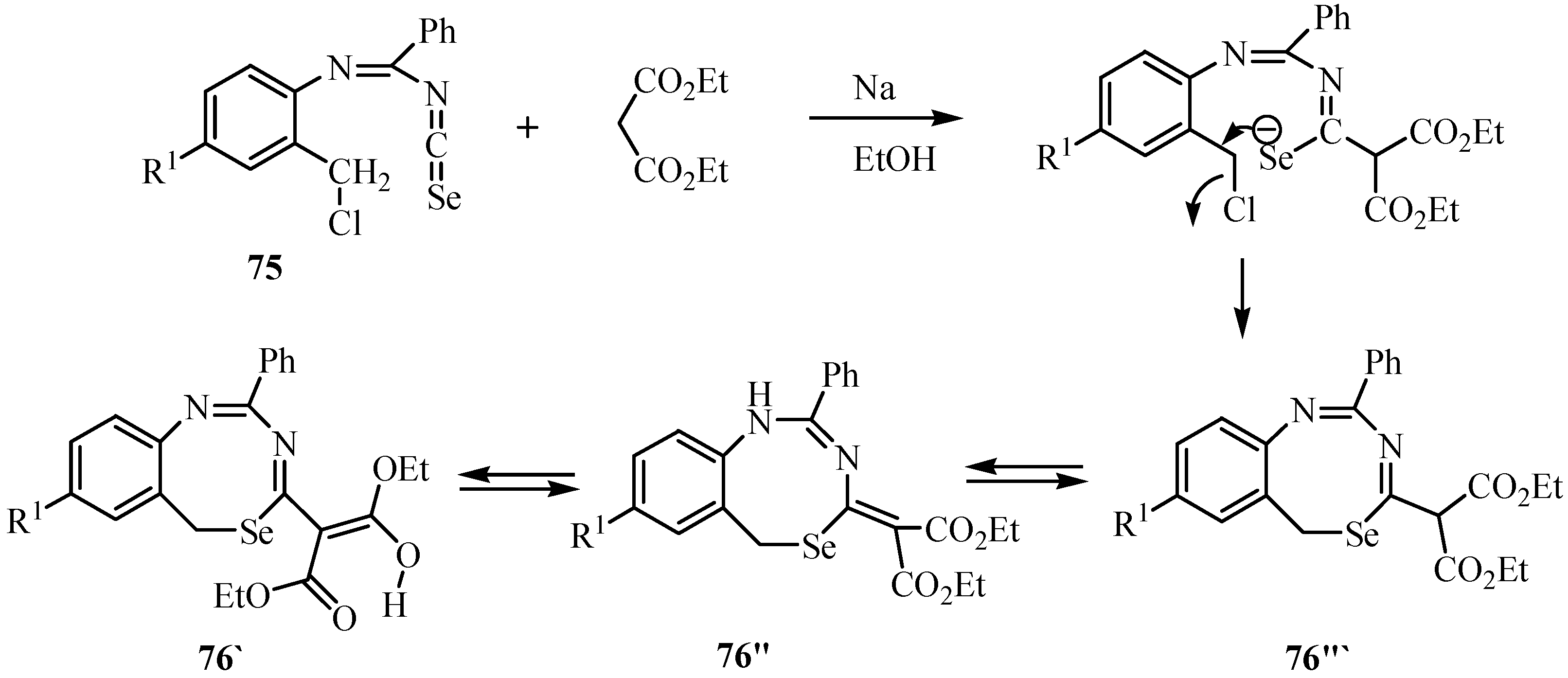
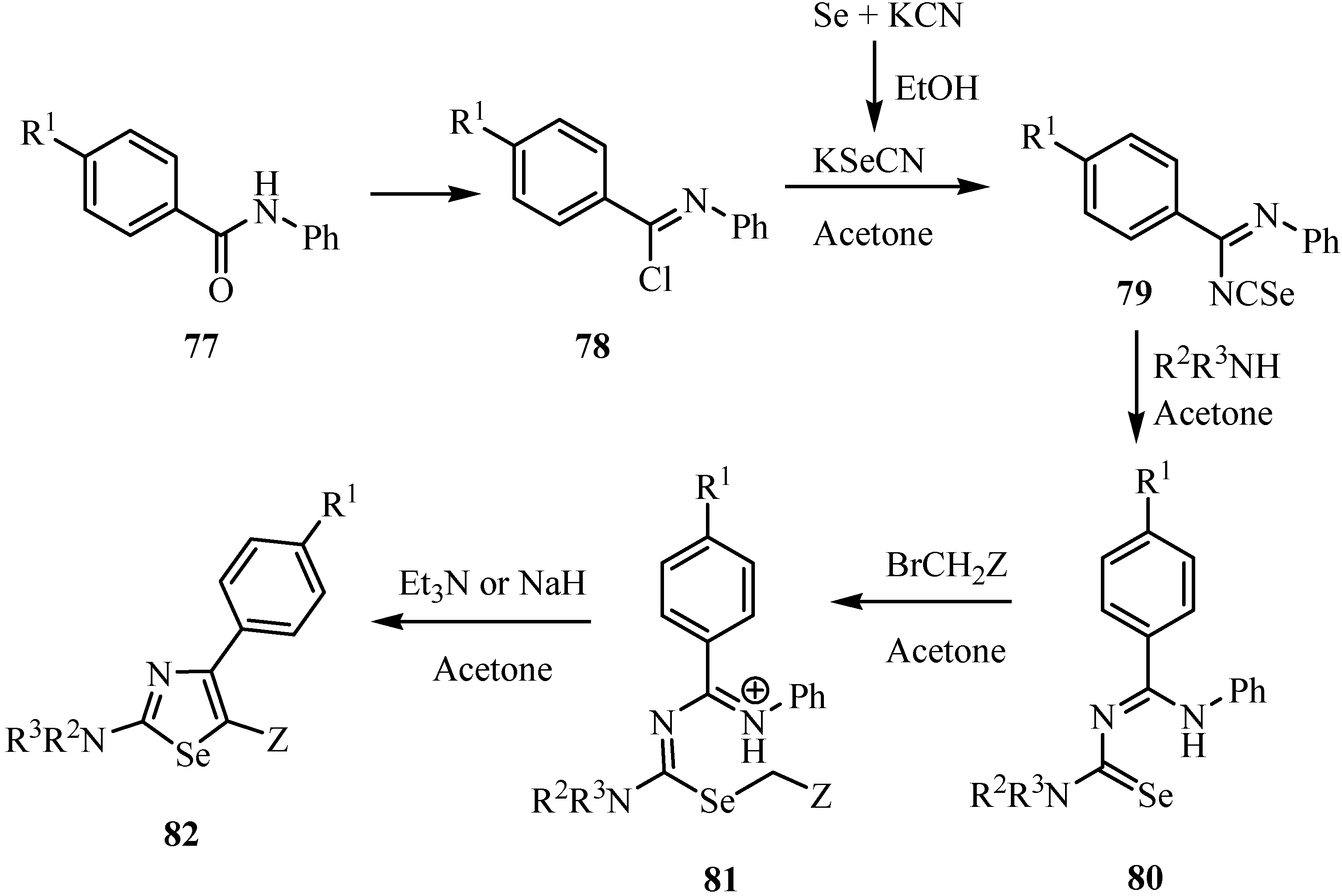
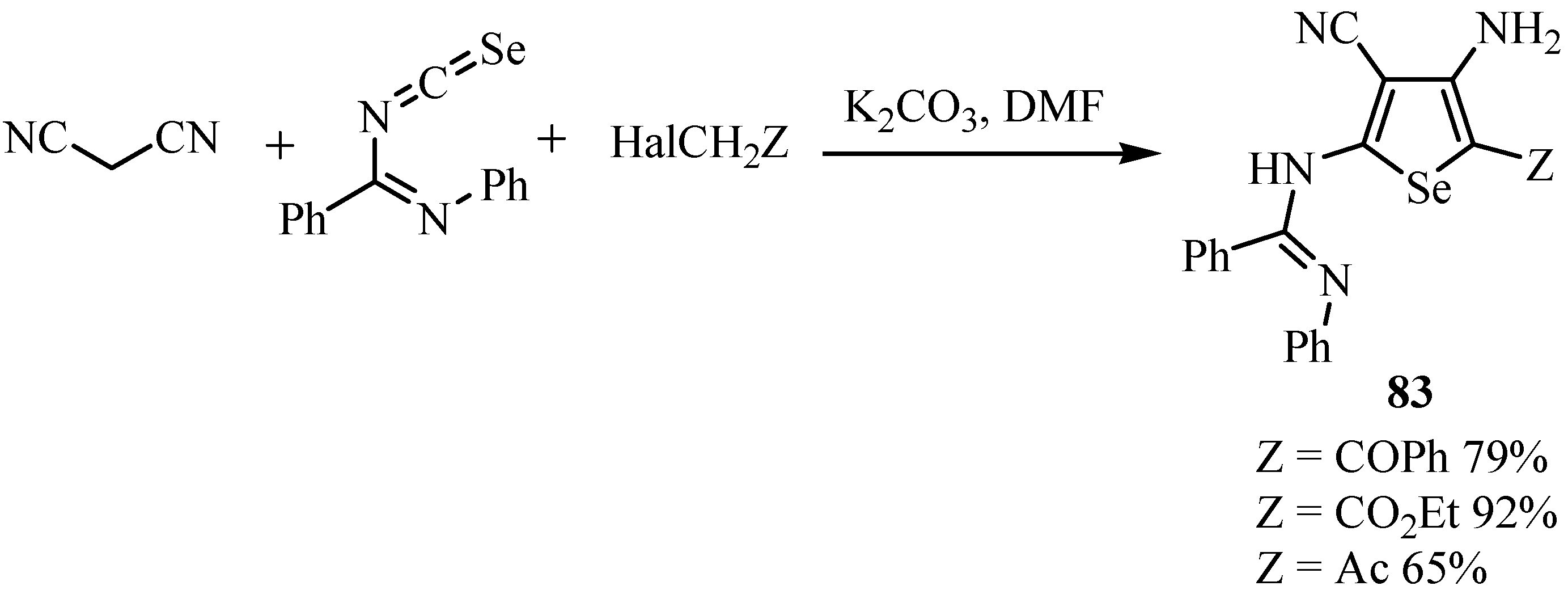

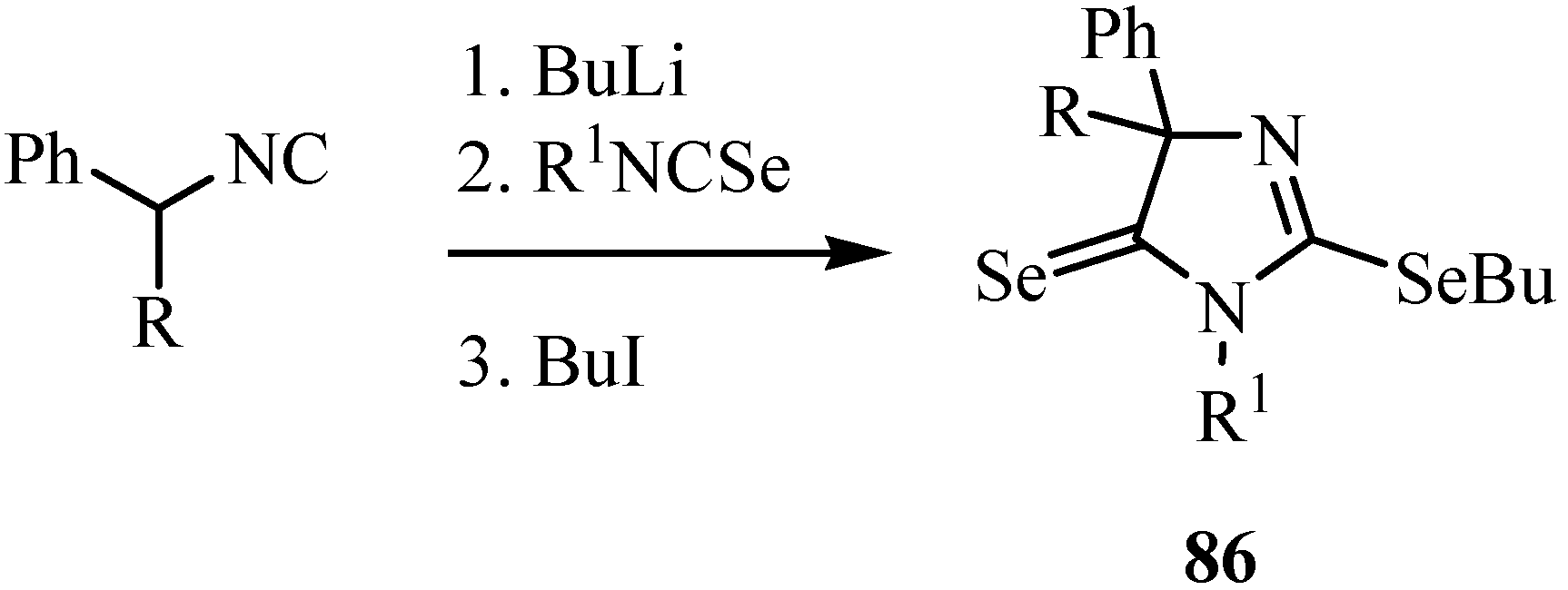

7. Reactions with azodicaboxylates and diazomethanes
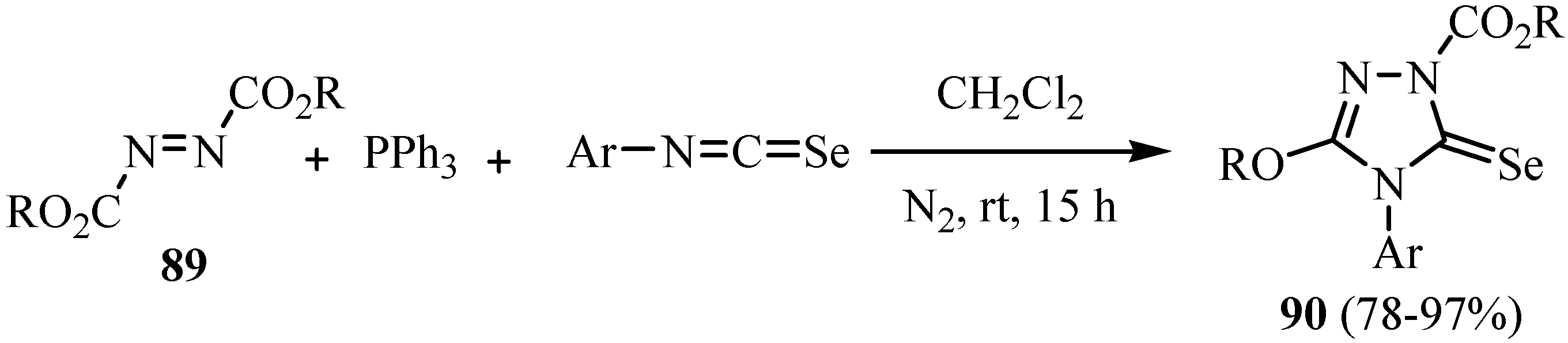
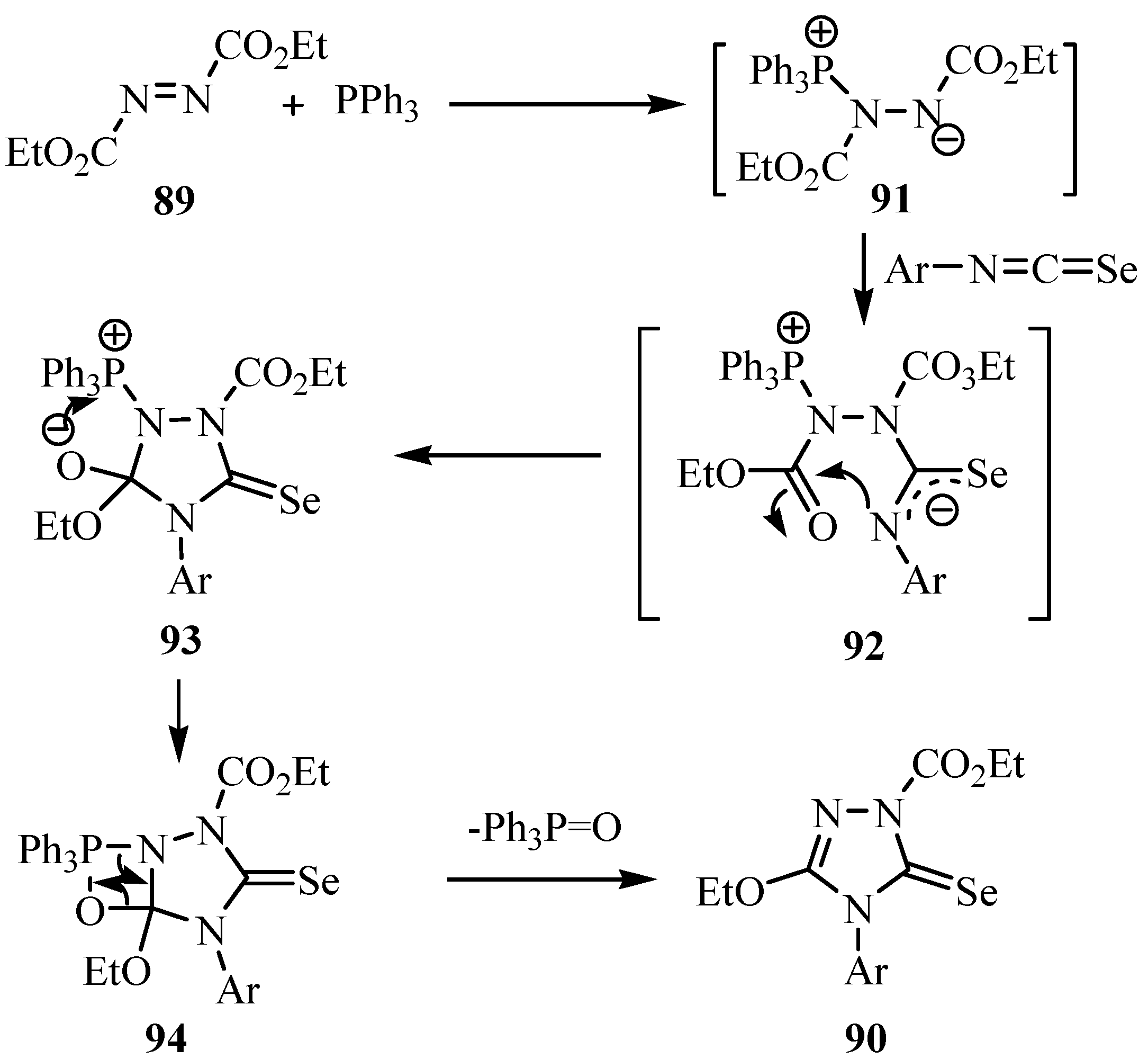
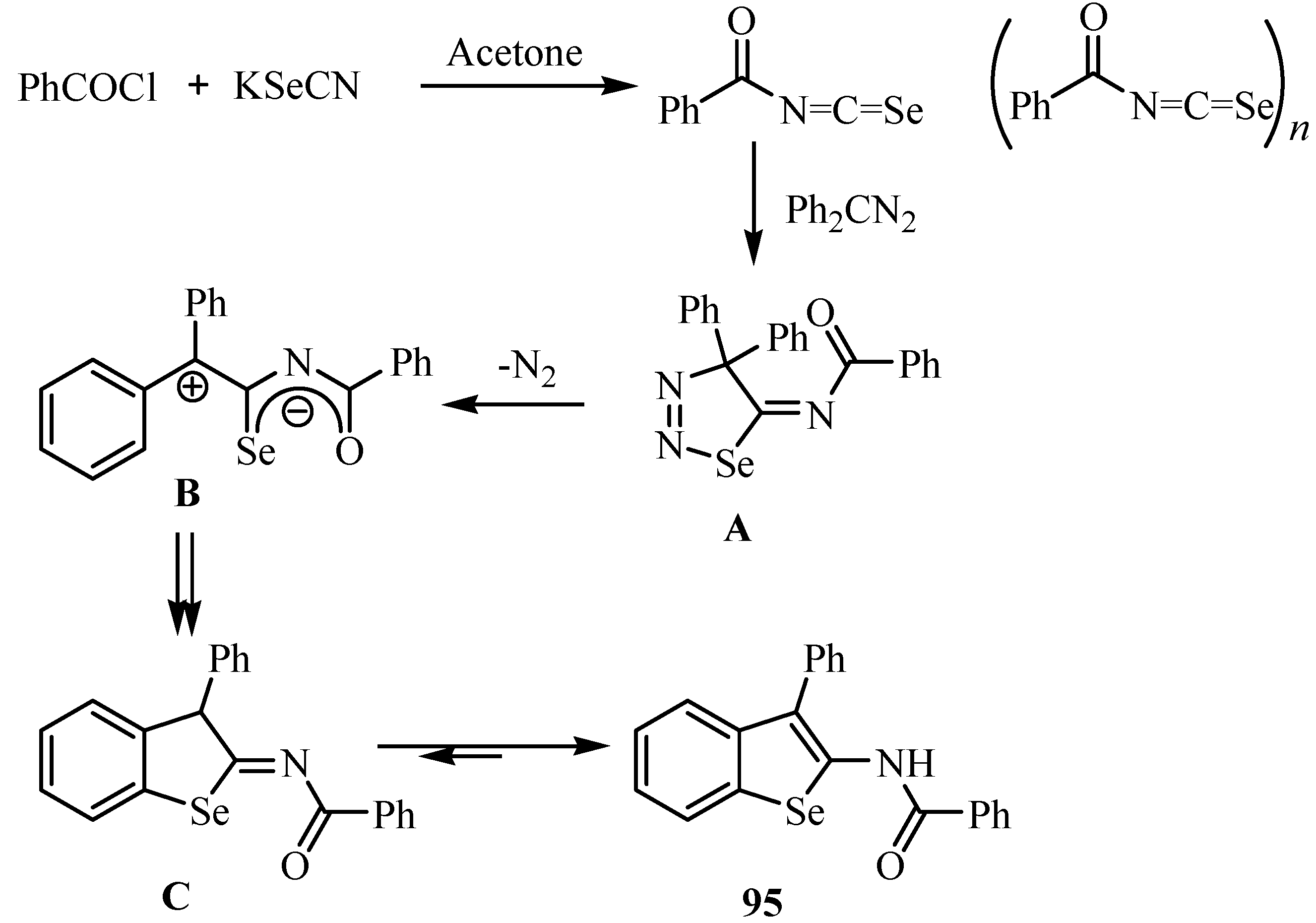

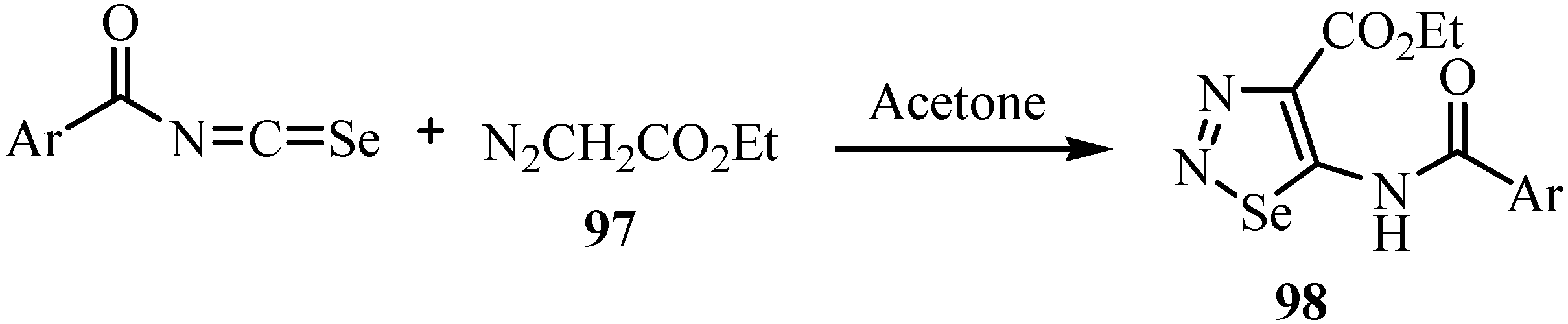
8. Cycloaddition reactions
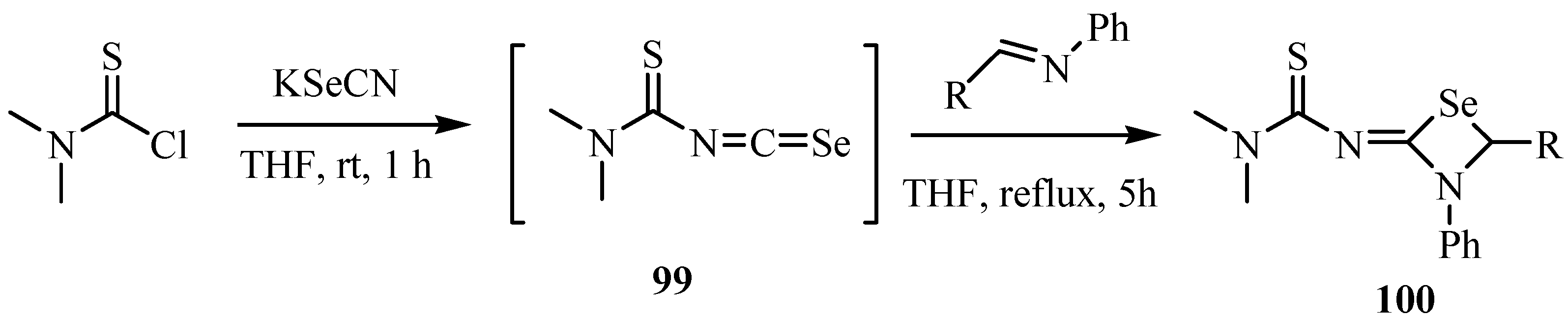
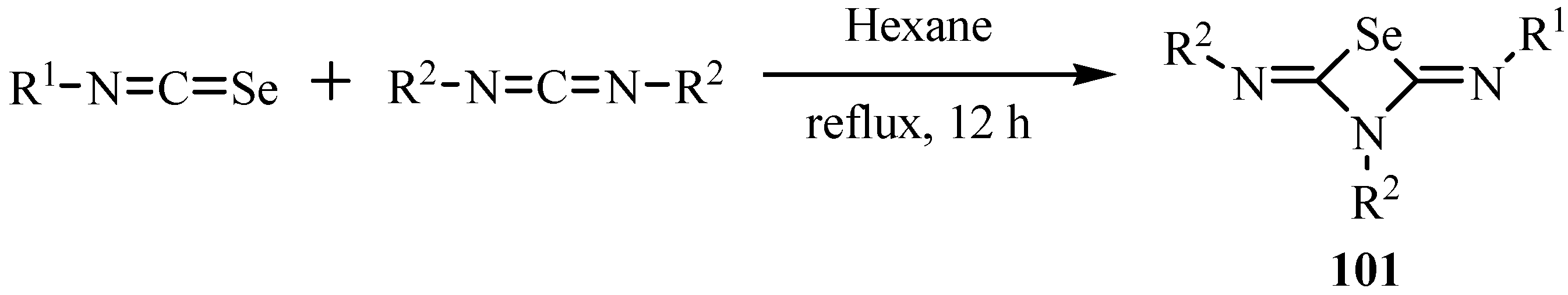

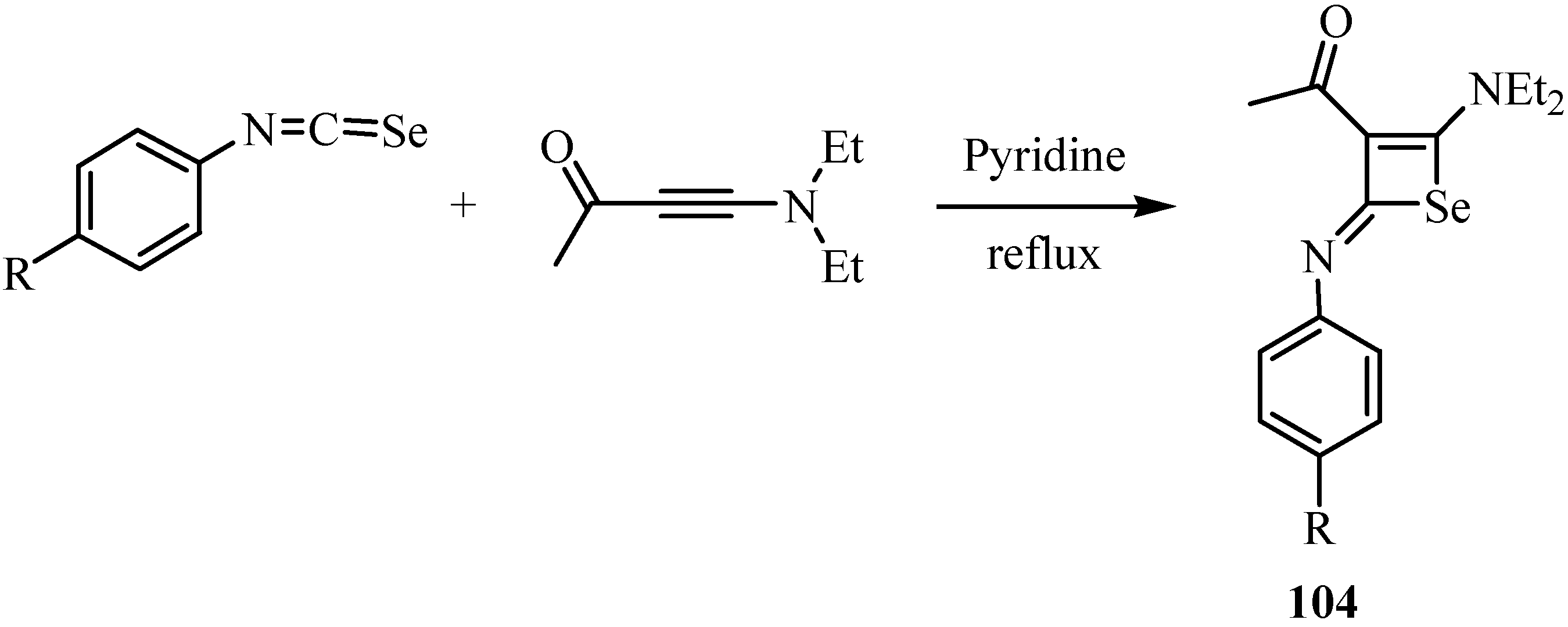
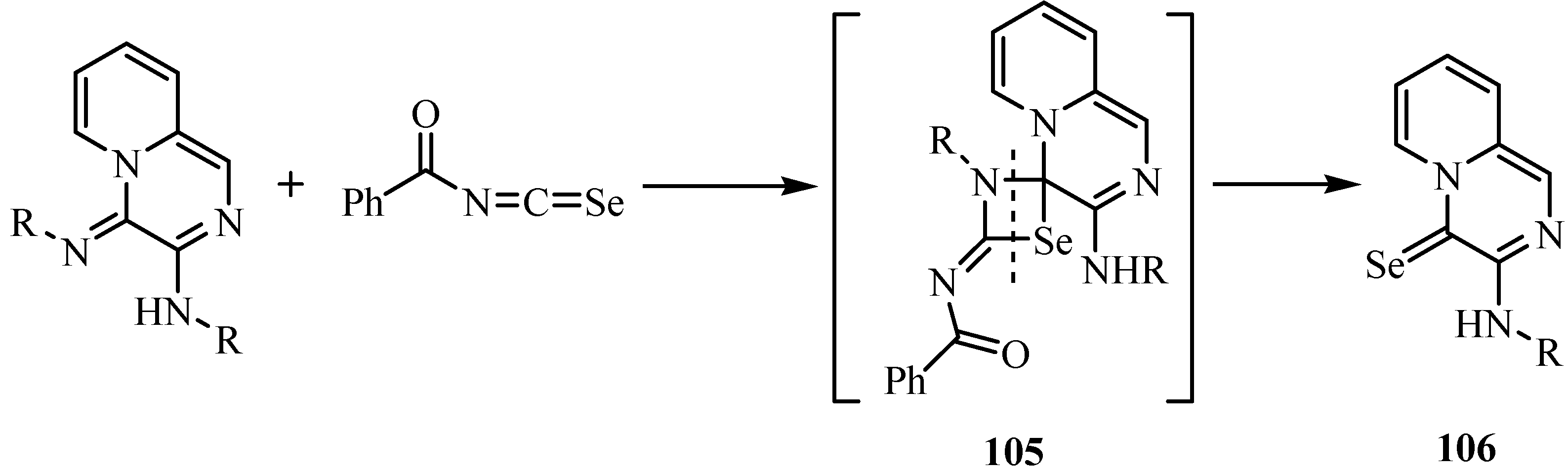
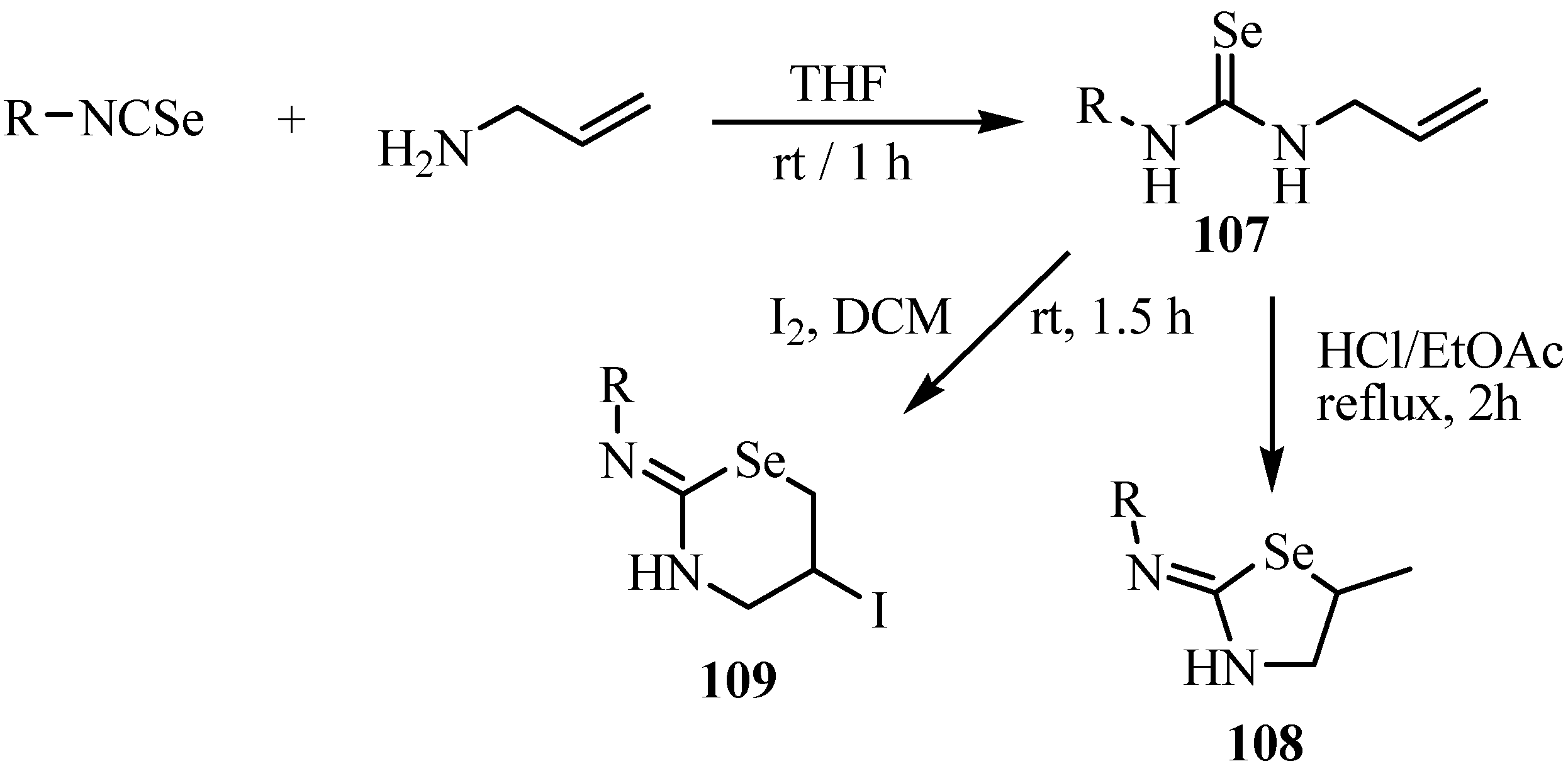
9. Iodo- and acid-catalyzed cyclization reactions
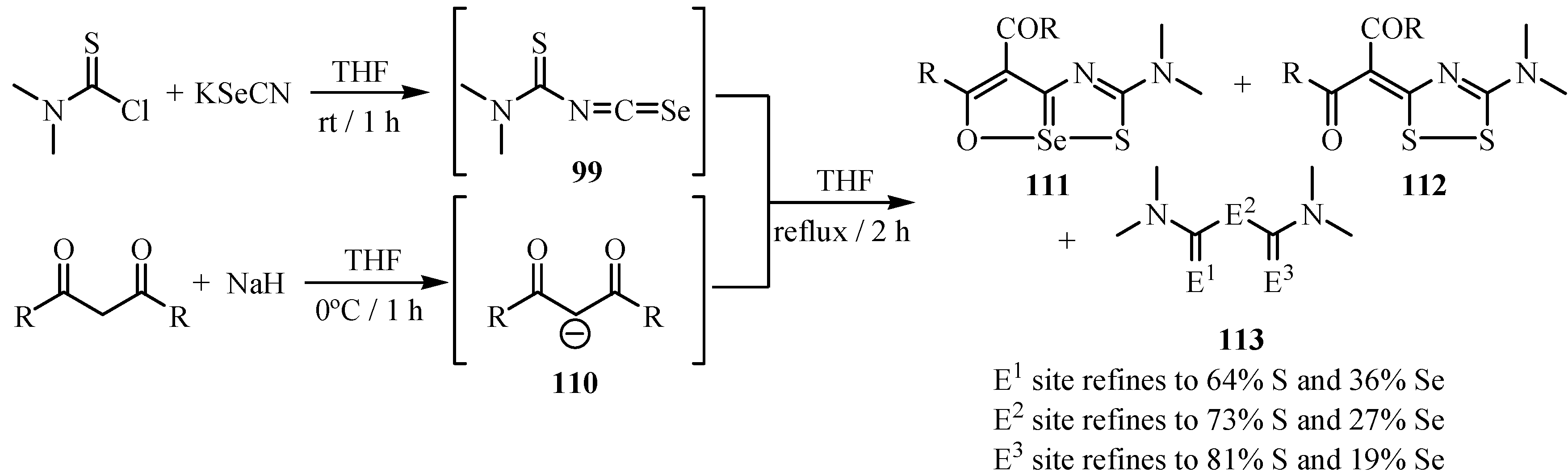
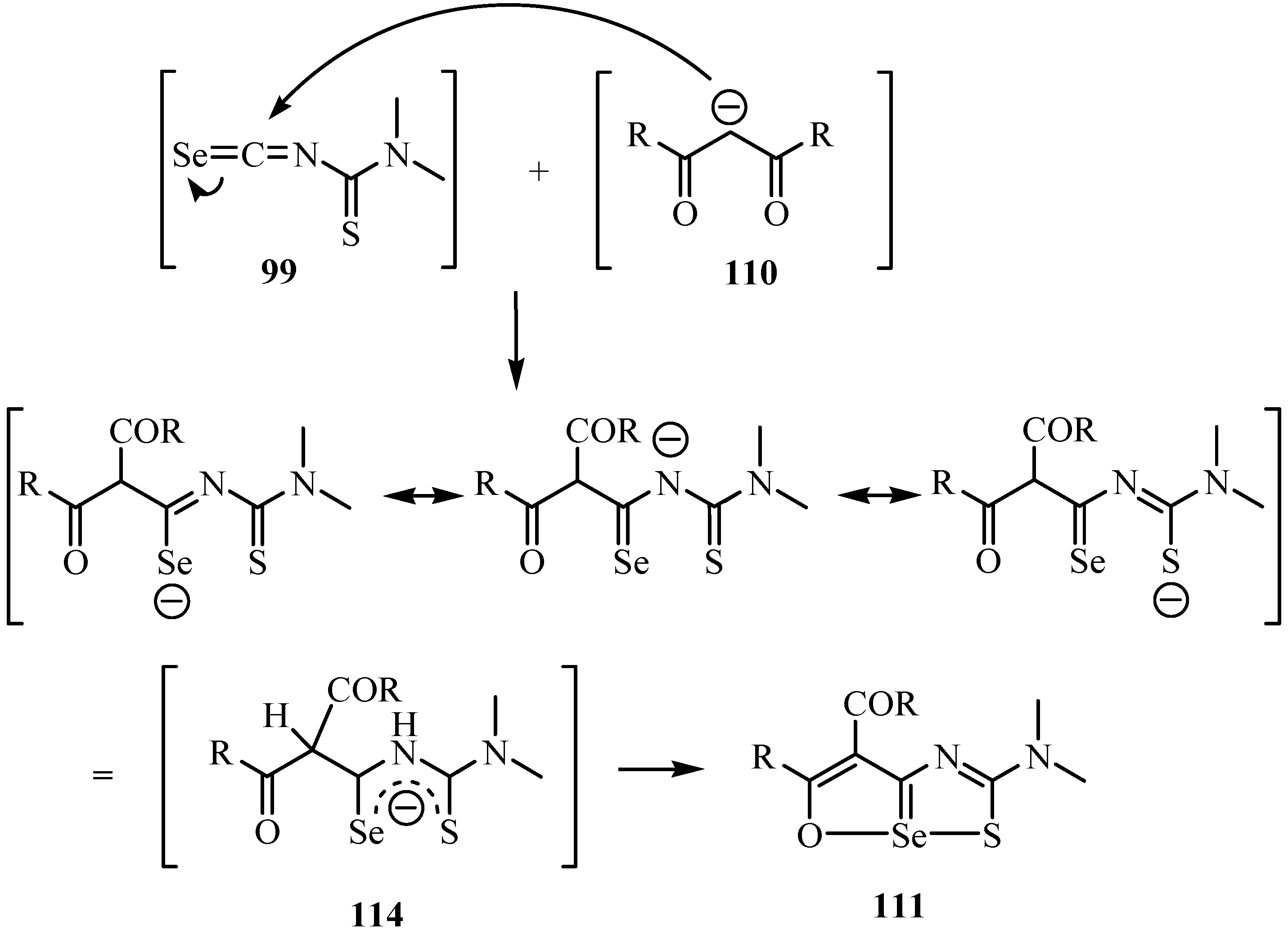
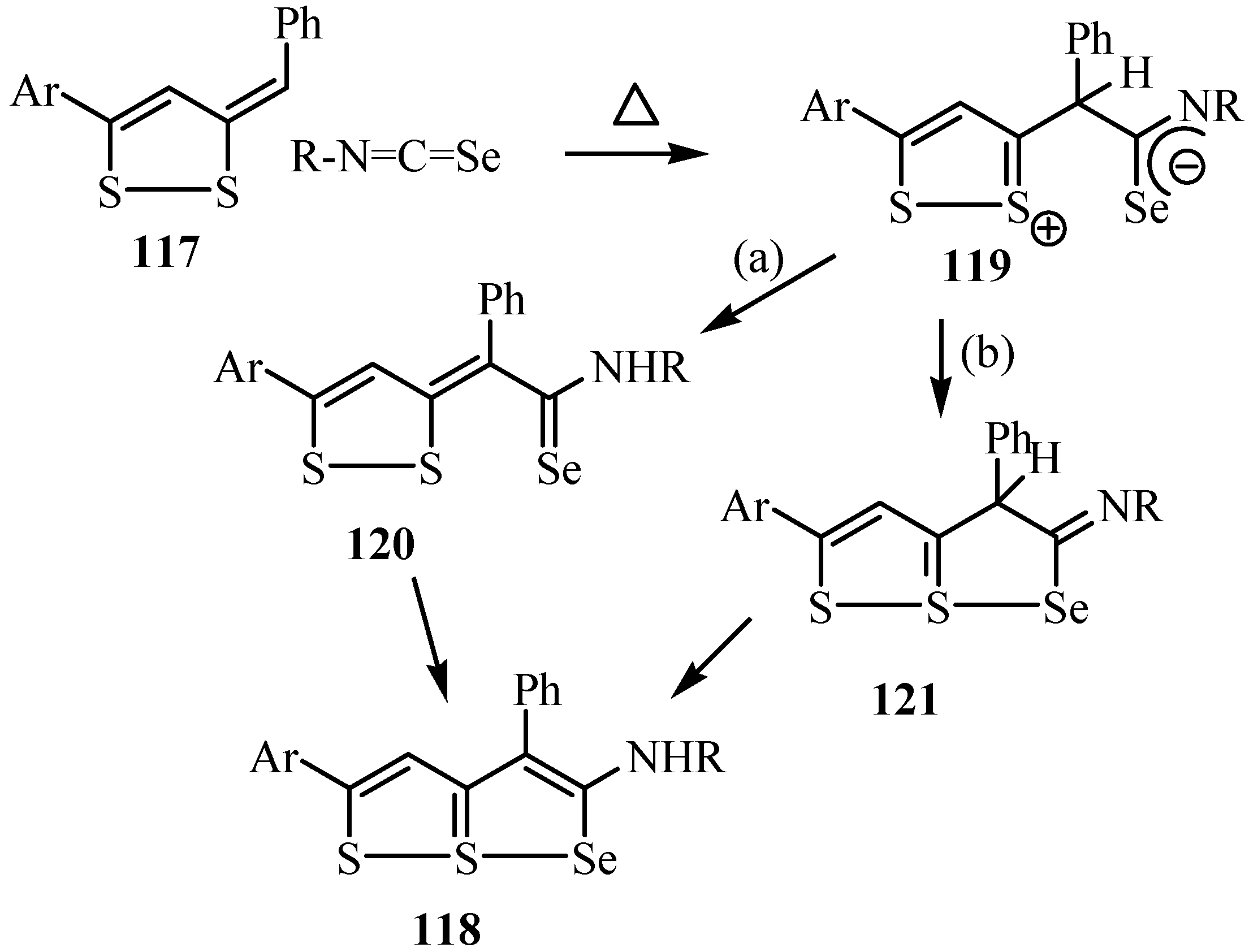
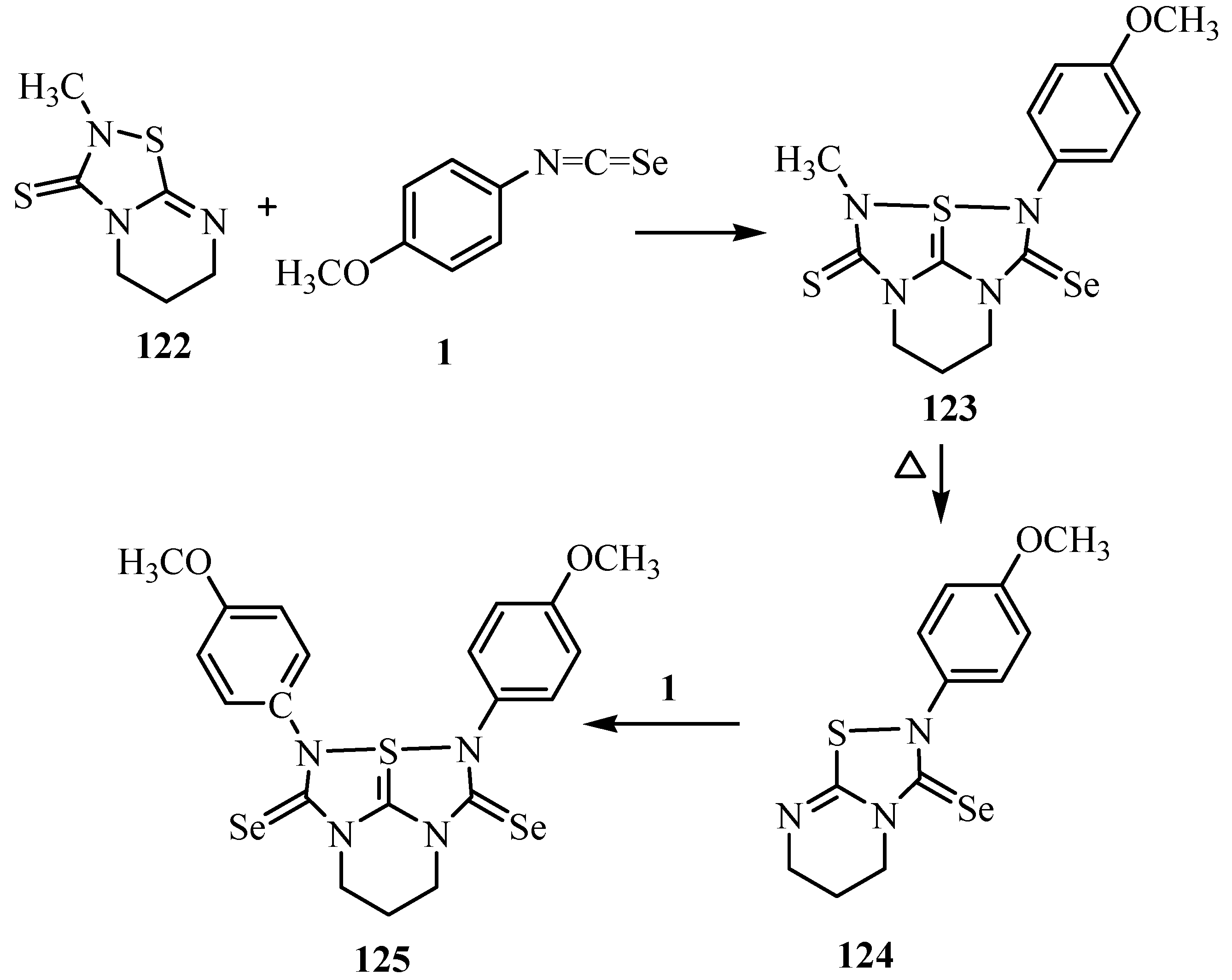
10. Synthesis of pentalenes
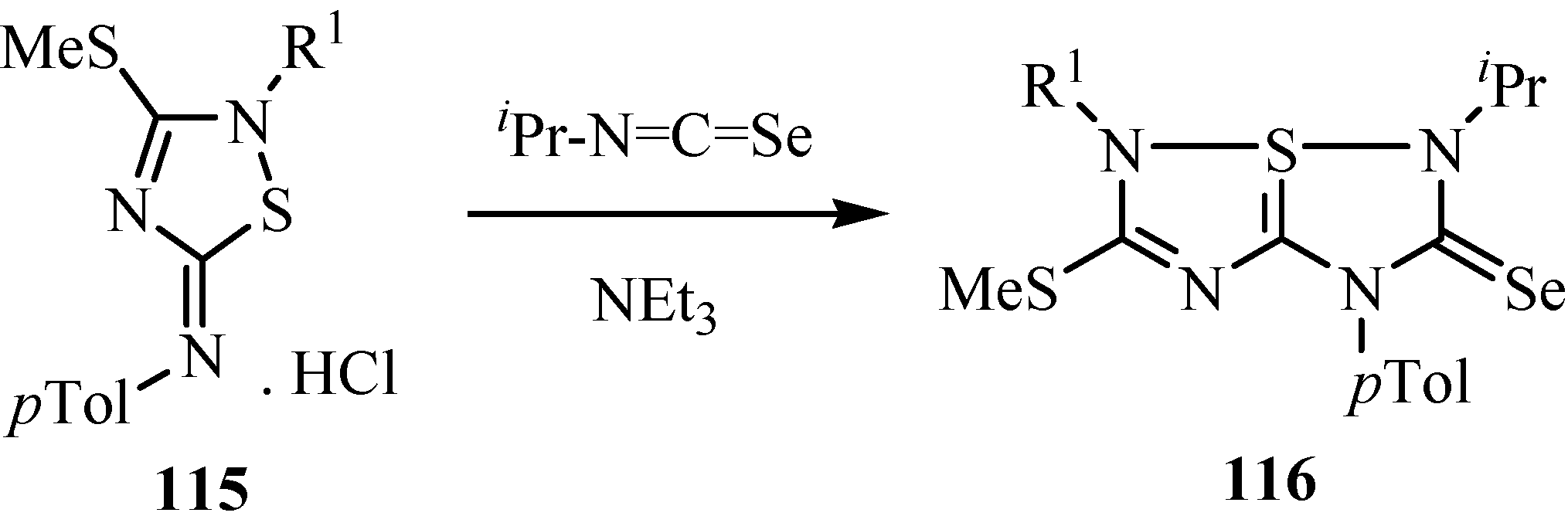
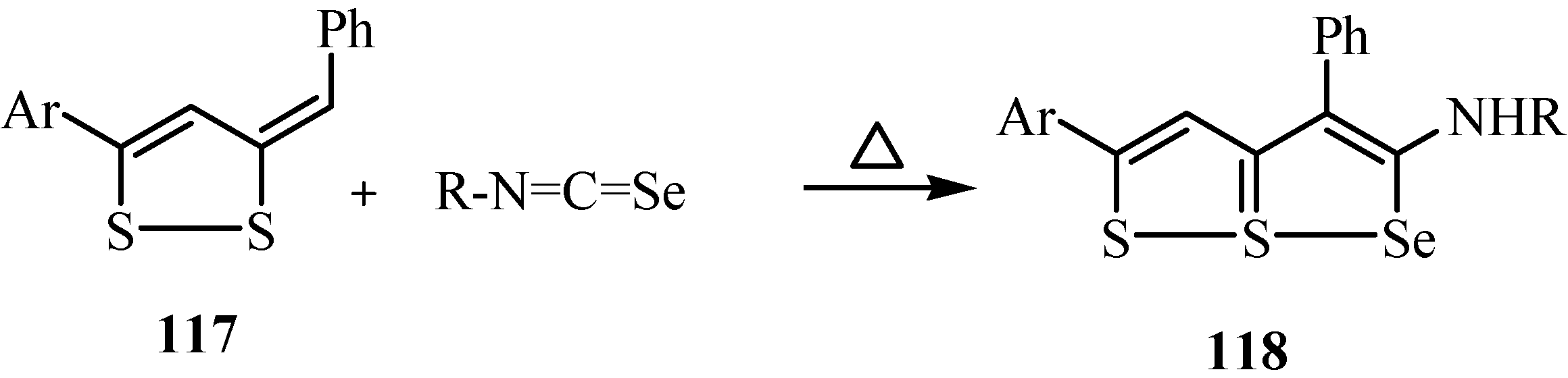
11. Conclusions
Acknowledgments
References and Notes
- Berzelius, J. J. Afhandl. Fys. Kemi Mineral. 1818, 6, 42.
- Wöhler, F.; Siemens, C. Ueber das Selenmercaptan. Ann. Chem. 1847, 61, 360–362. [Google Scholar] [CrossRef]
- Srivastava, P. C.; Robins, R. K. Synthesis and antitumor activity of 2-β-D-ribofuranosylselenazole-4-carboxamide and related derivatives. J. Med. Chem. 1983, 26, 445–448. [Google Scholar] [CrossRef] Wu, W.; Murakami, K.; Koketsu, M.; Yamada, Y.; Saiki, I. Induction of apoptosis in human gastric adenocarcinoma cells by two novel organoselenium compounds and TS-2 and TS-6. Anticancer Res. 1999, 19, 5375–5382. [Google Scholar]
- El-Bayoumy, K.; Sinha, R. Mechanisms of mammary cancer chemopre prevention by organoselenium compounds. Mutat. Res. 2004, 551, 181–197. [Google Scholar] [CrossRef] Patrick, L. Selenium biochemistry and cancer: a review of the literature. Altern. Med. Chem. 2004, 9, 239–258. [Google Scholar] Block, E. Recent results in the organosulfur and organoselenium chemistry of genus Allium and Brassica plants. Relevance for cancer prevention. Adv. Exp. Med. Biol. 1996, 401, 155–169. [Google Scholar] [CrossRef] Koketsu, M.; Ishihara, H.; Wu, W.; Murakami, K.; Saiki, I. 1,3-Selenazine derivatives induce cytotoxicity and DNA fragmentation in human HT-1080 fibrosarcoma cells. Eur. J. Pharm. Sci. 1999, 9, 157–161. [Google Scholar]
- Block, E.; Bird, S.; Tyson, J. F.; Uden, P. C.; Zhang, X.; Denoyer, E. The search for anticarcinogenic organoselenium compounds from natural sources. Phosphorus Sulfur Silicon Relat. Elem. 1998, 136, 1–10. [Google Scholar]
- May, S. W. Selenium-based pharmacological agents: an update. Exp. Opin. Invest. Drugs 2002, 11, 1261–1269. [Google Scholar] [CrossRef]
- Parnham, M. J.; Graf, E. Pharmacology of synthetic organic selenium compounds. Prog. Drug. Res. 1991, 36, 9–47. [Google Scholar]
- Koketsu, M.; Ishihara, H.; Hatsu, M. Novel compounds, 1,3-selenazine derivatives, as antibacterial agents against Escherichia coli and Staphylococcus aureus. Res. Commun. Mol. Pathol. Pharmacol. 1998, 101, 179–186. [Google Scholar]
- May, S. W.; Wang, L.; Gill-Woznichak, M. M.; Browner, R. F.; Ogonowski, A. A.; Smith, J. B.; Pollock, S. H. An orally active selenium-based antihypertensive agent with restricted CNS permeability. J. Pharm. Exp. Ther. 1997, 283, 470–477. [Google Scholar]
- Göbel, T.; Gsell, L.; Hüter, O. F.; Maienfisch, P.; Naef, R.; O’Sullivan, A. C.; Pitterna, T.; Rapold, T.; Seifert, G.; Sern, M.; Szczepanski, H.; Wadsworth, D. J. Synthetic approaches towards CGA 293'343: a novel broad-spectrum insecticide. Pestic. Sci. 1999, 55, 355–357. [Google Scholar] [CrossRef] El-Desoky, S. I.; Bondock, S. B.; Etman, H. A.; Fadda, A. A.; Metwally, M. A. Synthesis of some new thiazole derivatives of pharmaceutical interest. Sulfur Lett. 2003, 26, 127–135. [Google Scholar] [CrossRef] Lamberth, C. Sulfur chemistry in crop protection. J. Sulfur Chem. 2004, 25, 39–62. [Google Scholar] [CrossRef] Kedar, R. M.; Vidhale, N. N.; Chincholkar, M. M. Synthesis of new heterocycles from simple chalcones and their antimicrobial study. Orient. J. Chem. 1996, 12, 301–304. [Google Scholar] Metwally, M. A.; Abdel-Latif, E.; Amer, F. A.; Kaupp, G. Versatile 2-amino-4-substituted-1,3-thiazoles: synthesis and reactions. J. Sulfur Chem. 2004, 25, 63–85. [Google Scholar] Erol, D. D.; Aytemir, M. D.; Yulug, N. Synthesis and antibacterial and antifungal properties of thiazolinoethyl-2(3H)-benzoxazolone derivatives. II. Eur. J. Med. Chem. 1996, 31, 731–734. [Google Scholar] [CrossRef]
- Bulka, E.; Ahlers, K.-D.; Tućek, E. Synthese und IR-spektren von aryl-isoselenocyanaten. Chem Ber. 1967, 100, 1367–1372. [Google Scholar] Bulka, E. The Chemistry of Cyanates and Thiocyanates; Patai, S., Ed.; Wiley & Sons: New York, 1972. [Google Scholar]
- Barton, D. H. R.; Parekh, S. I.; Tajbakhsh, M.; Theodorakis, E. A.; Tse, C.-L. A convenient and high yielding procedure for the preparation of isoselenocyanates. Synthesis and reactivity of O-alkylselenocarbamates. Tetrahedron 1994, 50, 639–654. [Google Scholar]
- Bakhsh, M. T.; Behshtiha, Y. S.; Heravi, M. M. A facile method for the preparation of isoselenocyanates. J. Chem. Soc. Pak. 1996, 18, 159. [Google Scholar] Su, W. K.; Liang, X. R. An efficient and convenient route to some isoselenocyanates via reaction of formamides with bis(trichloromethyl) carbonate and selenium. J. Indian Chem. Soc. 2003, 80, 645–647. [Google Scholar]
- Fernández-Bolaños, J. G.; López, Ó.; Ulgar, V.; Maya, I.; Fuentes, J. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett. 2004, 45, 4081–4084. [Google Scholar] [CrossRef]
- Henriksen, L.; Ehrbar, U. One-step synthesis of alkyl and aryl isoselenocyanates from primary amines. Synthesis 1976, 519. [Google Scholar] [CrossRef]
- Tarantelli, T.; Pecile, C. Triphenylmethyl isoselenocyanate. Ann. Chim. (Rome) 1962, 52, 75–79. [Google Scholar] Pederson, C. T. Preparation of some 4-substituted selenosemicarbazides. Acta. Chem. Scan. 1963, 17, 1459–1461. [Google Scholar] [CrossRef]
- Stolte, H. Ber, Dtsch. Chem. Ges. 1886, 19, 2350. [CrossRef] Collard-Charon, C.; Renson, M. Synthesis of substituted selenosemicarbazides. I. Synthesis of isoselenocyanic esters. Bull. Soc. Chim. Belg. 1962, 71, 531–540. [Google Scholar] [CrossRef]
- Suzuki, H.; Usuki, M.; Hanafusa, T. A Photochemical route to some substituted benzyl isoselenocyanates. Synthesis 1979, 705–707. [Google Scholar] [CrossRef]
- Koketsu, M.; Suzuki, N.; Ishihara, H. Preparation of isoselenocyanate and synthesis of carbodiimide by oxidation of selenourea. J. Org. Chem. 1999, 64, 6473–6475. [Google Scholar] [CrossRef]
- Douglas, I. B. Acylselenoureas. J. Am. Chem. Soc. 1937, 59, 740–742. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Aoki, H.; Ishihara, H. The preparation of acylselenourea and selenocarbamate using isoselenocyanate. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2699–2708. [Google Scholar] [CrossRef]
- Hashim Nizar, P. N.; Parkash, S.; Chauhan, S. M. S. Synthesis of 3-cyclohexyl-1-alkylselenoureas and 2-(N-nitrosocyclohexylamino)-2-selenazoline. J. Indian Chem. Soc. 1997, 74, 161–162. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,3-selenazolidine and perhydro-1,3-selenazine derivatives. Eur. J. Org. Chem. 2005, 3128–3137. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. First synthesis of a selenazepane. Tetrahedron Lett. 2005, 46, 6723–6725. [Google Scholar]
- Azerbaev, I. N.; Tsoi, L. A.; Salimbaeva, A. D.; Cholpankulova, S. T.; Ryskieva, G. A.; Kalkabaeva, L. T.; Aitkhozhaeva, M. Zh. Reactions of acetylene amines with isocyanates, isothiocyanates, and isoselenocyanates. Trudy Instit. Khim. Nauk Akad. Nauk Kazak.SSR 1980, 52, 128–146. [Google Scholar]
- Koketsu, M.; Sakai, T.; Kiyokuni, T.; Garud, D. R.; Ando, H.; Ishihara, H. One-pot synthesis of 2-imino-1,3-selenazolidines by reaction of isoselenocyanates with propargylamine. Heterocycles 2006, 68, 1607–1615. [Google Scholar] [CrossRef]
- Banert, K.; Toth, C. Synthesis and reactions of vinyl isoselenocyanates. Angew. Chem. Int. Ed. Engl. 1995, 34, 1627–1629. [Google Scholar] [CrossRef]
- Banert, K.; Hückstädt, H.; Vrobel, K. Synthesis and reactions of isothiocyanate-substituted allenes and 1,3-butadienes. Angew. Chem. Int. Ed. Engl. 1992, 31, 90–92. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Suzuki, K.; Yano, A.; Matsumoto, M.; Kubo, T.; Minato, H.; Arai, Y.; Tsuji, J.; Watanabe, N. Novel and orally bioavailable inducible nitric oxide synthase inhibitors: synthesis and evaluation of optically active 4,5-dialkyl-2-iminoselenazolidine derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1361–1366. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Yano, A.; Ido, M.; Matsumoto, M.; Kawasaki, M. 4,5-Disubstituted-1,3-oxazolidin-2-imine derivatives: a new class of orally bioavailable nitric oxide synthase inhibitor. Bioorg. Med. Chem. Lett. 2004, 14, 313–316. [Google Scholar] [CrossRef]
- Ueda, S.; Terauchi, H.; Yano, A.; Matsumoto, M.; Kubo, T.; Kyoya, Y.; Suzuki, K.; Ido, M.; Kawasaki, M. 4,5-Dialkylsubstituted 2-imino-1,3-thiazolidine derivatives as potent inducible nitric oxide synthase inhibitors. Bioorg. Med. Chem. 2004, 12, 4101–4116. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Derivatives from isoselenocyanates: Synthesis of 2-phenyl-6H-[5,1,3]benzoselenadiazocine. Helv. Chim. Acta 2004, 87, 1452–1466. [Google Scholar] [CrossRef]
- Koketsu, M.; Takahashi, A.; Ishihara, H. A facile preparation of selenohydantoins using isoselenocyanate. J. Heterocycl. Chem. 2007, 44, 79–81. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 2-arylaminoselenazolo[5,4-b]pyridines. Heterocycles 2003, 61, 569–579. [Google Scholar] [CrossRef]
- Kristian, P.; Koščik, D.; Gonda, J. Heterocycles with pyrido[3,2-e]-1,3-selenazine and pyrido[3,4-e]-1,3-selenazine ring systems. Collect. Czech. Chem. Commu. 1983, 48, 3567–3574. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1H-5-selena-1,3,6-triazaaceanthrylene derivatives. Helv. Chim. Acta 2003, 86, 3235–3243. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hara, S.; Nagata, N.; Ikeda, K.; Mizuno, Y. Synthesis of new 1-chalcogenapurines by the reaction of 5-aminoimidazole-4-carbonitrile with isochalcogenocyanates. Heterocycles 1995, 41, 47–56. [Google Scholar] [CrossRef]
- Atanassov, P. K.; Linden, A.; Heimgartner, H. Synthesis of 4-(phenylamino)quinazoline-2(1H)-selones and diselenides from isoselenocyanates: Dimroth rearrangement of an intermediate. Helv. Chim. Acta 2004, 87, 1873–1887. [Google Scholar] [CrossRef]
- EI Ashry, E. S. H.; EI Kilany, Y.; Rashed, N.; Assafir, H. Dimroth rearrangement: Translocation of heteroatoms in heterocyclic rings and its role in ring transformations of heterocycles. Adv. Heterocycl. Chem. 1999, 75, 79–167. [Google Scholar] [CrossRef] Fujii, T.; Itaya, T. The Dimroth rearrangement in the adenine series: A review updated. Heterocycles 1998, 48, 359–390. [Google Scholar] [CrossRef] Itaya, T.; Ito, N.; Kanai, T.; Fujii, T. Purines. LXXV. Dimroth rearrangement, hydrolytic deamination, and pyrimidine-ring breakdown of 7-alkylated 1-alkoxyadenines: N(1)-C(2) versus N(1)-C(6) bond fission. Chem. Pharm. Bull. 1997, 45, 832–841. [Google Scholar] [CrossRef]
- Taylor, E. C.; Ravindranathan, R. V. Reaction of anthranilonitrile and N-methylanthranilonitrile with phenyl isocyanate and phenyl isothiocyanate. J. Org. Chem. 1962, 27, 2622–2627. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of selenosemicarbazides and 1,2,4-triazoles. Heterocycles 2006, 68, 1191–1200. [Google Scholar] [CrossRef]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Use of hydrazine for the synthesis of 1,3,4-selenadiazine derivatives. Helv. Chim. Acta 2006, 89, 1322–1329. [Google Scholar] [CrossRef]
- Athayde-Filho, P. F. D.; Simas, A. M.; Goncalves, S. M. C.; Miller, J. Synthesis and characterization of mesoionic 1,3,4-triazolium-2-selenolates. Phosphorus Sulfur Silicon Relat. Elem. 2000, 161, 115–121. [Google Scholar] [CrossRef]
- Stefaniak, L.; Jazwiuski, J. Chem. Heterocycl. Compd. 1995, 31, 1027. [CrossRef]
- Bocian, W.; Jazwinski, J.; Stefaniak, L. Multinuclear 77Se, 15N, 14N, 13C and 1H NMR study of mesoionic 1,3,4-triazolium-5-selenolates and related compounds. Polish J. Chem. 1995, 69, 85–89. [Google Scholar]
- Bartels-Keith, J.R.; Burgess, M.; Stevenson, J. M. Carbon-13 nuclear magnetic resonance studies of heterocycles bearing carbon-sulfur and carbon-selenium bonds: 1,3,4-thiadiazole, 1,3,4-selenadiazole, and tetrazole derivatives. J. Org. Chem. 1977, 42, 3725–3731. [Google Scholar] [CrossRef]
- Duarte, H.C. Master’s Thesis, Universidade Estadual de Campinas, 1979.
- Asanuma, Y.; Fujiwara, S.; Shin-ike, T.; Kambe, N. Selenoimidoylation of alcohols with selenium and isocyanides and its application to the synthesis of selenium-containing heterocycles. J. Org. Chem. 2004, 69, 4845–4848. [Google Scholar]
- Fujiwara, S.-i; Shikano, Y.; Shin-ike, T.; Kambe, N.; Sonoda, N. Stereoselective synthesis of new selenium-containing heterocycles by cyclocarbonylation of aminoalkynes with carbon monoxide and selenium. J. Org. Chem. 2002, 67, 6275–6278. [Google Scholar]
- Mizuno, T.; Nakamura, F.; Ishino, Y.; Nishiguchi, I.; Hirashima, T.; Ogawa, A.; Kambe, N.; Sonoda, N. Facile stereoselective synthesis of 4-alkylidene-2-oxo-1,3-oxathiolanes from 2-alkyn-1-ols, carbon monoxide, and sulfur. Synthesis 1989, 770–771. [Google Scholar]
- Silks, L. A.; Peng, J.; Odom, J. D.; Dunlap, R. B. Synthesis of (4S,5R)-(–)-4-methyl-5-phenyloxazolidine-2-selone: a chiral auxiliary reagent capable of detecting the enantiomers of (R,S)-lipoic acid by 77Se nuclear magnetic resonance spectroscopy. J. Chem. Soc., Perkin Trans. 1 1991, 2495–2498. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Synthesis of 2-selenoxo-1,3-thiazolidin-4-ones and 2-selenoxo-1,3-thiazinan-4-ones from isoselenocyanates. Heterocycles 2005, 65, 1903–1915. [Google Scholar] [CrossRef]
- Shafiee, A.; Fanaii, G. A facile synthesis of N-(4-aryl-1,3-dithiol-2-ylidene)-amides, N-(4- or 5-aryl-1,3-thiaselenol-2-ylidene)-amides, and N-(4-aryl-1,3-diselenol-2-ylidene)-amides. Synthesis 1984, 512–514. [Google Scholar] [CrossRef]
- Zmitrovich, N. I.; Petrov, M. L.; Potekhin, K. A.; Balashova, E.V. Synthesis, crystal and molecular structure of unsubstituted 2-phenylimino-1,3-diselenol. Zh. Obshchei Khim. 1996, 66, 1684–1687. [Google Scholar]
- Zmitrovich, N. I.; Petrov, M. L. α,β-Unsaturated thiolates and their analogs in cycloaddition reactions. XXIII. A convenient one-pot synthesis of 2-phenylimino-1,3-thiaselenoles and -1,3-diselenoles. Zh. Org. Khim. 1996, 32, 1870–1874. [Google Scholar]
- Koketsu, M.; Yamamura, Y.; Ishihara, H. Synthesis of 2-selenoxoperhydro-1,3-selenazin-4-ones and 2-selenoxo-1,3-selenazolidin-4-ones via diselenocarbamate intermediates. Synthesis 2006, 2738–2742. [Google Scholar] [CrossRef]
- Suchár, G.; Štefko, R. Synthesis of tetrahydro-1,3,5-selenodiazine-2-selenones. Chem. Zvesti 1982, 36, 419–422. [Google Scholar]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: synthesis of 2-methylidene-1,3-selenazolidine derivatives. Tetrahedron 2006, 62, 3344–3354. [Google Scholar]
- Murai, T. Thio-, seleno-, telluro-amides. Top. Curr. Chem. 2005, 251, 247–272. [Google Scholar] Baskakov, Yu. A.; Volovnik, L. L.; Vasil’ev, A. F.; Aryutkina, N. L.; Tibanov, P. V.; Negrebetskii, V. V. Herbicidal derivatives of hydroxylamine. XXXIV. Reaction of haloacetic acid halides with hydroxylamine derivatives of thiourea. Khim. Geterotsikl. Soedin. 1971, 3, 104–107. [Google Scholar] Velkov, Z. Thioamides - some properties and preparations. Bulg. Chem. Commun. 2003, 35, 227–230. [Google Scholar] Jagodzinski, T. S. Thioamides as useful synthons in the synthesis of heterocycles. Chem. Rev. 2003, 103, 197–228. [Google Scholar] Mitchell, S. C.; Steventon, G. B. Thiourea and its biological interactions. Sulfur Rep. 1994, 16, 117–137. [Google Scholar] [CrossRef] Bobbitt, J. M.; Bourque, A. J. Synthesis of heterocycles using aminoacetals. Heterocycles 1987, 25, 601–616. [Google Scholar] [CrossRef]
- Kaválek, J.; Jirman, J.; Štĕrba, J. V. Kinetics and mechanism of rearrangement and methanolysis of acylphenylthioureas. Collect. Czech. Chem. Commun. 1985, 50, 766–778. [Google Scholar] [CrossRef] Kaválek, J.; Novak, J.; Štĕrba, V. Kinetics of hydrolysis and rearrangements of S-acylthiouronium salts. Collect. Czech. Chem. Commun. 1982, 47, 2702–2710. [Google Scholar] [CrossRef] Pratt, R. F.; Bruice, T. C. Reactions of S-acylisothioureas. II. Effects of structure and stereochemistry on the rates of hydrolysis, thiol elimination, and S to N acyl migration in acylic systems. J. Am. Chem. Soc. 1972, 94, 2823–2837. [Google Scholar] [CrossRef] Bruice, T. C.; Pratt, R. F. Reactions S-acylisothioureas. I. S- to N-acyl migrations in S-benzoylisothiobiotin and analogs. Biochemistry 1971, 10, 3178–3185. [Google Scholar] [CrossRef]
- Klika, K. D.; Janovec, L.; Imrich, J.; Suchár, G.; Kristian, P.; Sillanpää, R.; Pihlaja, K. Regioselective synthesis of 2-imino-1,3-thiazolidin-4-ones by treatment of N-(anthracen-9-yl)-N`-ethylthiourea with bromoacetic acid derivatives. Eur. J. Org. Chem. 2002, 1248–1255. [Google Scholar]
- Zhou, Y.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,3-selenazoles from N-phenylimidoyl isoselenocyanates. Helv. Chim. Acta 2000, 83, 1576–1598. [Google Scholar]
- Teller, J.; Dehne, H.; Zimmermann, T.; Fischer, G.W.; Olk, B. Novel synthesis of 2-dialkylamino-4-arylthiazoles. Z. Chem. 1989, 29, 255. [Google Scholar] Teller, J.; Dehne, H.; Zimmermann, T.; Fischer, G.W.; Olk, B. Substituierte 2-amino-thiazole aus α-thiocyanato-acetophenonen und dialkylaminen. J. Prakt. Chem. 1990, 332, 453–460. [Google Scholar] [CrossRef] Zimmermann, T.; Fischer, G.W.; Teller, J.; Dehne, H.; Olk, B. Substituierte N,N`-bis(thiazol-2-yl)-diaminoalkane aus α-thiocyanato-acetophenonen und N,N`-dialkyl-diamino-alkanen. J. Prakt. Chem. 1990, 332, 723–730. [Google Scholar] [CrossRef]
- Sommen, G.; Comel, A.; Kirsch, G. New synthesis of selenophenes and condensed ring systems from ketene dithioacetals. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 939–943. [Google Scholar] [CrossRef]
- Maeda, H.; Kambe, N.; Sonoda, N.; Fujiwara, S.; Shin-ike, T. Synthesis of 1,3-selenazoles and 2-imidazolin-5-selones from isoselenocyanates and isocyanides. Tetrahedron 1997, 53, 13667–13680. [Google Scholar]
- Becher, J.; Frandsen, E. G.; Dreier, C.; Henriksen, L. Derivatives and reactions of glutacondialdehyde. VII. Reaction of the glutacondialdehyde anion with heterocumulenes. Acta Chem. Scand. B 1977, 31, 843–847. [Google Scholar]
- Favero, F.; Sommen, G. L.; Linden, A.; Heimgartner, H. Synthesis of 5-selenoxo-1,2,4-triazole-1-carboxylates from isoselenocyanates and azodicarboxylates. Heterocycles 2006, 67, 749–762. [Google Scholar] [CrossRef]
- Bittner, S.; Assaf, Y.; Krief, A.; Pomerantz, M.; Ziemnicka, B. T.; Smith, C. G. Synthesis of N-acyl-, N-sulfonyl-, and N-phosphinylphospha(PV)azenes by a redox-condensation reaction using amides, triphenylphosphine, and diethyl azodicarboxylate. J. Org. Chem. 1985, 50, 1712–1718. [Google Scholar] [CrossRef] Mukaiyama, T. Oxidation-reduction condensation. Angew. Chem., Int. Ed. Engl. 1976, 15, 94–103. [Google Scholar] [CrossRef] Mitsunobu, O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1981, 1–28. [Google Scholar] [CrossRef] Fauduet, H.; Burgada, R. Condensation of spirophosphoranes containing phosphorus-hydrogen bond with diethyl azodicarboxylate. C.R. Acad. Sci. Ser. C 1980, 291, 81–83. [Google Scholar] Itzstein, M. V.; Jenkins, I. D. The reaction of diols with triphenylphosphine and di-isopropyl azodicarboxylate. Part 1. Formation of cyclic phosphoranes from 1,3- and 1,4- diols. J. Chem. Soc., Perkin Trans. 1 1986, 437–445. [Google Scholar] [CrossRef] Camp, D.; Jenkins, I. D. The mechanism of the Mitsunobu esterification reaction. Part II. The involvement of (acyloxy)alkoxyphosphoranes. J. Org. Chem. 1989, 54, 3049–3054. [Google Scholar] [CrossRef] Itzstein, M. V.; Jenkins, I. D. The mechanism of the Mitsunobu reaction. II. Dialkoxytriphenylphosphoranes. Aust. J. Chem. 1983, 36, 557–563. [Google Scholar] Niclas, H.-J.; Martin, D. Eine einfache synthese von phosphin-imiden unter verwendung von azodicarbonsäure-dialkylestern. Tetrahedron Lett. 1978, 19, 4031–4032. [Google Scholar] [CrossRef]
- Hughes, D. L. Org. Reactions 1992, 42, 335–656.
- Ľabbé, G.; Dekerk, J.-P.; Martens, C.; Toppet, S. Chemistry of N-sulfonyl-substituted thiiranimines. J. Org. Chem. 1980, 45, 4366–4371. [Google Scholar] [CrossRef]
- Suchár, G.; Kristian, P. Synthesis, properties, and reactions of heterodienes. III. Cycloaddition reactions of isoselenocyanates with enamines and diazomethane. Chem. Zvesti 1975, 29, 244–249. [Google Scholar]
- Zhou, Y.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Synthesis of 1,2,3-selenadiazole derivatives. Helv. Chim. Acta 2000, 83, 539–553. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Ishihara, H. Synthesis of 1,3-selenazetidine derivatives from imines and thiocarbamoyl isoselenocyanate. Heterocycles 2006, 68, 2107–2112. [Google Scholar] [CrossRef]
- Sommen, G. L.; Linden, A.; Heimgartner, H. Selenium-containing heterocycles from isoselenocyanates: Cycloaddition of carbodiimides to selenazetidines. Helv. Chim. Acta 2005, 88, 766–773. [Google Scholar] [CrossRef]
- Koketsu, M.; Yamamura, Y.; Ando, H.; Ishihara, H. Synthesis of 1,3-selenazetidines and 4H-1,3,5-oxadiazines using acyl isoselenocyanates. Heterocycles 2006, 68, 1267–1273. [Google Scholar] [CrossRef]
- Atanassov, P.K.; Linden, A.; Heimgartner, H. Synthesis of 3-acetyl-N-aryl-4-diethylaminoselenet-2(2H)-imines from 4-diethylamino-3-butyn-2-one and aryl isoselenocyanates. Heterocycles 2004, 62, 521–533. [Google Scholar]
- Yoo, C. Y.; Choi, E. B.; Pak, C. S. Preparation of 2H-thietimines from the reaction of 4-dialkylamino-3-butyn-2-one with aryl isothiocyanates. Synlett 2001, 361–364. [Google Scholar]
- Billert, T.; Beckert, R.; Döring, M.; Görls, H. Beiträge zur Chemie der pyrido[1,2-a]pyrazine - reaktivität gegenüber heterocumulenen der kohlensäurereihe und ketenen. J. Prakt. Chem. 1999, 341, 332–341. [Google Scholar] [CrossRef]
- Koketsu, M.; Kiyokuni, T.; Sakai, T.; Ando, H.; Ishihara, H. Carbonate extension. Synthesis of 1,3-selenazines and 1,3-selenazolidines via intramolecular addition of N-allylselenoureas. Chem. Lett. 2006, 35, 626–627. [Google Scholar] [CrossRef]
- Tamaru, Y.; Mizutani, M.; Furukawa, Y.; Kawamura, S.; Yoshida, Z.; Yanagi, K.; Minobe, M. 1,3-Asymmetric induction: highly stereoselective synthesis of 2,4-trans-disubstituted γ-butyrolactones and γ-butyrothiolactones. J. Am. Chem. Soc. 1984, 106, 1079–1085. [Google Scholar] Cardillo, G.; Orena, M.; Sandri, S. A new synthetic approach to 3-amino-1,2-diols from allylic alcohols via trichloroacetimidates. J. Chem. Soc., Chem. Commun. 1983, 1489–1490. [Google Scholar] [CrossRef] Bongini, A.; Cardillo, G.; Orena, M.; Porzi, G.; Sandri, S. Regio- and stereocontrolled synthesis of epoxy alcohols and triols from allylic and homoallylic alcohols via iodocarbonates. J. Org. Chem. 1982, 47, 4626–4633. [Google Scholar] Bartlett, P. A.; Meadows, J. D.; Brown, E. G.; Morimoto, A.; Jernstedt, K. K. Carbonate extension. A versatile procedure for functionalization of acyclic homoallylic alcohols with moderate stereocontrol. J. Org. Chem. 1982, 47, 4013–4018. [Google Scholar] [CrossRef]
- Koketsu, M.; Otsuka, T.; Swenson, D.; Ishihara, H. The synthesis of 1-thia-6-oxa-6aλ4-seleno-3-azapentalene and a 3H-1,2,4-dithiazole. Org. Biomol. Chem. 2007, 5, 613–616. [Google Scholar] [CrossRef]
- Morel, G.; Marchand, E.; Sinbandhit, S.; Toupet, L. 5-Chloro-3-methylthio-1,2,4-thiadiazol-2-ium chlorides as useful synthetic precursors to a variety of 6aλ4-thiapentalene systems. Heteroatom. Chem. 2003, 14, 95–105. [Google Scholar] [CrossRef]
- Lai, L.-L.; Reid, D. H.; Nicol, R. H.; Rhodes, J. B. Reactions of fused dihydro-1,2,4-thiadiazoles with isocyanates and isothiocyanates to give 6aλ4-thia-1,3,4,6-tetraazapentalene derivatives. Heteroatom. Chem. 1994, 5, 149–157. [Google Scholar] [CrossRef] Lai, L.-L.; Reid, D. H. Reactions of fused dihydro-1,2,4-thiadiazoles with isoselenocyanates giving 6aλ4-thia-1,3,4,6-tetraazapentalene derivatives and 5,10-dihydro-1,2,4-thiaselenazolo[4,5-b][2,4]benzodiazepines. Heteroat. Chem. 1996, 7, 97–109. [Google Scholar] [CrossRef] Lai, L.-L.; Reid, D. H. Synthesis of 6aλ4-thia-1,6-diselena-3,4-diazapentalenes, 1,6,6aλ4-triselena-3,4-diazapentalenes, 6aλ4-thia-1,3,4,6-tetraazapentalenes, and 6aλ4-Selena-1,3,4,6-tetraazapentalenes from cyclic thioureas and selenoureas. Heteroatom. Chem. 1997, 8, 13–27. [Google Scholar] [CrossRef]
- Goerdeler, J.; Löbach, W. Ringöffnende Cycloadditionen, VI. Reaktionen von 5-imino-∆3-1,2,4-thiadiazolinen mit heterocumulenen (Präparative gesichtspunkte). Chem Ber. 1979, 112, 517–531. [Google Scholar] [CrossRef]
- Ding, Y.; Kong, J.; Reid, D. H. Trithia- and dithiaselenapentalenes from benzylidene-1,2-dithioles and heterocumulenes. Heteroatom. Chem. 1997, 8, 233–244. [Google Scholar] [CrossRef]
- Matsumura, N.; Konishi, T.; Hayashi, H.; Yasui, M.; Iwasaki, F.; Mizuno, K. Synthesis and properties of novel macrocyclic compounds bearing thiourea moieties by use of chemical feature of hypervalent sulfur. J. Heterocyclic Chem. 2002, 39, 189–202. [Google Scholar] [CrossRef]
- Sample Availability: Not applicable.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Garud, D.R.; Koketsu, M.; Ishihara, H. Isoselenocyanates: A Powerful Tool for the Synthesis of Selenium-Containing Heterocycles. Molecules 2007, 12, 504-535. https://doi.org/10.3390/12030504
Garud DR, Koketsu M, Ishihara H. Isoselenocyanates: A Powerful Tool for the Synthesis of Selenium-Containing Heterocycles. Molecules. 2007; 12(3):504-535. https://doi.org/10.3390/12030504
Chicago/Turabian StyleGarud, Dinesh Ramesh, Mamoru Koketsu, and Hideharu Ishihara. 2007. "Isoselenocyanates: A Powerful Tool for the Synthesis of Selenium-Containing Heterocycles" Molecules 12, no. 3: 504-535. https://doi.org/10.3390/12030504



