Synthesis of 5-Acetoxymethyl- and 5-Hydroxymethyl-2-vinyl-furan
Abstract
:Introduction
Results and Discussion
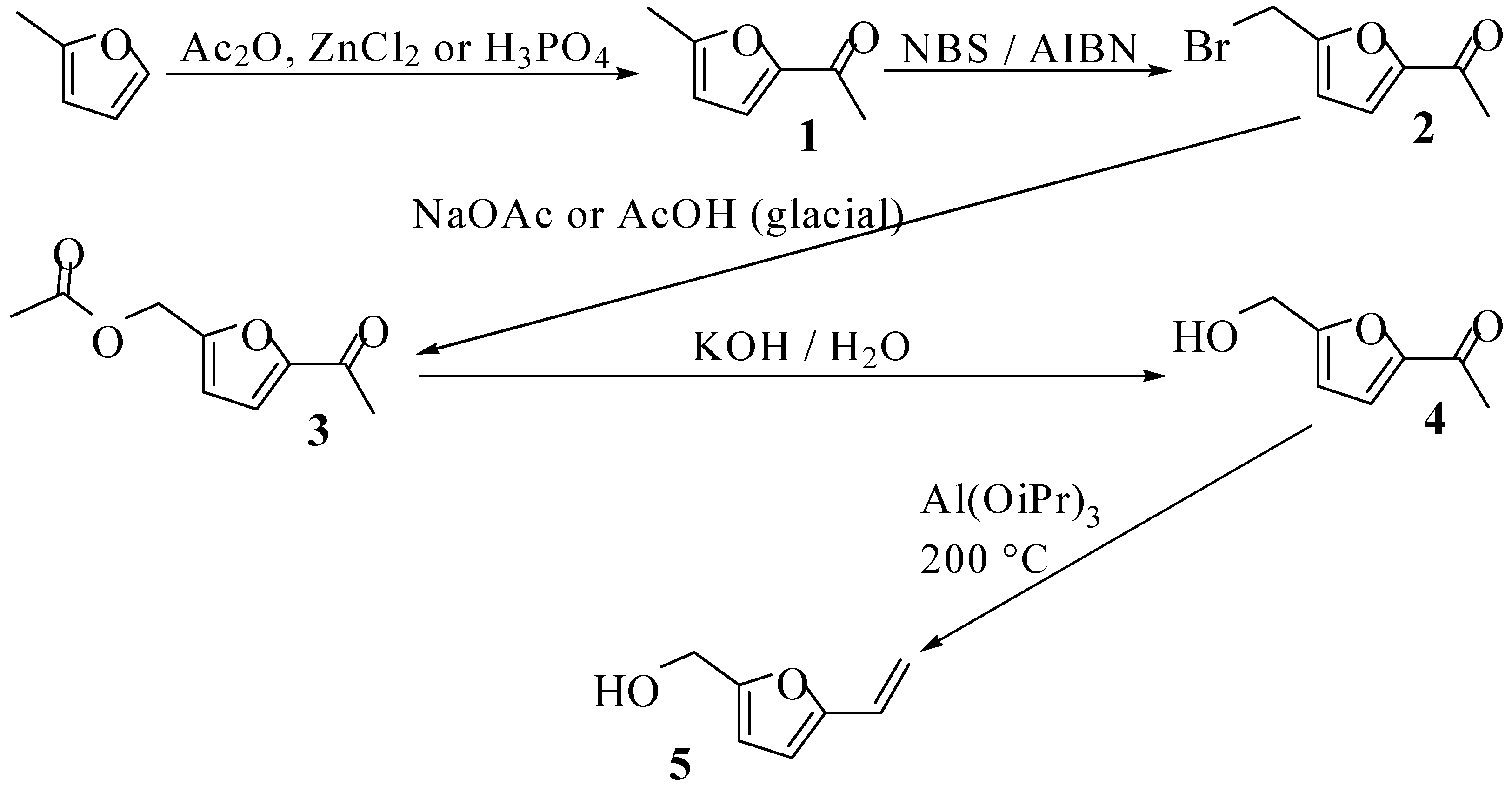
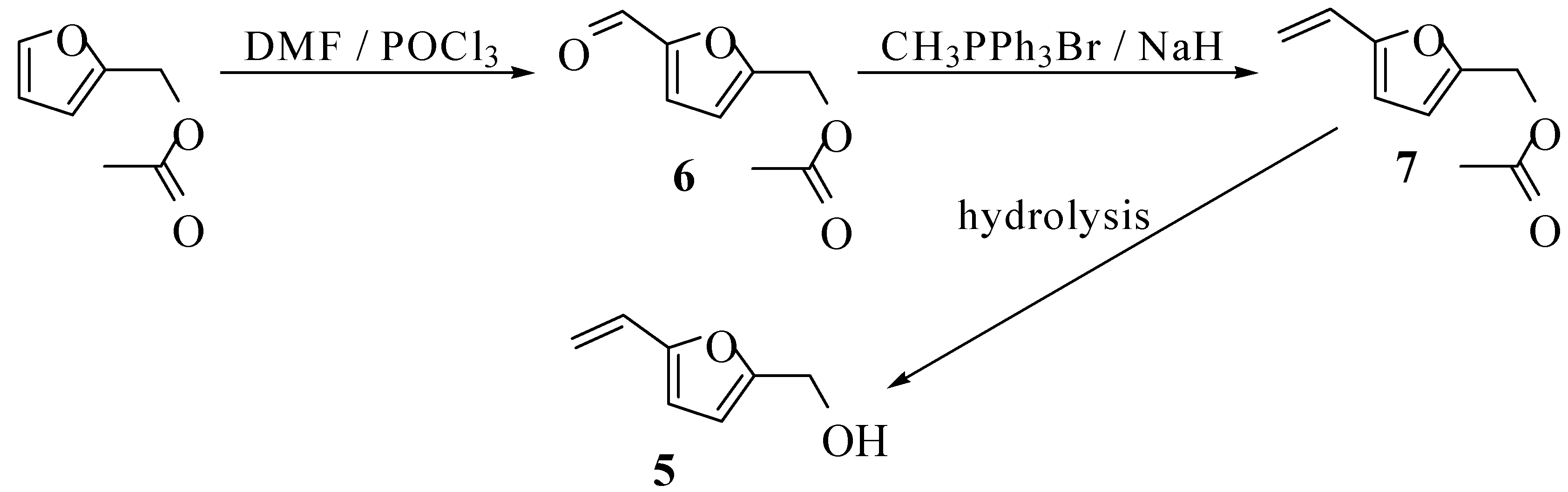
Conclusions
Experimental
General
5-Methyl-2-acetylfuran (1)
5-Acetyl-2-furfuyl acetate (3)
5-Acetyl furfuryl alcohol (4)
5-Hydroxymethylvinylfuran (5)

5-Formylfurfuryl acetate (6)

5-Acetoxymethyl-2-vinylfuran (7)

Acknowledgments
References
- Dzhemilev, U. M.; Gubaidullin, L. Y.; Tolstikov, B. A. Reaction of 2-vinylfuran with formaldehyde catalyzed by palladium complexes. Russ. Chem. Bull. 1976, 25, 683–684. [Google Scholar]
- Moreau, C.; Mohamed Naceur Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar]
- Farrar, M. W.; Levine, R. J. Condensations effected by boron fluoride complexes. III. The acylation of certain substituted thiophenes and furans. J. Am. Chem. Soc. 1950, 72, 3695–3698. [Google Scholar] [CrossRef]
- Itoh, T.; Hall, H. K., Jr. 7-Chloro-7-phenyl-8,8-dicyanoquinodimethane. A Novel Initiator for Cationic Polymerizations. Macromolecules 1990, 23, 4879–4881. [Google Scholar] [CrossRef]
- Benfaremo, N.; Cava, M. P. Studies in anthracycline synthesis: simple Diels-Alder routes to pachybasin, ω-hydroxypachybasin, aloe-emodin, and fallacinol. J. Org. Chem. 1985, 50, 139–141. [Google Scholar] [CrossRef]
- Wilds, A. L. Reduction with aluminium alkoxides (The Meerwein-Ponndorf-Verley reduction). Org. React. 1944, 2, 178–223. [Google Scholar]
- Mowry, D. T.; Renoll, M.; Huber, F. W. Vinyl aromatic compounds. I. The vapor phase dehydration of arylmethylcarbinols. J. Am. Chem. Soc. 1946, 68, 1105–1109. [Google Scholar]
- Semple, J. E.; Wang, P. C.; Lysenko, Z.; Joullie, M. M. Total Synthesis of (+)-Furanomycin and Stereoisomers. J. Am. Chem. Soc. 1980, 25, 7505–7510. [Google Scholar]
- Achmatowicz, O.; Burzynska, M. H. Stereospecific synthesis of methyl D,L-hex-2-ulopyranosides from furan compounds. Tetrahedron 1982, 38, 3507–3513. [Google Scholar]
- Klein, L. L. Structural effects on intramolecular furan cycloadditions. J. Org. Chem. 1985, 50, 1770–1773. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mehner, A.; Montero, A.L.; Martinez, R.; Spange, S. Synthesis of 5-Acetoxymethyl- and 5-Hydroxymethyl-2-vinyl-furan. Molecules 2007, 12, 634-640. https://doi.org/10.3390/12030634
Mehner A, Montero AL, Martinez R, Spange S. Synthesis of 5-Acetoxymethyl- and 5-Hydroxymethyl-2-vinyl-furan. Molecules. 2007; 12(3):634-640. https://doi.org/10.3390/12030634
Chicago/Turabian StyleMehner, Alexander, Ana L Montero, Ricardo Martinez, and Stefan Spange. 2007. "Synthesis of 5-Acetoxymethyl- and 5-Hydroxymethyl-2-vinyl-furan" Molecules 12, no. 3: 634-640. https://doi.org/10.3390/12030634



