Litchi Flavonoids: Isolation, Identification and Biological Activity
Abstract
:1. Introduction
2. Extraction and purification of flavonoids
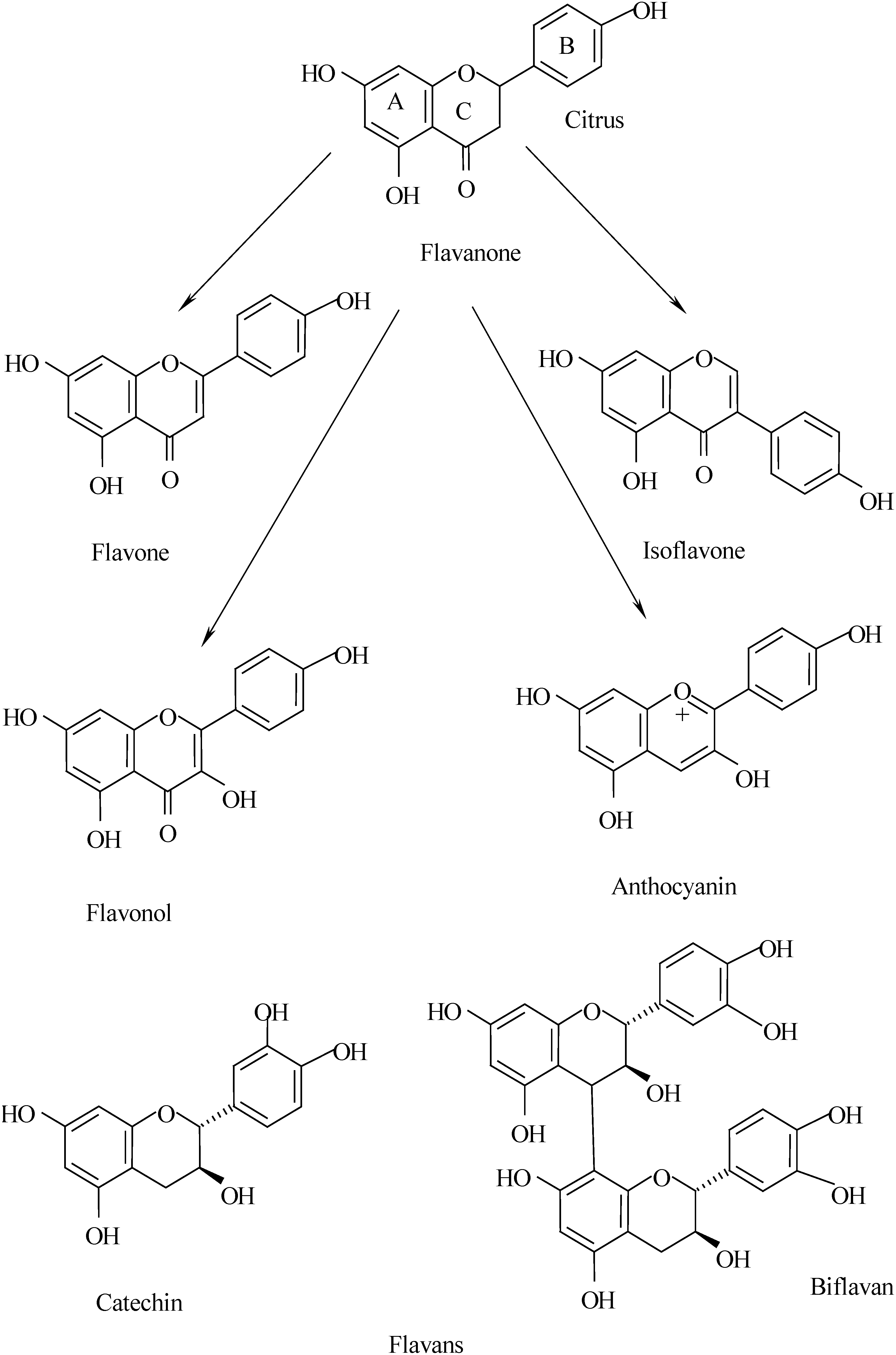
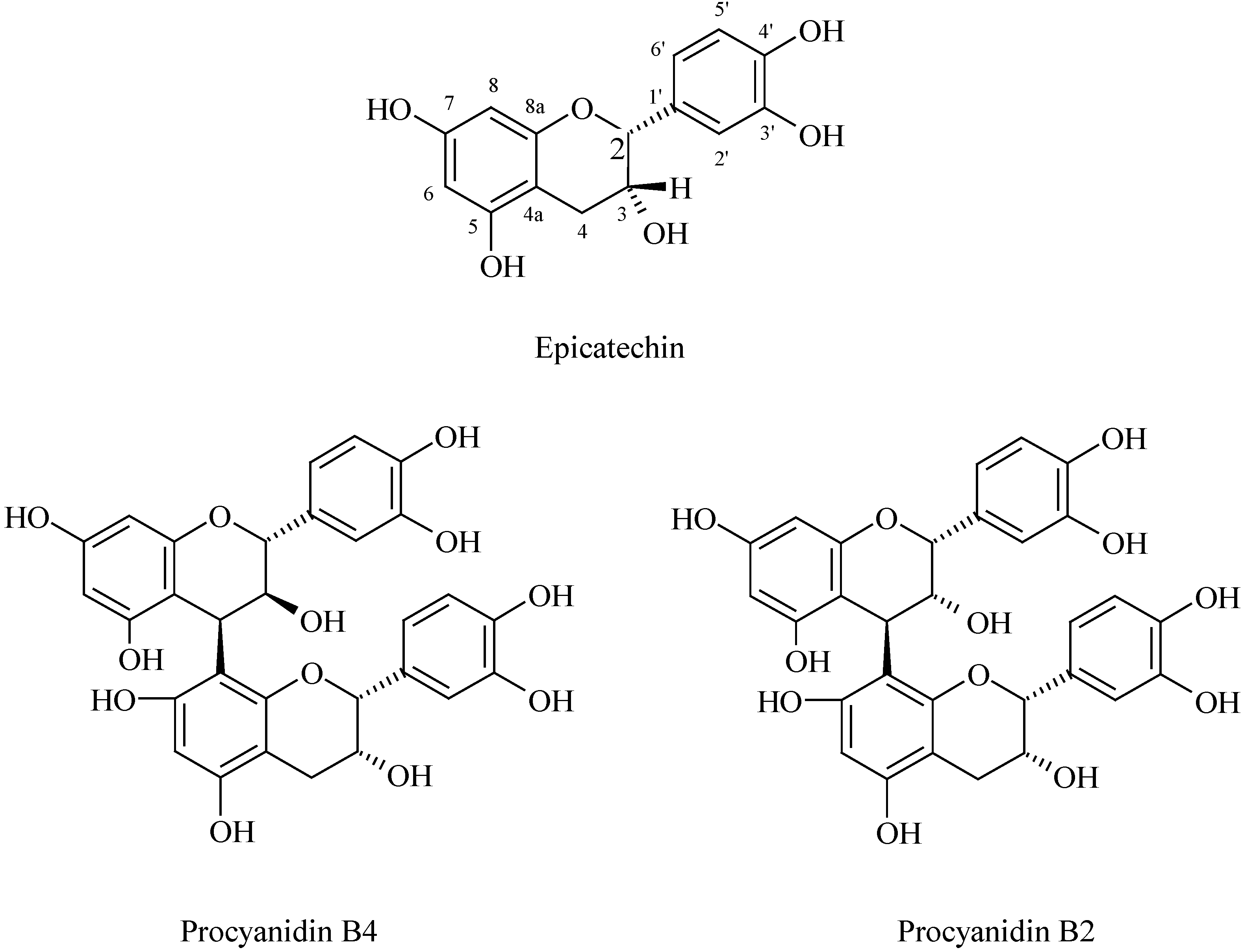
3. Identification of flavonoids
4. Analysis of flavonoids
5. Flavonoid contents
| Phenolic compounds | Area (%) |
|---|---|
| Gallic acid | 0.4 |
| Procyanidin B1 | 1.0 |
| (+)-Catechin | 1.6 |
| (+)-Gallocatechin | 1.1 |
| Procyanidin B4 | 5.3 |
| Procyanidin B2 | 11.4 |
| (−)-Epicatechin | 32.5 |
| (−)-Epigallocatechin | 10.9 |
| (−)-Epicatechin-3-gallate | 0.3 |
| Cyanidin-3-glucoside | 31.7 |
| Malvidin-3-glucoside | 3.8 |
| Solvent fraction | Flavonoid content (mg/g dried weight) | Percent flavonoids |
|---|---|---|
| Hexane | 0.04 | 0.2 |
| Ethyl acetate | 21.3 | 83.1 |
| Butanol | 3.5 | 13.6 |
| Water | 0.8 | 3.1 |
6. Stability of flavonoids

7. Functions of flavonoids
8. Pharmacological properties of flavonoids
8a. Antioxidant activity
| Amount of anthocyanins (μg) | DPPH scavenging activity (%) | Hydroxyl radical scavenging activity (%) | Superoxide anion scavenging activity (%) |
|---|---|---|---|
| 20 | 91 | 89 | 83 |
| 100 | 93 | 93 | 89 |
8b. Potential anticancer activity
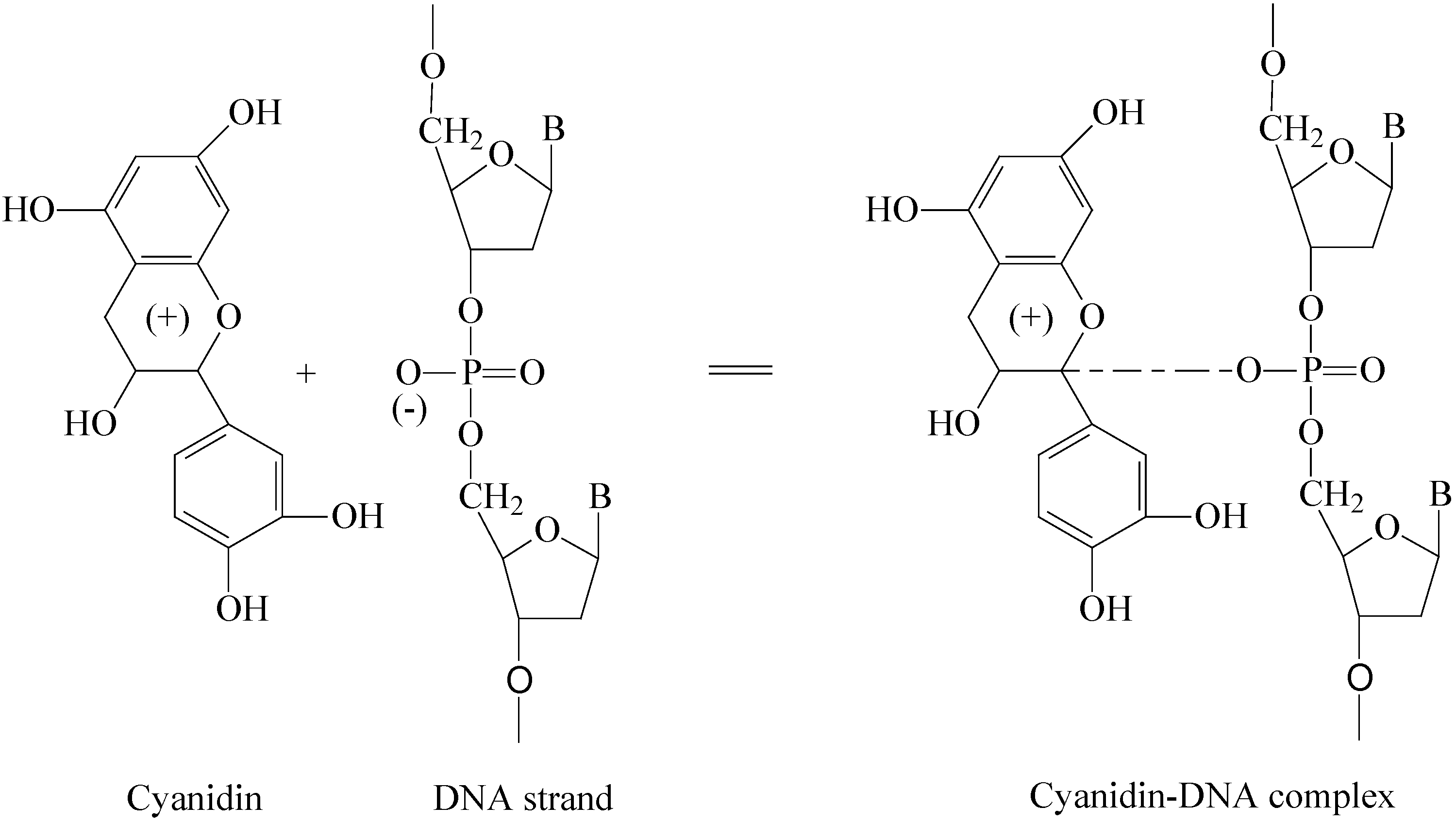
8c. Interaction with DNA
9. Prospects
Acknowledgments
References
- Ghosh, S.P. World trade in litchi: past, present and future. Acta Hort. 2001, 558, 23–30. [Google Scholar]
- Jiang, Y.M.; Wang, Y.; Song, L.L.; Liu, H.; Lichter, A.; Kerdchoechuen, O.; Joyce, D.C.; Shi, J. Production and postharvest characteristics and technology of litchi fruit: an overview. Aust. J. Exp. Agric. 2006, 46, 1541–1556. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Human Nutr. 2004, 59, 113–122. [Google Scholar]
- Clifford, A.H.; Cuppett, S.L. Review: anthocyanins − nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Harborne, J.B. The Flavonoids: Advances in Research since 1980; Chapman and Hall: London, 1988. [Google Scholar]
- Tripoli, E.; Guardia, M.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, (in press). [Google Scholar]
- Cook, N.C.; Samman, S. Review: flavonoids − chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr. 2004, 134, 1529–1535. [Google Scholar]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharm. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Lyons-Wall, P.M.; Samman, S. Flavonoids - dietary perspectives and health benefits. Nutr. Soc. Aust. 1997, 21, 106–114. [Google Scholar]
- Ma, T.E.; Celestino, S.; Julián, C.R. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.D.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Duan, X.W.; Jiang, Y.M.; Su, X.G.; Zhang, Z.Q.; Shi, J. Antioxidant property of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1382–1388. [Google Scholar]
- Zhao, M.; Yang, B.; Wang, J.S.; Li, B.Z.; Jiang, Y.M. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 2006, 98, 737–742. [Google Scholar]
- Zhang, D.L.; Grigor, J.M.; Quantick, P.C. Changes in phenolic compounds in litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Postharv. Biol. Technol. 2000, 19, 165–172. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.M.; Martín-Ortega, G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agr. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Pang, X.Q.; Ji, Z.L.; Jiang, Y.M. Role of anthocyanin degradation in litchi pericarp browning. Food Chem. 2001, 75, 217–221. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Pang, X.Q.; Yang, C.; Ji, Z.L.; Jiang, Y.M. Purification and structural analysis of anthocyanins from litchi pericarp. Food Chem. 2004, 84, 601–604. [Google Scholar] [CrossRef]
- Prasad, U.S.; Jha, O.P. Changes in pigmentation patterns during litchi ripening: flavonoid production. Plant Biochem. J. 1978, 5, 44–49. [Google Scholar]
- Lee, H.S.; Wicker, L. Anthocyanin pigments in the skin of lychee fruit. J. Food Sci. 1991, 56, 466–468. [Google Scholar] [CrossRef]
- Lee, H.S.; Wicker, L. Quantitative changes in anthocyanin pigments of lychee fruit during refrigerated storage. Food Chem. 1991, 40, 263–270. [Google Scholar] [CrossRef]
- Sarni-Manchado, L.; Roux, E.; Guerneve, L.; Lozano, Y.; Cheynier, V. Phenolic composition of litchi fruit pericarp. J. Agr. Food Chem. 2000, 48, 5995–6002. [Google Scholar]
- Glässgen, W.E.; Wray, S.D.; Metzger, J.W.; Seitz, H.U. Anthocyanins from cell suspension cultures of Daucus carota. Phytochemistry 1992, 31, 1593–1601. [Google Scholar] [CrossRef]
- Careri, M.; Mangia, A.; Musci, M. Overview of the applications of liquid chromatography mass spectrometry interfacing systems in food analysis: naturally occurring substances in food. J. Chromatogr. A 1998, 794, 263–297. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Prenzler, P.; Antolovich, M. Applications of mass spectrometry to plant phenols. TAC-Trends Anal. Chem. 1999, 18, 362–372. [Google Scholar]
- Mouly, P.; Gaydou, E.M.; Auffray, A. Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography. J. Chromatogr. A 1998, 800, 171–179. [Google Scholar] [CrossRef]
- Jiang, Y.M. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Duan, X.W.; Joyce, D.C.; Zhang, Z.Q.; Li, J.R. Advances in understanding enzymatic browning of harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rept. 2001, 18, 310–333. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Review: flavanones, chalcones and dihydrochalcones − nature, occurrence and dietary burden. J. Sci. Food Agr. 2000, 80, 1073–1080. [Google Scholar]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Cooke, D.; Steward, W.P.; Gescher, A.J.; Marczylo, T. Anthocyans from fruits and vegetables – Does bright colour signal cancer chemopreventive activity? Eur. J. Cancer 2005, 41, 1931–1940. [Google Scholar] [CrossRef]
- Espin, J.C.; Soler-Rivas, C.; Wichers, H.J.; Garcia-Viguera, C. Anthocyanin-based natural colorants: a new source of antiradical activity for foodstuff. J. Agr. Food Chem. 2000, 48, 1588–1592. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agr. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar]
- Mann, J. Natural products in cancer chemotherapy: past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: a review of probable mechanisms of action and potential applications. Amer. J. Clin. Nut. 2001, 74, 418–425. [Google Scholar]
- Suolinna, E.M.; Buchsbaum, R.N.; Racker, E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975, 35, 1865–1872. [Google Scholar]
- Lottto, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Rad. Biol. Med. 2006, 41, 1727–1746. [Google Scholar]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Yochum, L.A.; Kushi, L.H.; Meyer, K.; Folsom, A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Amer. J. Epidemiol. 2000, 149, 943–949. [Google Scholar]
- Hirano, T.; Gotoh, M.; Oka, K. Natural flavonoids and lignans are potent cytostatic agents against human leukemia HL-60 cells. Life Sci. 1994, 55, 1061–1069. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Basu, S.; Sarkar, N.; Ghosh, A.C. Advances in cancer therapy with plant based natural products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W. Potential anticancer activity of litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2006, 239, 144–150. [Google Scholar]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y.M. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Wang, J.; Lin, P.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W.; Wei, Y. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol. Appl. Pharm. 2006, 215, 168–178. [Google Scholar] [CrossRef]
- Meiers, S.; Kemeny, M.; Weyand, U.; Gastpar, R.; Angerer, E.; Marko, D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J. Agric. Food Chem. 2001, 49, 958–962. [Google Scholar] [CrossRef]
- Mas, T.; Susperregui, J.; Berke, B.; Cheze, C.; Moreau, S.; Nuhrich, A.; Vercauteren, J. DNA triplex stabilization property of natural anthocyanins. Phytochemistry 2000, 53, 679–687. [Google Scholar]
- Sample availability: Not applicable.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Li, J.; Jiang, Y. Litchi Flavonoids: Isolation, Identification and Biological Activity. Molecules 2007, 12, 745-758. https://doi.org/10.3390/12040745
Li J, Jiang Y. Litchi Flavonoids: Isolation, Identification and Biological Activity. Molecules. 2007; 12(4):745-758. https://doi.org/10.3390/12040745
Chicago/Turabian StyleLi, Jiangrong, and Yueming Jiang. 2007. "Litchi Flavonoids: Isolation, Identification and Biological Activity" Molecules 12, no. 4: 745-758. https://doi.org/10.3390/12040745