Molecularly Imprinted Polymers for 5-Fluorouracil Release in Biological Fluids
Abstract
:Introduction
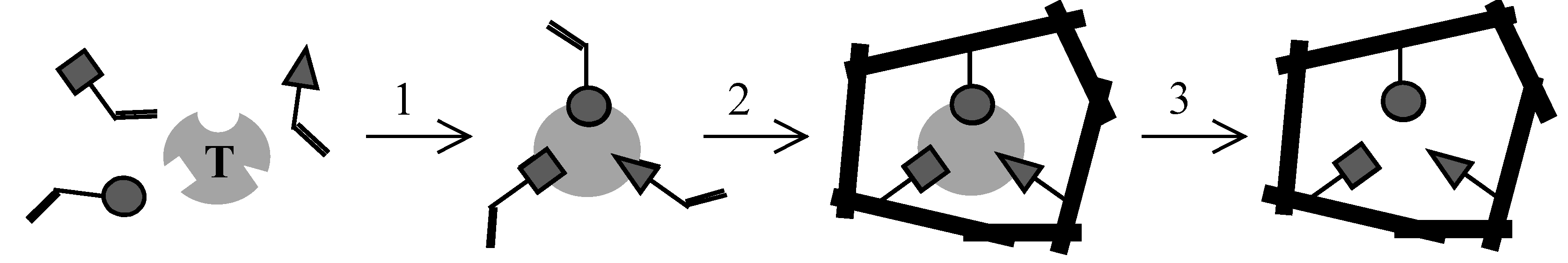

Results and Discussion
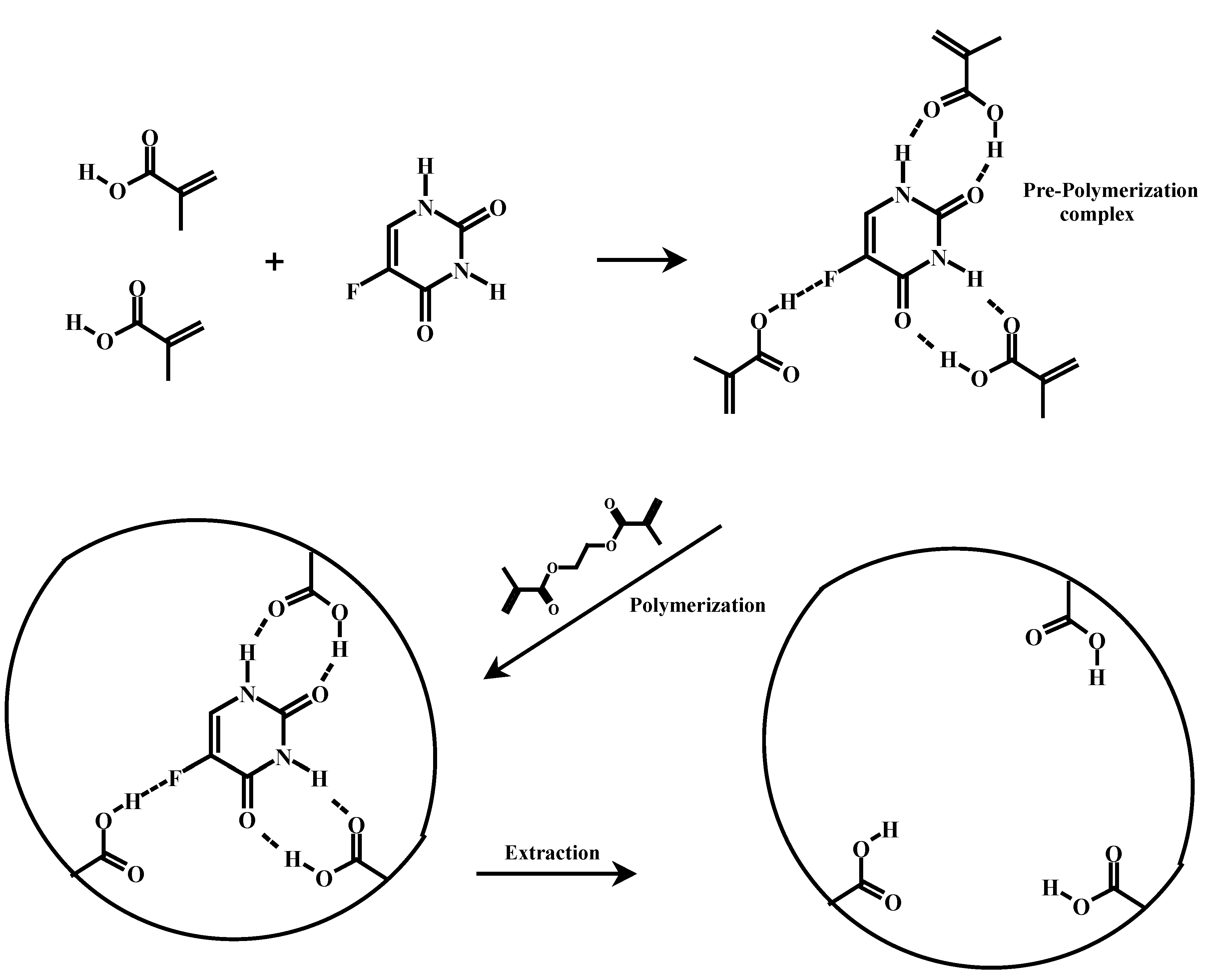
Rationale of MIP preparation and choice
| Polymer | 5 – FU (mmol) | MAA (mmol) | EGDMA (mmol) | AIBN (mg) |
|---|---|---|---|---|
| MIP-1 | 2.0 | 8.0 | 32.0 | 103 |
| MIP-2 | 2.0 | 8.0 | 40.0 | 103 |
| MIP-3 | 2.0 | 16.0 | 20.0 | 103 |
MIP and NIP recognition properties
| Matrix | 5-CFU bound | Matrix | 5-CFU bound |
|---|---|---|---|
| MIP-1 | 5 ± 3 % | NIP-1 | 5 ± 3% |
| MIP-2 | 10 ± 3% | NIP-2 | 7 ± 3% |
| MIP-3 | 30 ± 3% | NIP-3 | 10 ± 3% |
| Matrix | 5-CFU bound pH = 1.0 | 5-CFU bound pH = 7.4 | Matrix | 5-CFU bound pH = 1.0 | 5-CFU bound pH = 7.4 |
|---|---|---|---|---|---|
| MIP-1 | 15 ± 3 % | 10 ± 3 % | NIP-1 | 15 ± 3 % | 10 ± 3 % |
| MIP-2 | 19 ± 3 % | 12 ± 3 % | NIP-2 | 20 ± 3 % | 8 ± 3 % |
| MIP-3 | 35 ± 3 % | 27 ± 3 % | NIP-3 | 6 ± 3 % | 9 ± 3 % |
| Matrix | Uracil bound (organic solvent) | Uracil bound pH = 1.0 | Uracil bound pH = 7.4 |
|---|---|---|---|
| MIP-3 | 16 ± 3 % | 11% ± 3 % | 9 ± 3 % |
| NIP-3 | 15 ± 3 % | 10 ± 3 % | 10 ± 3 % |
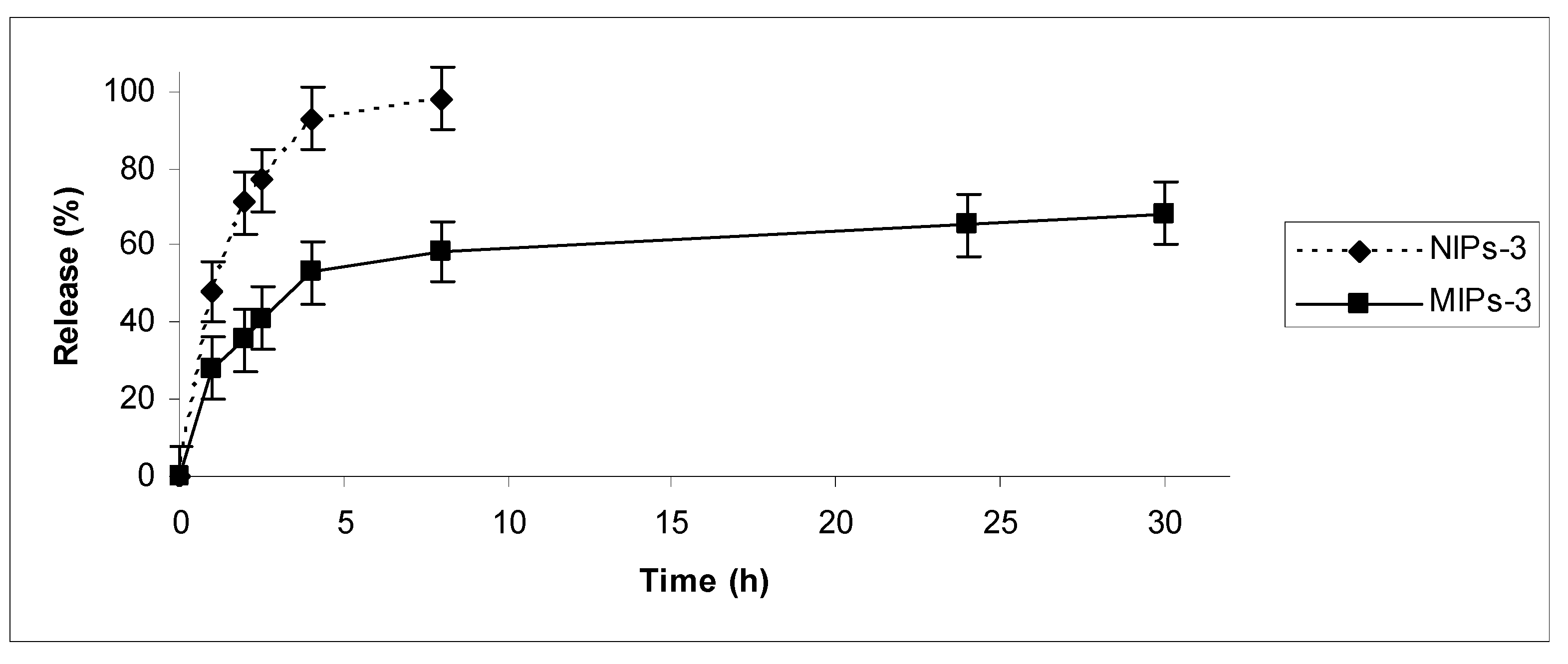
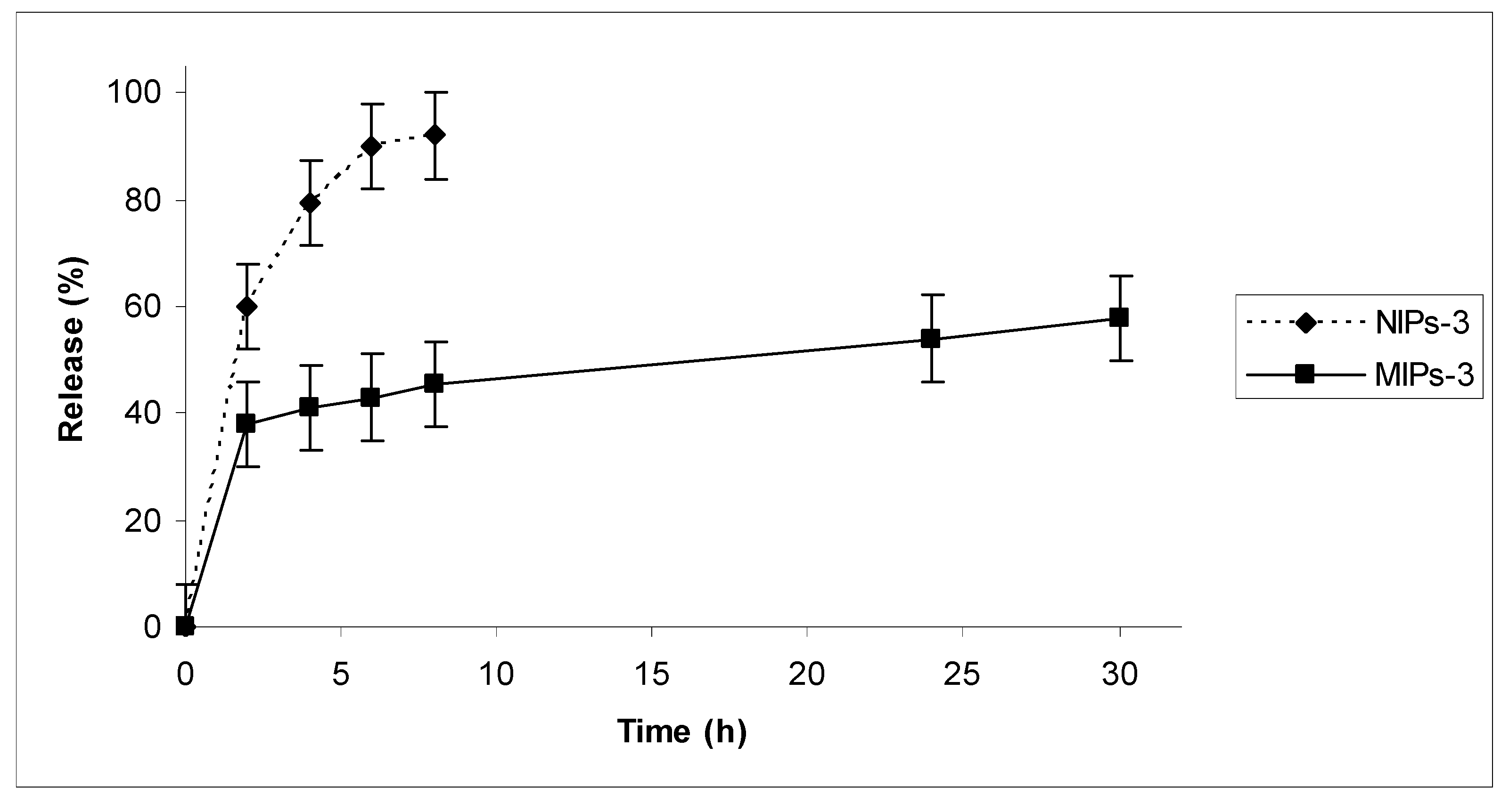
Drug release in vitro
Conclusions
Experimental
General
Synthesis of 5-FU molecularly imprinted polymer
Binding experiments
Drug Loading by the Soaking Procedure
In vitro release studies
HPLC Analysis
Acknowledgments
References
- Caro, E.; Marce, R.M.; Cormack, P.A.G.; Sherrington, D.C.; Borrull, F. On-line solid-phase extraction with molecularly imprinted polymers to selectively extract substituted 4-chlorophenols and 4-nitrophenol from water. J. Chromatogr. A 2003, 995, 233–238. [Google Scholar] [CrossRef]
- Ye, L.; Yu, Y.; Mosbach, K. Towards the development of molecularly imprinted artificial receptors for the screening of estrogenic chemicals. Analyst 2001, 126, 760–765. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Peppas, N.A. Structural analysis and diffusional behavior of molecularly imprinted polymer networks for cholesterol recognition. Chem. Mat. 2005, 17, 6719–6727. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Cirillo, G.; Trombino, S.; Cassano, R.; Picci, N. Molecularly Imprinted polymers for selective adsorption of cholesterol from aqueous environment. E-polymers 2007, 13, 1–9. [Google Scholar]
- Hishiya, T.; Asanuma, H.; Komiyama, M. Molecularly imprinted cyclodextrin polymers as stationary phases of high performance liquid chromatography. Polym. J. 2003, 35, 440–445. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly imprinted polymers in analytical chemistry. Analyst 2001, 126, 747–756. [Google Scholar] [CrossRef]
- Theodoridis, G.; Manesiotis, P. Selective solid-phase extraction sorbent for caffeine made by molecular imprinting. J. Chromatogr. A 2002, 948, 163–169. [Google Scholar] [CrossRef]
- Haupt, K. Imprinted polymers - Tailor-made mimics of antibodies and receptors. Chem. Commun. 2003, 2, 171–177. [Google Scholar] [CrossRef]
- Svitel, J.; Surugiu, I.; Dzgoev, A.; Ramanathan, K.; Danielsson, B. Functionalized surfaces for optical biosensors: Applications to in vitro pesticide residual analysis. J. Mater. Sci.: Mater. Med. 2001, 12, 1075–1078. [Google Scholar]
- Nicholls, I.A.; Matsui, J.; Krook, M.; Mosbach, K. Some Recent Developments in the Preparation of Novel Recognition Systems: A Recognition Site for the Selective Catalysis of an Aldol Condensation using Molecular Imprinting and Specific Affinity Motifs for α-chymotrypsin using a Phage Display Peptide Library. J. Mol. Recognit. 1996, 9, 652–657. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar] [CrossRef]
- Suedee, R.; Srichana, T.; Martin, G.P. Evaluation of matrices containing molecularly imprinted polymers in the enantioselective-controlled delivery of β-blockers. J. Control Release 2000, 66, 135–147. [Google Scholar] [CrossRef]
- Cunliffe, D.; Kirby, A.; Alexander, C. Molecularly imprinted drug delivery systems. Adv. Drug Del. Rev. 2005, 57, 1836–1853. [Google Scholar]
- Allender, C.J.; Richardson, J.; Woodhouse, B.; Heard, C.M.; Brain, K.R. Pharmaceutical applications for molecularly imprinted polymers. Int. J. Pharm. 2000, 195, 39–43. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Muzzalupo, R.; Spizzirri, U.G.; Trombino, S.; Cassano, R.; Picci, N. Spherical Molecularly Imprinted Polymers (SMIPs) via a Novel Precipitation Polymerization in the Controlled Delivery of Sulfasalazine. Macr. Biosci. 2004, 4, 22–26. [Google Scholar]
- Puoci, F.; Iemma, F.; Cirillo, G.; Muzzalupo, R.; Spizzirri, U.G.; Picci, N. Molecularly imprinted polymers based on amidic functional monomers for selective recognition of cholesterol in aqueous media. Macromol. Ind. J. 2006, 2, 2–4. [Google Scholar]
- Yoneda, K.; Yamamoto, T.; Ueta, E.; Osaki, T. The inhibitory action of BOF-A2, a 5-fluorouracil derivative, on squamous cell carcinoma. Cancer Lett. 1999, 137, 17–25. [Google Scholar] [CrossRef]
- Johnson, K.R.; Young, K.K.; Fan, W. Antagonistic interplay between antimitotic and G1-S arresting agents observed in experimental combination therapy. Clin. Cancer Res. 1999, 5, 2559–2565. [Google Scholar]
- Fournier, E.; Passirani, C.; Colin, N.; Breton, P.; Sagodira, S.; Benoit, J.P. Development of novel 5-FU-loaded poly(methylidenemalonate 2.1.2)-based microspheres for the treatment of brain cancers. Eur. J. Pharm. Biopharm. 2004, 57, 189–197. [Google Scholar]
- Kugimiya, A.; Mukawa, T.; Takeuchi, T. Synthesis of 5-fluorouracil-imprinted polymers with multiple hydrogen bonding interactions. Analyst 2001, 126, 772–774. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Byrne, M.E. Configurational biomimesis in drug delivery: Molecular imprinting of biologically significant molecules. Adv. Drug Del. Rev. 2004, 56, 1599–1620. [Google Scholar]
- Grem, J.L.; Nguyen, D.; Monahan, B.P.; Kao, V.; Geoffrey, F.J. Sequence dependent antagonism between fluorouracil and paclitaxel in human breast cancer cells. Biochem. Pharmacol. 1999, 58, 477–486. [Google Scholar] [CrossRef]
- Karsson, J.G.; Andersson, L.I.; Nicholls, I.A. Probing the molecular basis for ligand-selective recognition in molecularly imprinted polymers selective for the local anaesthetic bupivacaine. Anal. Chim. Acta 2001, 435, 57–64. [Google Scholar] [CrossRef]
- Sellergren, B.; Shea, K. Chiral ion-exchange chromatography: Correlation between solute retention and a theoretical ion-exchange model using imprinted polymers. J. Chromatogr. A 1993, 654, 17–28. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Puoci, F.; Iemma, F.; Cirillo, G.; Picci, N.; Matricardi, P.; Alhaique, F. Molecularly Imprinted Polymers for 5-Fluorouracil Release in Biological Fluids. Molecules 2007, 12, 805-814. https://doi.org/10.3390/12040805
Puoci F, Iemma F, Cirillo G, Picci N, Matricardi P, Alhaique F. Molecularly Imprinted Polymers for 5-Fluorouracil Release in Biological Fluids. Molecules. 2007; 12(4):805-814. https://doi.org/10.3390/12040805
Chicago/Turabian StylePuoci, Francesco, Francesca Iemma, Giuseppe Cirillo, Nevio Picci, Pietro Matricardi, and Franco Alhaique. 2007. "Molecularly Imprinted Polymers for 5-Fluorouracil Release in Biological Fluids" Molecules 12, no. 4: 805-814. https://doi.org/10.3390/12040805




